Abstract
Human leukocyte antigen-G is involved in immunotolerogenic, inflammatory and carcinogenic process. This study investigated serum soluble HLA-G (sHLA-G) levels in patients with chronic hepatitis B virus (HBV) infection according to the infection phases and clinical diagnoses. The study included 223 patients with chronic HBV infection [phases: 38 immune-tolerant (IT), 83 immune clearance (IC), 30 non/low-replicative (LR) and 72 HBeAg negative hepatitis (ENH); diagnoses: 38 asymptomatic HBV carriers (ASC), 98 chronic hepatitis (CH), 46 cirrhosis (LC) and 41 hepatocellular carcinoma (HCC)], 62 HBV infection resolvers and 66 healthy controls. The sHLA-G levels in patients were elevated compared with resolvers and healthy controls (P < 0.001). According to phases, sHLA-G levels were higher in IC and ENH than in IT (P = 0.017 and P = 0.001, respectively). Serum sHLA-G levels were also higher in ENH than in LR (P = 0.008). According to diagnoses, sHLA-G levels in HCC were significantly increased compared with LC, CH and ASC (P = 0.010, P < 0.001 and P < 0.001, respectively). Serum sHLA-G levels were higher in CH than in ASC (P = 0.039). The sHLA-G levels in IC, ENH and CH were correlated with alanine aminotransferase levels (P = 0.011, P = 0.010 and P < 0.001, respectively). It is concluded that sHLA-G is involved in the pathogenesis of chronic HBV infection and correlates with infection phases and clinical diseases, suggesting the value in evaluating disease activity and defining clinical diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatitis B virus (HBV) infection is a significant cause of a variety of clinical liver diseases such as acute and chronic hepatitis (CH), cirrhosis and hepatocellular carcinoma (HCC). Chronic HBV infection is a dynamic process of complex interactions between the virus and the host’s immune response, leading to various disease condition and progression [1, 2]. Typically, the natural course of chronic HBV infection includes several phases: an immune-tolerant (IT) phase, an immune clearance (IC) phase and an inactive or non/low-replicative (LR) phase [3, 4]. In addition, active hepatitis, referred as HBeAg negative hepatitis (ENH), may sometimes reoccur in individuals already in an inactive phase [3, 5–8]. Intricately, these phases may or may not necessarily and sequentially occur in an infected individual [9]. Furthermore, HBV infection may spontaneously resolve and manifest seroclearance of HBeAg, HBV DNA and HBsAg and seroconversion of anti-HBs in variously low percentages depending on many parameters from the host and the virus [10, 11]. The phases of chronic HBV infection are characterized by different levels of immunoregulatory and inflammatory responses of the hosts to various degrees of the HBV viral replication, in which natural killer (NK) cells [12–14], CD4+ and CD8+ T-lymphocytes [15, 16], dendritic cells [17] and regulatory T cells [18, 19] associated with innate and adaptive immune responses are all involved.
Human leukocyte antigen-G (HLA-G) is a non-classical class I major histocompatibility complex molecule. It was firstly found to be predominantly expressed on the surface of cytotrophoblast cells and is believed to function in establishing the tolerogenic microenvironment at the fetal–maternal interface through inhibiting natural killer cell-mediated lysis and influencing cytokine expression [20, 21]. Subsequent research demonstrated that HLA-G antigens can regulate NK and CD8+ T cell cytotoxicity, modulate the functions of CD4+ T-lymphocytes and dendritic cells and induce regulatory T cells, playing a tolerogenic role in innate and adaptive responses [22–24]. The expression of HLA-G molecules has been associated with a variety of disorders such as septic shock, autoimmune diseases, viral infections, tumors and organ transplantations [25–29], linking HLA-G molecules with the establishment of anti-inflammatory environments.
