Abstract
The function of the epithelial-to-mesenchymal transition (EMT) during hepatocellular carcinoma (HCC) progression is well established. However, the regulatory mechanisms modulating this phenomenon remain unclear. Homeobox B9 (HOXB9) has been proposed as an oncogene in many cancer developments, but its function and underlying mechanisms in HCC metastasis remain unknown. HOXB9 modulates EMT through the transforming growth factor-β1 (TGF-β1) pathway, which is a recognized regulator of EMT in HCC cells. The knockdown of HOXB9 decreased the migration and invasion of HCC cells. Conversely, the HOXB9 overexpression led to an increase in the above-mentioned phenotypes in HCC cells. Further analysis of HOXB9-regulated cellular functions showed the ability of this transcription factor to induce EMT. Moreover, we demonstrated that the TGF-β1 pathway is important in HOXB9-induced EMT in HCC cells. These findings define a novel cellular mechanism regulated by HOXB9, which controls EMT phenotype in HCC. This study is the first to illustrate the pivotal function of HOXB9 in regulating the metastatic behavior of HCC cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer deaths worldwide. The vast majority of HCCs arise in the context of chronic liver injury, inflammation, and hepatocyte proliferation provoked by different etiologies. HCCs have a highly variable clinical course and include several subgroups with distinct pathways of hepatocarcinogenesis [1]. These processes share common mechanisms with embryogenesis and can be considered as an aberrant form of organogenesis [2]. The transcription factors involved in early-stage liver development belong to the winged helix [3] and zinc-finger families [4], subsequently several of them are ascribed to the homeobox gene family [2].
Homeobox genes (HOX in human) are a family of homeodomain-containing transcription factors that determine cellular identity during development. In mammals, 39 HOX genes have been identified and organized into four paralogous clusters (A–D) located in four different chromosomes [5]. These genes are significantly involved in embryonic development, particularly for patterning the anterior-to-posterior axis from the level of the hindbrain to the end of the spine [6]. Over the past decades, several HOX genes were identified in normal tissues and tissues of people with different diseases and metabolic alterations [7]. Recently, numerous studies have demonstrated the deregulated HOX gene expression in cancer, including lung, prostate, breast, colon, bladder, and ovarian cancer [8–12].
Little is known about the involvement of HOX genes in liver cancerogenesis. Recent studies have demonstrated the implication of Homeobox B9 (HOXB9) in tumorigenesis. Several studies have suggested that HOXB9 protein is upregulated in breast tumor. By altering the microenvironment, the protein induces several tumorigenic phenotypes and promotes disease progression [13, 14]. HOXB9 expression has also been reported in lung cancer cell lines [11]. Hayashida et al. [14] demonstrated that in breast carcinoma, HOXB9 induces the expression of several angiogenic factors, such as vascular endothelial growth factor, basic fibroblast growth factor, interleukin-8, and angiopoietin-like-2, as well as human epidermal growth factor and transforming growth factor (TGF)-β1. The induced expression of these factors activates their respective pathways, thus causing increased cell motility and acquisition of mesenchymal phenotypes through epithelial-to-mesenchymal transition (EMT).
Epithelial-to-mesenchymal transition is a tightly controlled process that is critical for various biological events, such as embryomorphogenesis, fibrotic diseases, and tumor metastasis [15]. EMT provides cells the ability to migrate and invade. In tumor metastasis, carcinoma cells acquire a mesenchymal phenotype, leading to enhanced motility and the acquirement of the ability to evade apoptosis; these changes allow tumor cells to metastasize and establish secondary tumors at distant sites [16, 17]. Increasing numbers of signaling molecules implicated in the control of EMT during embryogenesis and cancer progression is observed [18–20]. The members of multipotent cytokine TGF-β superfamily have important functions in the control of cell proliferation, apoptosis, differentiation, and aging. TGF-β1 is the most widely used inducer of EMT for in vitro studies. Snail and slug zinc-finger transcriptional factors are known as repressors of E-cadherin, which acts as EMT inducers and therefore has critical functions in TGF-β1-induced EMT [21–23].
Although HOXB9 is known to be upregulated in many cancers, we are unsure whether the HOXB9 expression is enhanced in human HCC cell lines. The involvement of HOXB9 in EMT and the participation of HOXB9 as a target of TGF-β1 signaling are uncertain. We investigated the HOXB9 expression in human HCC cell lines and its functions in EMT regulation.
Materials and methods
Cell lines and antibodies
Human HCC cell lines (SMMC-7721, HCCLM3, SK-Hep-1, and Huh-7) were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). Normal liver cell lines such as HL-7702 and L-02 were obtained from China Center for Type Culture collection (CCTCC, Wuhan, Hubei, China). The cells were maintained in minimum essential medium (MEM) (Invitrogen, Carlsbad, CA, USA) supplemented with 10 % fetal bovine serum (FBS) (Invitrogen). Mouse monoclonal HOXB9, p-Smad2, Smad2, TGF-β1, Snail, Slug, and ZEB1 were purchased from Abcam. Mouse monoclonal β-actin antibody was the product of Santa Cruz Biotech (Santa Cruz, CA, USA).
Plasmids construct
Human cDNA of HOXB9 was cloned as previously reported [13]. The full-length cDNAs were subcloned into the multiple cloning sites of the pBabe plasmid, forming the pBabe HOXB9 expression plasmids. Short hairpin RNA (shRNA) targeting HOXB9-1(sense:5′-TCGACGCGAATCTCTCTTTGGCAAGTTCAAGAGACTTGCCAAAGAGAGATTCGTTTTTTGGAAT-3′; antisense:5′-CTAGATTCCAAAAAACGAATCTCTCTTTGGCAAGTCTCTTGAACTTGCCAAAGAGAGATTCGCG-3′) and HOXB9-2(sense:5′-TCGAGGCACCTAAGCATCAGATGGATTCAAGAGACTTGCCAAAGAGAGATTCGTTTTTTGGAAT-3′; antisense:5′-CTAGATTCCAAAAAAGGTAGACTACGAATCCACG CTCTTGAACTTGCCAAAGAGAGATTCGCG-3′) were initially inserted into the Sal I and Xba I sites of pSuper plasmid, forming the pSuper-shHOXB9-1 and pSuper-shHOXB9-2 plasmids.
Generation of stable cell lines
BEL-4705 cell line was transfected with the pBabe or pBabe-HOXB9 plasmid using the Lipofectamine 2000 according to the manufacturer’s instructions (Invitrogen). HepG2 cell line was transfected with the pSuper-Scramble or pSuper-shHOXB9-1 and pSuper-shHOXB9-2 plasmids using the Lipofectamine 2000. Stable transfectants were obtained after selection by puromycin (Invitrogen; 10 μg/ml) for 2 weeks. Expression of HOXB9 mRNA and protein in stable cell lines was analyzed by qRT-PCR and Western blot, respectively.
qRT-PCR
Total RNA was extracted using Trizol reagent, and cDNA was synthesized using SuperScript II Reverse Transcriptase (Invitrogen). qRT-PCR and data collection were performed with an ABI PRISM 7900HT sequence detection system. The primers used for the amplification of the indicated genes are listed in Table S1.
Immunoblot analysis
Cells and tumors were lysed in lysis buffer (1 % Triton X-100, 150 mM NaCl, 10 mM Tris–HCl, at pH 7.4, 1 mM EDTA, 1 mM EGTA, containing 2 mM NaF, 1 mM sodium orthovanadate, 10 μg ml−1 leupeptin, 10 μg ml−1 pepstatin, 10 μg ml−1 aprotinin, 10 μg ml−1 E 64, and 1 mM Pefabloc; EMD). Equal amounts of protein lysates were loaded and separated by SDS–PAGE and followed by Western blot.
Confocal immunofluorescence microscopy
Immunofluorescence analysis was performed as described previously [XX]. Cell lines were plated on culture slides (Costar, Cambridge, MA, USA) and after 24 h were rinsed with phosphate-buffered saline (PBS) and fixed in ice-cold methanol–acetone for 5 min at −20 °C. The cells were then blocked for 30 min in 10 % BSA (Sigma-Aldrich St. Louis, MO, USA) in PBS and then incubated with primary monoclonal antibodies in PBS for 2 h at room temperature. After three washes in PBS, the slides were incubated for 1 h in the dark with secondary goat anti-mouse, or goat anti rabbit antibodies (Invitrogen, Carlsbad, CA, USA). After three further washes, the slides were stained with 4,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich St. Louis, MO, USA) for 5 min to visualize the nuclei and examined using an Carl Zeiss confocal imaging system (Zeiss 780).