Alternative splicing of the primary HLA-G transcripts generates membrane-bound and soluble protein isoforms: HLA-G1 to HLA-G4 as membrane-bound and HLA-G5 to HLA-G7 as soluble molecules [30]. Like the cell-surface isoforms, soluble HLA-G (sHLA-G) can also inhibit NK cells and cytotoxic T-lymphocytes [31–33] and suppress CD4+ and CD8+ T cell alloproliferation [34]. Following binding to the CD158d (KIR2DL4) receptor on the surface of human resting NK cells and being then endocytosed, sHLA-G can trigger the expression of a set of chemokines and cytokines, inducing a proinflammatory and proangiogenic response [35].
Because chronic HBV infection is characterized by immunoregulatory and inflammatory responses and associates with the occurrence of HCC, and HLA-G is involved in immunosuppressive, inflammatory and carcinogenic process, the aim of this study was to evaluate serum sHLA-G levels in various phases and clinical diseases of patients with persistent HBV infection. For controls, we also analyzed sHLA-G levels in individuals resolved from HBV infection and in healthy controls without HBV infection.
Patients and methods
Patients and controls
The study was performed in patients with chronic HBV infection from the First Affiliated Hospital, School of Medicine, Xi’an Jiaotong University, a tertiary hospital in the Northwest China. All patients were negative for markers of hepatitis C virus (HCV) and human immunodeficiency virus (HIV). Coexistence of autoimmune, alcoholic or metabolic liver disease was excluded. Chronic patients who had been never treated with nucleos(t)ide analogs or interferon (IFN)-α were eligible for inclusion.
Resolvers from HBV infection were those who had normal liver biochemistries and were seropositive for anti-HBs and anti-HBc. Healthy controls were those who had normal liver biochemistries, had no history of hepatitis B, and were seropositive for anti-HBs only or seronegative for HBV markers.
Patients were classified according to the phase of chronic HBV infection as IT, immune clearance or immune reactive (IC), LR and ENH and to the clinical disease as asymptomatic HBV carrier status (ASC), CH, liver cirrhosis (LC) and HCC.
The phase of chronic HBV infection in each patient was determined by HBsAg and anti-HBc positivity, HBeAg/anti-HBe serostatus, and serum HBV DNA and alanine aminotransferase (ALT) levels [4]. The IT phase was defined as: HBsAg and anti-HBc positive, HBeAg positive, high viral load, serum ALT < 2 × upper limit of normal (ULN). The IC phase was defined as: HBsAg and anti-HBc positive, HBeAg positive, moderate to high level of viral load, serum ALT ≥ 2 × ULN. The LR phase was defined as: HBsAg and anti-HBc positive, HBeAg negative, HBV DNA < 2000 IU/ml, normal serum ALT. The ENH was defined as: HBsAg and anti-HBc positive, HBeAg negative, HBV DNA > 2000 IU/ml, serum ALT ≥ 2 × ULN or fluctuation.
The clinical diagnosis of chronic HBV infection in each patient was determined by history of HBV infection, HBsAg/anti-HBs, HBeAg/anti-HBe and anti-HBc serostatus, measurement of HBV DNA, biochemical liver function, α-fetoprotein (AFP) levels, and ultrasonography and/or computerized tomography (CT)/magnetic resonance imaging (MRI) as described previously [36, 37]. The ASC was defined as: HBsAg, HBeAg and anti-HBc positive, high viral load, normal serum ALT level. The CH was defined as: HBsAg and anti-HBc positive, HBeAg and anti-HBe positive or negative, moderate to high level of HBV DNA, ALT ≥ 2 × ULN or fluctuation, no evidence of LC and HCC. The LC was defined as: HBsAg and anti-HBc positive, HBeAg and anti-HBe positive or negative, detectable HBV DNA, ALT elevation or not, evidence of LC on liver biopsy and/or on ultrasonography and/or CT/MRI and gastroesophageal varices by endoscopy, no evidence of HCC. The HCC was defined as: HBsAg and anti-HBc positive, HBeAg and anti-HBe positive or negative, detectable HBV DNA, ALT elevation or not, evidence of HCC on liver biopsy and/or ultrasonography and/or CT/MRI with or without elevated AFP and gastroesophageal varices by endoscopy. The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee.