Wound-healing assay
Cells were seeded in 6-cm culture plates, and the cell monolayers were wounded by scratching with sterile plastic 200 μl micropipette tips and photographed using phase-contrast microscopy immediately and 48 h after wounding. The assays were independently performed in triplicate. The migration distance of each cell was measured after the photographs were converted to Photoshop files.
Cell invasion and motility assay
Invasion of cells was measured in Matrigel (BD Biosciences)-coated Transwell inserts (6.5 mm, Costar, Cambridge, MA, USA) containing polycarbonate filters with 8-μm pores. The inserts were coated with 50 μl of 1 mg/ml Matrigel matrix according to the manufacturer’s recommendations. About 2 × 105 cells in 200 μl of serum-free medium were plated in the upper chamber, whereas 600 μl of medium with 10 % FBS was added to lower well. After 24-h incubation, top cells were removed and bottom cells were counted. Cells that migrated to the lower surface of the membrane were fixed in 4 % paraformaldehyde and stained with 0.5 % crystal violet. For each membrane, five random fields were counted at 10× magnification. The mean was calculated, and data were presented as mean ± SEM from three independent experiments done in triplicate. Motility assays were performed using Transwell membrane inserts (6.5 mm, Costar, Cambridge) containing polycarbonate filters with 8-μm pores. Methods used in cell migration assay were similar to Matrigel invasion assay except that the Transwell insert was not coated with Matrigel.
Statistical analysis
Data were described as the mean ± SD and analyzed by Student’s two-tailed t test. The limit of statistical significance was P < 0.05. Statistical analysis was done with SPSS/Win11.0 software (SPSS, Inc., Chicago, Illinois, USA).
Results
HOXB9 was highly expressed in invasive HCC cell lines
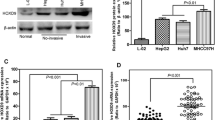
To develop in vitro models to examine the mechanistic function of HOXB9 in HCC biology, we determined the protein expression of HOXB9 in four human HCC cell lines (SMMC-7721, HCCLM3, SK-Hep-1, and Huh-7) and two normal human hepatocytes (HL-7702 and L-02) by Western blot analysis. Results showed that HOXB9 was highly expressed in three invasive HCC cells (HCCLM3, SK-Hep-1, and Huh-7) compared with the noninvasive HCC cell (SMMC-7721) and normal human hepatocytes (HL-7702 and L-02). Real-time reverse transcription-PCR (qRT-PCR) analysis confirmed that HOXB9 mRNA expression emulated the protein expression in these cell lines. Similar to the data shown in Fig. 1a, Huh-7 showed the highest HOXB9 mRNA expression and SMMC-7721 was among the HCC cells with the lowest HOXB9 mRNA expression (Fig. 1b).
Generation of stable cell lines
To test the oncogenic activity of HOXB9 in HCC, SMMC-7721 was selected for HOXB9-overexpression experiments, and Huh-7 cells were transfected with the pSuper-Scramble or pSuper-shHOXB9-1 and pSuper-shHOXB9-2 plasmids in knockdown experiments. The stable overexpression of HOXB9 in SMMC-7721 cell (designated as SMMC-7721-HOXB9) and the silencing of HOXB9 in Huh-7 cell (designated as Huh-7-shHOXB9-1 and Huh-7-shHOXB9-2) were retrovirally established. The HOXB9 levels in these resultant cell lines were confirmed by Western blot analysis (Fig. 2a, c) and qRT-PCR (Fig. 2b, d).
Generation of stable cell lines. HOXB9 protein expression was evaluated by Western blot analysis in Huh-7-pSuper-Scramble, Huh-7-shHOXB9-1, and Huh-7-shHOXB9-2 cells (a). HOXB9–mRNA expression was analyzed by qRT-PCR in Huh-7-pSuper-Scramble, Huh-7-shHOXB9-1, and Huh-7-shHOXB9-2 cells (b). HOXB9 protein expression was examined by Western blot analysis in SMMC-7721-pBabe and SMMC-7721-HOXB9 cells (c). HOXB9 mRNA expression was analyzed by qRT-PCR in SMMC-7721-pBabe and SMMC-7721-HOXB9 cells (d). **P < 0.01 is based on Student’s t test. All results are from three independent experiments. Error bars indicate standard deviation
HOXB9 induces EMT in HCC cells
To investigate whether HOXB9 positively regulates cell migration and invasion, we initially observed morphological changes and found that Huh-7-shHOXB9-1 and Huh-7-shHOXB9-2 cells reverted to an epithelial phenotype compared with their respective control cells (pSuper-Scramble) (Fig. 3a). This observation was further confirmed by the expression analyses of epithelial and mesenchymal markers (Fig. 3c). HOXB9 knockdown decreased the levels of mesenchymal markers (N-cadherin and vimentin) and increased the levels of epithelial markers (E-cadherin and α-catenin) in Huh-7-shHOXB9-1 and Huh-7-shHOXB9-2 cells compared with Huh-7-pSuper-Scr cells (Figs. 3c, 4). Moreover, the expression levels of mRNA correlated with the corresponding protein levels (Fig. 5a), indicating that HOXB9 affects the expression of epithelial and mesenchymal markers at the transcript level. Conversely, SMMC-7721-HOXB9 cells reverted to a mesenchymal phenotype compared to the control cells (Fig. 3b). Consistent with this finding, HOXB9 overexpression decreased the levels of epithelial markers and increased the levels of mesenchymal markers (Figs. 3d, 4, 5b).
HOXB9 regulates the transition between epithelial and mesenchymal phenotypes in HCC cells. Representative phase-contrast images of Huh-7-pSuper-Scramble, Huh-7-shHOXB9-1, and Huh-7-shHOXB9-2 cells showed HOXB9-modulated morphological changes (a). Representative phase-contrast images of SMMC-7721-pBabe and SMMC-7721-HOXB9 cells showed HOXB9-modulated morphological changes (b). Expression of epithelial and mesenchymal markers was evaluated by Western blot analysis in Huh-7-pSuper-Scramble, Huh-7-shHOXB9-1, and Huh-7-shHOXB9-2 cells (c). Expression of epithelial and mesenchymal markers was analyzed by Western blot analysis in SMMC-7721-pBabe and SMMC-7721-HOXB9 cells (d)
Expression of epithelial and mesenchymal markers was analyzed by immunofluorescence stains. Expression of epithelial and mesenchymal markers was analyzed by immunofluorescence stains in Huh-7-pSuper-Scramble, Huh-7-shHOXB9-1, and Huh-7-shHOXB9-2 cells (a). Expression of epithelial and mesenchymal markers was analyzed by immunofluorescence stains in SMMC-7721-pBabe and SMMC-7721-HOXB9 cells (b)
Expression of epithelial and mesenchymal markers was analyzed by qRT-PCR. Expression of epithelial and mesenchymal markers was analyzed by qRT-PCR in Huh-7-pSuper-Scramble, Huh-7-shHOXB9-1, and Huh-7-shHOXB9-2 cells (a). Expression of epithelial and mesenchymal markers was analyzed by qRT-PCR in SMMC-7721-pBabe and SMMC-7721-HOXB9 cells (b). **P < 0.01 is based on Student’s t test. All results are from three independent experiments. Error bars indicate standard deviation
HOXB9 promotes migratory and invasive capacities of HCC cells in vitro
The effect of HOXB9 in cell migration was initially assessed by wound-healing assay. Huh-7-shHOXB9-1 and Huh-7-shHOXB9-2 cells had significantly slower closure of the wound area compared with the control cells (Fig. 6a). This result was confirmed by Boyden’s chamber assay (Fig. 6c). Moreover, Huh-7-shHOXB9-1 and Huh-7-shHOXB9-2 cells showed a low degree of invasion through Matrigel (Fig. 6c). By contrast, HOXB9 overexpression dramatically increased the migratory and invasive capacity of SMMC-7721 cells (Fig. 6b, d), indicating that restoration of an epithelial phenotype through EMT may dampen or inhibit their mobility potential. These results indicated that HOXB9 promotes migratory and invasive behavior in HCC cells.