Determination of serum sHLA-G levels, HBV markers and liver biochemistry
Serum sHLA-G levels were determined using commercially available Human sHLA-G ELISA kit (Exbio/BioVendor, Praha, Czech Republic) according to the manufacturer’s instructions. HBV serologic markers including HBsAg, anti-HBs, HBeAg, anti-HBe and anti-HBc were performed using ELISA from Beijing Wantai Biological Pharmacy (Beijing, China). Biochemical liver function including ALT and aspartate aminotransferase (AST) levels (IU/l) was assayed on the Olympus AU5400 automatic biochemical analyzer (Olympus Corporation, Mishama, Japan). Serum HBV DNA levels (IU/ml) were quantitatively determined using HBV fluorescence polymerase chain reaction diagnostic kit manufactured by Da An Gene Co., Ltd. of Sun Yat-Sen University (Guangzhou, China) according to the instruction. Serum AFP levels (ng/ml) were measured using automated Eleceyes (Roche Diagnostics, Mannheim, Germany).
Statistical analysis
Data were expressed as the mean ± SD or median (range). Statistical analysis was performed by SPSS software version 16.0 (SPSS, Inc., Chicago, IL). Continuous and categorical variables were compared between groups, using the Mann–Whitney test or Kruskall–Wallis rank-sum test where appropriate. Correlations between variables were assessed using Spearman’s correlation coefficients. A P value < 0.05 was considered statistically significant.
Results
Two hundred and twenty-three treatment naïve patients with chronic HBV infection were recruited. Sixty-two HBV infection resolvers and 66 healthy individuals were recruited as controls. The demographics of the study subjects and the phases and clinical diagnoses of chronic HBV infection in the patients are presented in Table 1. All subjects were of Chinese Han ethnicity. The gender and age between HBV patients, HBV infection resolvers and healthy controls had no statistical difference (Table 1).
Serum sHLA-G levels in HBV patients were significantly elevated compared with HBV infection resolvers (P < 0.001) and healthy controls (P < 0.001, Table 1). Serum sHLA-G levels between HBV infection resolvers and healthy controls had no significant difference (P = 0.907, Table 1).
Serum sHLA-G levels according to the phases of chronic HBV infection
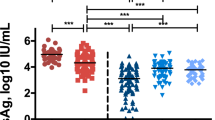
The classifications of patients’ phases were as follows: IT (n = 38), IC (n = 83), LR (n = 30) and ENH (n = 72) (Table 2). The demographics and serum sHLA-G levels in different phases are shown in Table 2. The distribution of gender among the IT, IC, LR and ENH had no significant difference. The patients in IT were younger than those in IC, LR and ENH (P < 0.001). The HBV DNA, ALT, AST, total bilirubin and albumin levels were significantly different between the patients in different phases. Serum HBV DNA levels were higher in IT and IC than in LR and ENH, serum ALT, AST and total bilirubin levels were higher in IC and ENH than in IT and LR, and albumin levels were lower in IC and ENH than in IT and LR (Table 2).
The serum sHLA-G levels in IC and ENH were higher than those in IT (P = 0.017 and P = 0.001, respectively, Fig. 1). Serum sHLA-G levels in ENH were also higher than those in LR (P = 0.008, Fig. 1).
Serum sHLA-G levels according to clinical diseases of chronic HBV infection
The clinical diagnoses of the patients were as follows: ASC (n = 38), CH (n = 98), LC (n = 46) and HCC (n = 41) (Table 3). The demographics and serum sHLA-G levels in different diagnoses of chronic HBV infection are shown in Table 3. The distribution of gender among the ASC, CH, LC and HCC had no significant difference. The HBV DNA, ALT, AST, total bilirubin and albumin levels were significantly different between the patients with different diagnoses. HBV DNA levels were higher in ASC patients than in CH patients and lower in HCC patients than in LC patients (P < 0.001). The ALT and AST levels in CH patients were higher than those in ASC, LC and HCC patients (P < 0.001). The total bilirubin levels in CH, LC and HCC patients were higher than those in ASC patients (P < 0.001). Serum albumin levels in LC and HCC patients were lower than those in ASC and CH patients (P < 0.001).