HOXB9 promotes migration and invasion of HCC cells. Huh-7-shHOXB9 and SMMC-7721-HOXB9 cells or control vector cells were subjected to wound-healing assay (a, b), transwell migration (c, d, upper panels), and Matrigel invasion assays (c, d, lower panels). The uncovered areas in the wound-healing assays were quantified as a percentage of the original wound area (a, b). Quantification of migrated cells through the membrane and invaded cells through Matrigel of each cell line are shown as proportions of their vector controls (c, d). **P < 0.01 is based on Student’s t test. All results are from three or four independent experiments. Error bars indicate standard deviation
TGF-β1 signaling is required for HOXB9-induced EMT
Diverse cell-signaling factors, including TGF-β1, Rac1, c-Src, Ras, and PI3K-Akt, have been implicated in EMT. TGF-β1 induces EMT via Smad2/3-dependent pathway. Thus, we explored the possibility of TGF-β1 to act as a pathway effector that controls EMT in HOXB9-induced HCC cells. The knockdown of HOXB9 in Huh-7 cells significantly reduced TGF-β1 protein expression (Fig. 7a). Consistent with the results in Huh-7 cells, the expression of TGF-β1 was elevated in SMMC-7721 cells overexpressing HOXB9 at protein levels (Fig. 7b). These data suggest that HOXB9 upregulates the TGF-β1 expression in HCC cells. To investigate the involvement of TGF-β1-dependent signaling in HOXB9-induced EMT, we examined the expression of phosho-Smad2. Consistent with TGF-β1 activation, phosho-Smad2 expression was elevated in SMMC-7721-HOXB9 cells compared with SMMC-7721-vector cells (Fig. 7b). The HOXB9 knockdown in Huh-7 cells significantly suppressed Smad2 phosphorylation (Fig. 7a). Previous studies have shown that TGF-β1 is pro-invasive by inducing EMT via induction of transcriptional repressors, including Slug, Snail, and ZEB1 [17, 24–26]. Thus, we measured the expression of these transcriptional repressors in HCC cells, which was either modulated by HOXB9 or not. As shown in Fig. 7b, Slug, Snail, and ZEB1 expressions were elevated in SMMC-7721-HOXB9 cells compared with SMMC-7721-vector cells. The knockdown of HOXB9 in Huh-7 cells significantly suppressed Slug, Snail, and ZEB1 expressions (Fig. 7a).
HOXB9 regulates TGF-β1 expression in HCC cells. The expression of TGF-β1, p-Smad2, Smad2, Slug, Snail, and ZEB1 was analyzed by Western blot analysis in Huh-7-pSuper-Scramble, Huh-7-shHOXB9-1, and Huh-7-shHOXB9-2 cells (a). The expression of TGF-β1, p-Smad2, Smad2, Slug, Snail, and ZEB1 was analyzed by Western blot analysis in SMMC-7721-pBabe and SMMC-7721-HOXB9 cells (b)
Transforming growth factor-β1 receptor signaling by using LY364947 suppressed the Smad2 phosphorylation in SMMC-7721-HOXB9 cells (Fig. 8a). The treatment with LY364947, an inhibitor of TGF-β1 receptor signaling, decreased the levels of mesenchymal markers (vimentin) and increased the levels of epithelial markers (E-cadherin) (Fig. 8b). LY364947 also decreased the expression levels of Slug, Snail, and ZEB1 (Fig. 8c). The suppression of TGF-β1 signaling reduced the migration and invasive property of SMMC-7721-HOXB9 cells (Fig. 8d, e). These results indicated that HOXB9 activates TGF-β1 signaling pathways implicated in the transformation of mesenchymal cell outcome, cellular motility, and invasive properties of HCC cells.