The serum sHLA-G levels in HCC patients were higher than those in LC (P = 0.010), CH (P < 0.001) and ASC patients (P < 0.001, Fig. 2). Serum sHLA-G levels in CH were also higher than those in ASC (P = 0.039, Fig. 2).
Correlations between serum sHLA-G and ALT as well as AFP levels
When all the HBV patients were analyzed, sHLA-G levels were significantly correlated with serum ALT levels (r = 0.146, P = 0.029).
When the HBV patients were analyzed according to infection phases, sHLA-G levels were significantly correlated with serum ALT levels in IC and ENH patients (P = 0.011 and P = 0.010, respectively, Fig. 3). The sHLA-G levels in IT and LR patients had no significant correlation with serum ALT levels (Fig. 3).
When the correlation between ALT and serum sHLA-G levels was analyzed according to clinical diagnoses, sHLA-G levels were significantly correlated with serum ALT levels in CH patients (P < 0.001, Fig. 4). The sHLA-G levels in ASC, LC and HCC patients had no significant correlation with serum ALT levels (Fig. 4).
In HCC patients, sHLA-G levels had no significant correlation with AFP levels (r = 0.264, P = 0.096).
Discussion
This study demonstrated that serum sHLA-G levels were elevated in chronic HBV infection and differed according to infection phases and clinical diseases. HLA-G has anti-inflammatory and immunosuppressive effects by inhibiting CD8+ T cell toxicity [38], CD4+ T cell alloreactivity [39] and NK cell-mediated cytolysis [21], and activating regulatory CD4+ T cells [40]. It is suggested that sHLA-G is involved in the immuno-inflammatory process of chronic HBV infection and the elevation of sHLA-G in chronic HBV infection is the consequence of the break of immune-tolerance status and the activation of immune response and inflammatory progress. In accord with these results, studies have shown that sHLA-G was markedly elevated in inflammatory and autoimmune diseases such as septic shock [25], HIV [28] and HCV infection [27], and systemic lupus erythematosis [26].
Recent studies showed that soluble and membrane-bound HLA-G expression can be observed on hepatocytes and biliary epithelial cells of patients with chronic HBV infection [41] and sHLA-G levels in HBV infection were much higher than those in normal controls [42, 43]. However, these studies did not correlate the sHLA-G levels with the infection phases. We carefully classified our patients according to current definition of the phases of chronic HBV infection. We showed significant correlation between serum sHLA-G and ALT levels in the active phases of chronic HBV infection (IC and ENH) and the diagnosis of CH. This correlation may have relevance to clinical practice. Currently, ALT level is one of the major parameters for evaluating disease activity and predicting treatment response in persistent HBV infection [44, 45]. Taking into account of the close correlation with disease phase and activity, it is plausible to foresee that sHLA-G may be used as another parameter for evaluating disease activity and predicting treatment response in HBV infection.
There has been increasing evidence showing the involvement of HLA-G in carcinogenesis [46]. In terms of HBV-associated HCC, the expression of HLA-G has been found to be a characteristic feature and correlate to disease progression and prognosis [24, 47, 48]. Serum levels of sHLA-G in the HCC were shown to be significantly increased [24, 43, 47]. In support of this, we showed that sHLA-G levels in HCC patients were the highest across all the clinical diagnoses of chronic HBV infection including LC, CH and ASC, suggesting the involvement of sHLA-G in tumor immune escape and the potential usefulness of its determination in diagnosing HCC. However, in accord with previous study [43], serum sHLA-G levels were not found to be associated with AFP levels in our study. The explanations for the lack of correlation between sHLA-G and AFP levels are uncertain. However, previous studies showed that the expressions of HLA-G were differently up-regulated in HCC tissues [49] and the HLA-G expression in tumors had no significant correlation with AFP level [47]. Therefore, sHLA-G elevation may reflect a major but not all of the characteristics of HCC and may be used as a complementary marker to AFP in presupposing the diagnosis of HCC.
The mechanisms of sHLA-G alteration and the causal relationship between this alteration and the disease phases and activities in chronic HBV infection are largely unclear. However, multiple mechanisms including activation of immune response, reaction of inflammatory process and carcinogenesis may be differently involved in various phases or disease conditions of the infection. In patients in phases of IC and ENH and with diagnosis of CH, the activations of immune response and inflammatory process are probably the major reasons for sHLA-G elevation. In addition to immuno-inflammatory response, the carcinogenesis of HCC may synergistically induce the elevation of sHLA-G, contributing to the striking elevation of sHLA-G in HCC patients. Understanding the changing serum sHLA-G levels across the phases and disease conditions of chronic HBV infection may represent a step forward in not only investigating the influence of the host immune response but also designing immunotherapeutic strategy against chronic HBV infection as well as HCC [46].
Our study has some limitations. One limitation is its cross-sectional design, as it would have been more useful to follow patients longitudinally through different phases of chronic HBV infection. Another limitation is the lack of dynamic evaluation of the serum sHLA-G levels in relation to treatment response given the implication of this parameter to the immune and inflammatory status of the infected individuals. Larger prospective studies are required to evaluate longitudinal changes in serum sHLA-G levels and the potentials in predicting and monitoring the treatment response of both immune-modulator therapy such as IFN-α and antiviral therapy such as oral nucleos(t)ide analogs.
In conclusion, this study demonstrates significant differences in the serum sHLA-G levels across the different phases and clinical diseases of chronic HBV infection. Since sHLA-G may participate in the immuno-inflammatory process and carcinogenesis of HCC in chronic HBV infection, the levels of sHLA-G are closely correlated with infection phase and disease activity and strikingly elevated in HCC, and quantitation of serum sHLA-G levels is non-invasive, easy to perform and relatively inexpensive, it is suggested that determination of serum sHLA-G levels may be used as an immuno-inflammatory and carcinogenic seromarker to evaluate the disease activity and complementarily diagnose HCC in chronic HBV infection. The role of sHLA-G in predicting disease prognosis, refining treatment scheme and monitoring treatment response needs to be investigated.
References
Fattovich G, Brollo L, Giustina G et al (1991) Natural history and prognostic factors for chronic hepatitis type B. Gut 32:294–298
Locarnini S (2004) Molecular virology of hepatitis B virus. Semin Liver Dis 24(Suppl 1):3–10
Liaw YF, Chu CM (2009) Hepatitis B virus infection. Lancet 373:582–592
European Association For The Study Of The Liver (2009) EASL clinical practice guidelines: management of chronic hepatitis B. J Hepatol 50:227–242
Hsu YS, Chien RN, Yeh CT et al (2002) Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology 35:1522–1527
Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF (2004) Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. Am J Med 116:829–834
Fattovich G, Bortolotti F, Donato F (2008) Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol 48:335–352
Chu CM, Liaw YF (2007) Spontaneous relapse of hepatitis in inactive HBsAg carriers. Hepatol Int 1:311–315
Hadziyannis SJ, Vassilopoulos D (2001) Hepatitis B e antigen-negative chronic hepatitis B. Hepatology 34:617–624