TGF-β1 signaling is required for HOXB9-induced EMT. Western blot analysis of p-Smad2 and Smad2 protein levels in LY364947-treated SMMC-7721-HOXB9 cells and their controls (a). Western blot analysis of E-cadherin and Vimentin protein levels in LY364947-treated SMMC-7721-HOXB9 cells and their controls (b). Western blot analysis of Slug, Snail, and ZEB1 protein levels in LY364947-treated SMMC-7721-HOXB9 cells and their controls (c). LY364947-treated SMMC-7721-HOXB9 cells and the controls were subjected to a wound-healing assay (d). LY364947-treated SMMC-7721-HOXB9 cells and the controls were subjected to transwell migration and Matrigel invasion assays (e). The uncovered areas in the wound-healing assays were quantified as a percentage of the original wound area (d). Quantification of migrated cells through the membrane and invaded cells through Matrigel of each cell line is shown as proportions of their vector controls (e). **P < 0.01 is based on Student’s t test. All results are from three or four independent experiments. Error bars indicate standard deviation
Discussion
Hepatocellular carcinoma is historically characterized by rapidly infiltrating growth and early metastasis. Given these pathologic features, approximately 60–80 % of HCC patients are diagnosed at an advanced stage wherein no curative treatments are available. [27–29]. Achieving improvements in therapy requires a thorough understanding of the molecular mechanisms that regulate HCC invasion and metastasis.
Epithelial-to-mesenchymal transition is an essential process in developmental biology. The process was initially identified in normal tissue development, such as during embryogenesis and organogenesis. However, recent results identified EMT functions and its regulatory factors as major contributors to wound-healing by fibrosis and invasive tumor cell behavior [30–32]. In the EMT process, epithelial cells undergo phenotypic conversion to mesenchymal cells by activating the expression of EMT marker genes. The major function of these genes is transcriptional repression of the main epithelial molecules, notably the cell–cell adhesion protein E-cadherin, leading to a morphological change from cytoskeletal rearrangement. Complementary to the epithelial gene repression is simultaneously induced expression of mesenchymal genes coding for N-cadherin, vimentin. The EMT has been shown to contribute to tumor formation and metastasis of HCC; however, the mechanisms by which EMT is regulated in HCC have not been completely elucidated [33–35].
HOX genes regulate several cellular processes, including angiogenesis and maintenance of cell fate [8, 36]. HOXB9 is included in a cluster of homeobox genes, and the encoded protein functions as a sequence-specific transcription factor. Previous studies have shown that HOXB9 was expressed differently in normal and cancer tissues. However, the function of the altered HOXB9 expression in the progression of malignancies remains elusive, and its involvement in liver cancer invasion and metastasis is unclear. The possible significance of HOXB9 in HCC is still uncertain. Therefore, we examined the expression of HOXB9 in HCC cell lines and then explored the mechanism of HOXB9 to promote liver tumorigenicity by EMT.
In this research, in vitro studies in the cell line models of overexpression or suppression of HOXB9 expression showed that the overexpression of HOXB9 increased the migration and invasion of HCC cells. On the contrary, HOXB9 knockdown decreased the cancer-associated phenotypes in HCC cells. HOXB9 induced EMT in HCC cells. Further mechanistic studies in HCC cells identified TGF-β1 as a target of HOXB9 and showed that TGF-β1 signaling mediates HOXB9-induced EMT. Thus, a mechanism regulated by HOXB9 controlling EMT in HCC cells was identified. These results are correlated with previous reports, suggesting that HOXB9 promotes recurrence and metastasis in other tumor types, such as breast cancer [14]. Our findings demonstrated a central function for HOXB9 in the regulation of EMT in liver cancer and expand the repertoire of pathways that modulate this cellular process.
Transforming growth factor-β1 triggered the increase in mesenchymal markers and the decrease in epithelial markers. TGF-β1 also constructs a preferential environment for phenotypic transition and induces the invasiveness of cancer cells. Smad2 and Smad3 are directly phosphorylated by TGF-β type I receptor kinases in the TGF-β1 signaling pathway. TGF-β1 stimulation caused the phosphorylation of Smad2 and Smad3 on the SSXS motif in the C terminal residue, which resulted in the formation of a complex with Smad4 common mediator and nuclear translocation [21]. Results show that HOXB9 strongly upregulated TGF-β1 in human liver cancer cells, and TGF-β1 induction contributed to the phosphorylation of Smad2. Thus, the regulation of TGF-β1-Smad signaling by HOXB9 is an important pharmacologic tool for the chemoprevention of invasion and metastasis in cancer.