Liu J, Yang HI, Lee MH et al (2010) Incidence and determinants of spontaneous hepatitis B surface antigen seroclearance: a community-based follow-up study. Gastroenterology 139:474–482
Chen CJ, Yang HI (2011) Natural history of chronic hepatitis B REVEALed. J Gastroenterol Hepatol 26:628–638
Kakimi K, Guidotti LG, Koezuka Y, Chisari FV (2000) Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med 192:921–930
Fisicaro P, Valdatta C, Boni C et al (2009) Early kinetics of innate and adaptive immune responses during hepatitis B virus infection. Gut 58:974–982
Mondelli MU, Varchetta S, Oliviero B (2010) Natural killer cells in viral hepatitis: facts and controversies. Eur J Clin Invest 40:851–863
TrehanPati N, Geffers R, Sukriti S et al (2009) Gene expression signatures of peripheral CD4+ T cells clearly discriminate between patients with acute and chronic hepatitis B infection. Hepatology 49:781–790
Das A, Hoare M, Davies N et al (2008) Functional skewing of the global CD8 T cell population in chronic hepatitis B virus infection. J Exp Med 205:2111–2124
Op den Brouw ML, Binda RS, van Roosmalen MH et al (2009) Hepatitis B virus surface antigen impairs myeloid dendritic cell function: a possible immune escape mechanism of hepatitis B virus. Immunology 126:280–289
Stoop JN, van der Molen RG, Baan CC et al (2005) Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology 41:771–778
Xu D, Fu J, Jin L et al (2006) Circulating and liver resident CD4+ CD25+ regulatory T cells actively influence the antiviral immune response and disease progression in patients with hepatitis B. J Immunol 177:739–747
Kovats S, Main EK, Librach C, Stubblebine M, Fisher SJ, DeMars R (1990) A class I antigen, HLA-G, expressed in human trophoblasts. Science 248:220–223
Rouas-Freiss N, Gonçalves RM, Menier C, Dausset J, Carosella ED (1997) Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc Natl Acad Sci USA 94:11520–11525
Carosella ED, Moreau P, Lemaoult J, Rouas-Freiss N (2008) HLA-G: from biology to clinical benefits. Trends Immunol 29:125–132
Baricordi OR, Stignani M, Melchiorri L, Rizzo R (2008) HLA-G and inflammatory diseases. Inflamm Allergy Drug Targets 7:67–74
Lin A, Chen HX, Zhu CC et al (2010) Aberrant human leucocyte antigen-G expression and its clinical relevance in hepatocellular carcinoma. J Cell Mol Med 14:2162–2171
Monneret G, Voirin N, Krawice-Radanne I et al (2007) Soluble human leukocyte antigen-G5 in septic shock: marked and persisting elevation as a predictor of survival. Crit Care Med 35:1942–1947
Rosado S, Perez-Chacon G, Mellor-Pita S et al (2008) Expression of human leukocyte antigen-G in systemic lupus erythematosus. Hum Immunol 69:9–15
Weng PJ, Fu YM, Ding SX, Xu DP, Lin A, Yan WH (2011) Elevation of plasma soluble human leukocyte antigen-G in patients with chronic hepatitis C virus infection. Hum Immunol 72:406–411
Donaghy L, Gros F, Amiot L et al (2007) Elevated levels of soluble non-classical major histocompatibility class I molecule human leucocyte antigen (HLA)-G in the blood of HIV-infected patients with or without visceral leishmaniasis. Clin Exp Immunol 147:236–240
Lila N, Carpentier A, Amrein C, Khalil-Daher I, Dausset J, Carosella ED (2000) Implication of HLA-G molecule in heart-graft acceptance. Lancet 355:2138
Carosella ED, Dausset J, Kirszenbaum M (1996) HLA-G revisited. Immunol Today 17:407–409
Le Gal FA, Riteau B, Sedlik C et al (1999) HLA-G-mediated inhibition of antigen-specific cytotoxic T lymphocytes. Int Immunol 11:1351–1356
Contini P, Ghio M, Poggi A et al (2003) Soluble HLA-A, -B, -C and -G molecules induce apoptosis in T and NK CD8+ cells and inhibit cytotoxic T cell activity through CD8 ligation. Eur J Immunol 33:125–134