In conclusion, results show that HOXB9 overexpression promotes migration and invasion of HCC cells, which may contribute to HCC recurrence. Furthermore, HOXB9 induces EMT through direct upregulation of TGF-β1 EMT inducer. TGF-β1-Smad signaling is involved in the mechanism by which HOXB9 induces EMT in HCC. These data implicated that HOXB9 has an important function in mediating HCC progression and may serve as a therapeutic target for HCC.
References
Bruix J, Boix L, Sala M, Llovet JM (2004) Focus on hepatocellular carcinoma. Cancer Cell 5(3):215–219
Zaret KS (2002) Regulatory phases of early liver development: paradigms of organogenesis. Nat Rev Genet 3(7):499–512
Lai E, Prezioso VR, Tao WF, Chen WS, Darnell JE Jr (1991) Hepatocyte nuclear factor 3 alpha belongs to a gene family in mammals that is homologous to the Drosophila homeotic gene fork head. Genes Dev 5(3):416–427
Bossard P, Zaret KS (1998) GATA transcription factors as potentiators of gut endoderm differentiation. Development 125(24):4909–4917
Abate-Shen C (2002) Deregulated homeobox gene expression in cancer: cause or consequence? Nat Rev Cancer 2(10):777–785
Nagel S, Burek C, Venturini L, Scherr M, Quentmeier H, Meyer C, Rosenwald A, Drexler HG, MacLeod RA (2007) Comprehensive analysis of homeobox genes in Hodgkin lymphoma cell lines identifies dysregulated expression of HOXB9 mediated via ERK5 signaling and BMI1. Blood 109(7):3015–3023
Nunes FD, de Almeida FC, Tucci R, de Sousa SC (2003) Homeobox genes: a molecular link between development and cancer. Pesqui Odontol Bras 17(1):94–98
Cantile M, Cindolo L, Napodano G, Altieri V, Cillo C (2003) Hyperexpression of locus C genes in the HOX network is strongly associated in vivo with human bladder transitional cell carcinomas. Oncogene 22(41):6462–6468
Chen F, Capecchi MR (1999) Paralogous mouse Hox genes, Hoxa9, Hoxb9, and Hoxd9, function together to control development of the mammary gland in response to pregnancy. Proc Natl Acad Sci USA 96(2):541–546
Miller GJ, Miller HL, van Bokhoven A, Lambert JR, Werahera PN, Schirripa O, Lucia MS, Nordeen SK (2003) Aberrant HOXC expression accompanies the malignant phenotype in human prostate. Cancer Res 63(18):5879–5888
Nguyen DX, Chiang AC, Zhang XH, Kim JY, Kris MG, Ladanyi M, Gerald WL, Massague J (2009) WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell 138(1):51–62
Vider BZ, Zimber A, Hirsch D, Estlein D, Chastre E, Prevot S, Gespach C, Yaniv A, Gazit A (1997) Human colorectal carcinogenesis is associated with deregulation of homeobox gene expression. Biochem Biophys Res Commun 232(3):742–748
Shrestha B, Ansari KI, Bhan A, Kasiri S, Hussain I, Mandal SS (2012) Homeodomain-containing protein HOXB9 regulates expression of growth and angiogenic factors, facilitates tumor growth in vitro and is overexpressed in breast cancer tissue. FEBS J 279(19):3715–3726
Hayashida T, Takahashi F, Chiba N, Brachtel E, Takahashi M, Godin-Heymann N, Gross KW, Vivanco M, Wijendran V, Shioda T, Sgroi D, Donahoe PK, Maheswaran S (2010) HOXB9, a gene overexpressed in breast cancer, promotes tumorigenicity and lung metastasis. Proc Natl Acad Sci USA 107(3):1100–1105
Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Investig 119(6):1420–1428
Franco-Chuaire ML, Magda Carolina SC, Chuaire-Noack L (2013) Epithelial-mesenchymal transition (EMT): principles and clinical impact in cancer therapy. Invest Clin 54(2):186–205
Wang Y, Wen M, Kwon Y, Xu Y, Liu Y, Zhang P, He X, Wang Q, Huang Y, Jen KY, Labarge MA, You L, Kogan SC, Gray JW, Mao JH, Wei G (2014) CUL4A induces epithelial-mesenchymal transition and promotes cancer metastasis by regulating ZEB1 expression. Cancer Res 74(2):520–531