Rouas-Freiss N, Khalil-Daher I, Riteau B et al (1999) The immunotolerance role of HLA-G. Semin Cancer Biol 9:3–12
Bahri R, Hirsch F, Josse A et al (2006) Soluble HLA-G inhibits cell cycle progression in human alloreactive T lymphocytes. J Immunol 176:1331–1339
Rajagopalan S, Bryceson YT, Kuppusamy SP et al (2006) Activation of NK cells by an endocytosed receptor for soluble HLA-G. PLoS Biol 4:e9
Lok AS, McMahon BJ (2001) Chronic hepatitis B. Hepatology 34:1225–1241
Duan S, Zhang G, Han Q et al (2011) CTLA-4 exon 1 +49 polymorphism alone and in a haplotype with −318 promoter polymorphism may confer susceptibility to chronic HBV infection in Chinese Han patients. Mol Biol Rep 38:5125–5132
Fournel S, Aguerre-Girr M, Huc X et al (2000) Cutting edge: soluble HLA-G1 triggers CD95/CD95 ligand-mediated apoptosis in activated CD8+ cells by interacting with CD8. J Immunol 164:6100–6104
Lila N, Rouas-Freiss N, Dausset J, Carpentier A, Carosella ED (2001) Soluble HLA-G protein secreted by allo-specific CD4+ T cells suppresses the allo-proliferative response: a CD4+ T cell regulatory mechanism. Proc Natl Acad Sci USA 98:12150–12155
Paul P, Cabestre FA, Ibrahim EC et al (2000) Identification of HLA-G7 as a new splice variant of the HLA-G mRNA and expression of soluble HLA-G5, -G6, and -G7 transcripts in human transfected cells. Hum Immunol 61:1138–1149
Souto FJ, Crispim JC, Ferreira SC et al (2011) Liver HLA-G expression is associated with multiple clinical and histopathological forms of chronic hepatitis B virus infection. J Viral Hepat 18:102–105
Shi WW, Lin A, Xu DP et al (2011) Plasma soluble human leukocyte antigen-G expression is a potential clinical biomarker in patients with hepatitis B virus infection. Hum Immunol 72:1068–1073
Park Y, Park Y, Lim HS, Kim YS, Hong DJ, Kim HS (2012) Soluble human leukocyte antigen-G expression in hepatitis B virus infection and hepatocellular carcinoma. Tissue Antigens 79:97–103
Brook MG, Karayiannis P, Thomas HC (1989) Which patients with chronic hepatitis B virus infection will respond to alpha-interferon therapy? A statistical analysis of predictive factors. Hepatology 10:761–763
Chien RN, Liaw YF, Atkins M (1999) Pretherapy alanine transaminase level as a determinant for hepatitis B e antigen seroconversion during lamivudine therapy in patients with chronic hepatitis B. Asian Hepatitis Lamivudine Trial Group. Hepatology 30:770–774
Amiot L, Ferrone S, Grosse-Wilde H, Seliger B (2011) Biology of HLA-G in cancer: a candidate molecule for therapeutic intervention? Cell Mol Life Sci 68:417–431
Wang Y, Ye Z, Meng XQ, Zheng SS (2011) Expression of HLA-G in patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 10:158–163
Cai MY, Xu YF, Qiu SJ et al (2009) Human leukocyte antigen-G protein expression is an unfavorable prognostic predictor of hepatocellular carcinoma following curative resection. Clin Cancer Res 15:4686–4693
Chen A, Shen Y, Xia M et al (2011) Expression of the nonclassical HLA class I and MICA/B molecules in human hepatocellular carcinoma. Neoplasma 58:371–376
Acknowledgments
This work was supported in part by funding from the National Natural Science Foundation of China (Grant no. 30972758).
Conflict of interest
No conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Qunying Han and Na Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Han, Q., Li, N., Zhu, Q. et al. Association of serum soluble human leukocyte antigen-G levels with chronic hepatitis B virus infection. Clin Exp Med 14, 35–43 (2014). https://doi.org/10.1007/s10238-012-0214-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-012-0214-5