Lim J, Thiery JP (2012) Epithelial-mesenchymal transitions: insights from development. Development 139(19):3471–3486
Le Bras GF, Taubenslag KJ, Andl CD (2012) The regulation of cell–cell adhesion during epithelial-mesenchymal transition, motility and tumor progression. Cell Adh Migr 6(4):365–373
Gomes LR, Terra LF, Sogayar MC, Labriola L (2011) Epithelial-mesenchymal transition: implications in cancer progression and metastasis. Curr Pharm Biotechnol 12(11):1881–1890
Katsuno Y, Lamouille S, Derynck R (2013) TGF-beta signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol 25(1):76–84
Drabsch Y, ten Dijke P (2012) TGF-beta signalling and its role in cancer progression and metastasis. Cancer Metastasis Rev 31(3–4):553–568
Fuxe J, Karlsson MC (2012) TGF-beta-induced epithelial-mesenchymal transition: a link between cancer and inflammation. Semin Cancer Biol 22(5–6):455–461
Xiong M, Jiang L, Zhou Y, Qiu W, Fang L, Tan R, Wen P, Yang J (2012) The miR-200 family regulates TGF-beta1-induced renal tubular epithelial to mesenchymal transition through Smad pathway by targeting ZEB1 and ZEB2 expression. Am J Physiol Renal Physiol 302(3):F369–F379
Naber HP, Drabsch Y, Snaar-Jagalska BE, ten Dijke P, van Laar T (2013) Snail and Slug, key regulators of TGF-beta-induced EMT, are sufficient for the induction of single-cell invasion. Biochem Biophys Res Commun 435(1):58–63
Kang H, Lee M, Jang SW (2013) Celastrol inhibits TGF-beta1-induced epithelial-mesenchymal transition by inhibiting Snail and regulating E-cadherin expression. Biochem Biophys Res Commun 437(4):550–556
Sun VC, Sarna L (2008) Symptom management in hepatocellular carcinoma. Clin J Oncol Nurs 12(5):759–766
Yang JD, Harmsen WS, Slettedahl SW, Chaiteerakij R, Enders FT, Therneau TM, Orsini L, Kim WR, Roberts LR (2011) Factors that affect risk for hepatocellular carcinoma and effects of surveillance. Clin Gastroenterol Hepatol 9(7):617–623.e611
Cabrera R, Nelson DR (2010) Review article: the management of hepatocellular carcinoma. Aliment Pharmacol Ther 31(4):461–476
Chang AC, Fu Y, Garside VC, Niessen K, Chang L, Fuller M, Setiadi A, Smrz J, Kyle A, Minchinton A, Marra M, Hoodless PA, Karsan A (2011) Notch initiates the endothelial-to-mesenchymal transition in the atrioventricular canal through autocrine activation of soluble guanylyl cyclase. Dev Cell 21(2):288–300
Larue L, Bellacosa A (2005) Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene 24(50):7443–7454
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133(4):704–715
Lai W, Liu L, Zeng Y, Wu H, Xu H, Chen S, Chu Z (2013) KCNN4 channels participate in the EMT induced by PRL-3 in colorectal cancer. Med Oncol 30(2):566
Na DC, Lee JE, Yoo JE, Oh BK, Choi GH, Park YN (2011) Invasion and EMT-associated genes are up-regulated in B viral hepatocellular carcinoma with high expression of CD133-human and cell culture study. Exp Mol Pathol 90(1):66–73
Zheng X, Gai X, Wu Z, Liu Q, Yao Y (2013) Metastasin leads to poor prognosis of hepatocellular carcinoma through partly inducing EMT. Oncol Rep 29(5):1811–1818
Kapur RP, Gershon MD, Milla PJ, Pachnis V (2004) The influence of Hox genes and three intercellular signalling pathways on enteric neuromuscular development. Neurogastroenterol Motil 16(Suppl 1):8–13
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC H1617).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Lin Sha and Lei Dong have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sha, L., Dong, L., Lv, L. et al. HOXB9 promotes epithelial-to-mesenchymal transition via transforming growth factor-β1 pathway in hepatocellular carcinoma cells. Clin Exp Med 15, 55–64 (2015). https://doi.org/10.1007/s10238-014-0276-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-014-0276-7