Abstract
Hepatic ischaemia-reperfusion injury is a serious problem that occurs during various surgical operations such as liver transplantation, surgical revascularization, and partial organ resection. Different pharmacological agents have been used for the protection of organ function and for extending the tolerable ischaemic interval after the ischaemic insult. We aimed to determine the presence of 8-hydroxydeoxyguanosine (8-OHdG) in the DNA from liver undergoing ischaemia-reperfusion, and also to evaluate the protective effects of N-acetylcysteine (NAC) and EGb761 (Ginkgo biloba extract) against hepatic oxidative DNA damage. A total of 40 rats were divided into four groups of 10 animals each (sham-operation group, control group, NAC group, and EGb761 group). Oxidative damage to DNA was evaluated by measuring the increase in 8-OHdG formation in liver tissue and also the effects of NAC and EGb761 pretreatment. Hepatic ischaemia for 90 min followed by reperfusion caused a marked increase in tissue levels of 8-OHdG, thiobarbituric acid-reactive substance, serum ALT, AST and LDH activities compared to sham-operated group. Pretreatment with both NAC and EGb761 clearly diminished 8-OHdG formation and lipid peroxidation. These findings suggest that antioxidant molecules such as NAC and EGb761 may be useful in preventing postischaemic reperfusion injury in hepatic tissue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatic ischaemia-reperfusion (IR) injury is an important problem that occurs during various surgical operations such as liver transplantation, surgical revascularization, and partial organ resection [1]. The release of oxygen-derived free radicals during the reperfusion period has recently been implicated as the mediator of reperfusion injury in a variety of organs including the liver [2]. Therefore, endogenous and exogenous antioxidant molecules may be of critical importance in protecting against the development of reperfusion injury following an ischaemic insult.
Various pharmacological agents have been used for the protection of organ function and for extending the tolerable ischaemic interval after ischaemic insult [3, 4]. N-acetylcysteine (NAC) is a thiol-containing compound that interacts and detoxifies free radicals by nonenzymatic reactions either by conjugation or reduction, and is deacetylated to form cysteine, which supports glutathione biosynthesis [5]. There are several studies in which the protective effects of NAC on hepatic IR injury have been investigated, but the findings do not seem to be conclusive [6–10]. EGb761 is an extract of Ginkgo biloba standardized to 24% ginkgo-flavone glycosides and 6% terpenoids, both of which are known to scavenge hydroxyl (HO−), peroxide (HOO−) and superoxide radicals (O2 −) [11].
Recent studies have provided considerable support for the in vitro and in vivo protective effects of EGb761 in IR injury [12]. To our knowledge, however, there is a lack of information in the literature on the comparative protective effects of NAC and EGb761 against hepatic oxidative DNA damage induced by the liver IR in rats.
8-Hydroxydeoxyguanosine (8-OHdG) is a DNA base-modified product generated by reactive oxygen species [13]. It has also been reported that 8-OHdG may be a good marker of oxidative DNA damage if it can be analysed with high sensitivity [14]. Although a variety of experimental models of ischaemic injury have shown that free radicals induce postischaemic oxidative damage to DNA [15–17], there is very little information in the literature about the protective effects of NAC and EGb761 against the formation of 8-OHdG in DNA.
In this study, we aimed to determine the presence of 8-OHdG in the DNA from liver that had undergone IR, and also to evaluate the protective effects of NAC and EGb761 against hepatic oxidative DNA damage. For this purpose, we designed an experimental study associated with hepatic IR injury in rats, and measured 8-OHdG and malondialdehyde (MDA) levels (as a thiobarbituric acid-reactive substance, TBARS) in hepatic tissue from rats in both the ischaemic and reperfusion phases. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) activities were also measured to check the effects of NAC and EGb761 on these hepatic function tests in ischaemia and reperfusion phases.
Materials and methods
Animals and surgical procedure
Male Wistar-albino rats weighing 225 ± 25 g were used in this study. The experimental protocol was approved by the medical ethics committee, and all animals received humane care in compliance with the guidelines of Ataturk University Research Council’s criteria. Using aseptic procedures, surgical operations were carried out under anaesthesia induced by thiopental sodium administered intraperitoneally (i.p.) at a dose of 25 mg/kg. The rats were divided into the following four groups. Rats of group 1 (sham-operated, n = 10) underwent laparotomy via a median abdominal incision. Blood samples were taken from the vena cava, and samples of tissue from the liver. Rats of group 2 (control group, n = 10) were treated i.p. with 0.5 ml saline 30 min before surgery. Rats of group 3 (NAC group, n = 10) were treated i.p. with NAC at a dose 250 mg/kg 30 min before surgery (Fluimucil ampule, Zambo, Spain). Rats of group 4 (EGb761 group, n = 10) received i.p. 100 mg/kg EGb761 (Tebokan gutt; Abdi Ibrahim, Istanbul, Turkey) 30 min before surgery. The doses of NAC and EGb761 were chosen according to previous studies [18, 19].
In rats of groups 2, 3 and 4 after induction of anaesthesia, a catheter was placed into the right jugular vein. Left hepatic lobar ischaemia was induced by clamping the left hepatic artery and the left portal branch. A single dose of heparin (100 U/100 g) and Ringer’s lactate solution (0.5 ml over 15 min) were infused through the jugular catheter during ischaemia. After 90 min of ischaemia, blood and tissue samples were taken from the jugular catheter and the left lobe of the liver (respectively). The ischaemic period was followed by reperfusion for 90 min. After reperfusion, blood and liver tissue samples were again taken from the jugular catheter and the left lobe of the reperfused liver (respectively). At the end of each experiment, all animals were killed with a lethal dose of pentobarbital.
Tissue treatment and biochemical assays
Liver tissue samples were palpated to remove blood, washed with ice-cold 0.9% NaCl solution, blotted on filter paper, quickly weighed, and homogenized in ice-cold 1.15% KCl solution (10% w/v) using an Omni tissue homogenizer. Tissue homogenates were centrifuged at 18,000g for 15 min at 4°C, and then the supernatants were stored at −80°C until the biochemical analyses were carried out. Blood samples were collected in Vacutainers without additive, and centrifuged (3,500g for 5 min) to obtain the serum. Then, AST, ALT, LDH activities in the serum were measured by conventional methods using an automated analyser (AU-2700; Olympus, Tokyo, Japan). Protein levels of the homogenates were determined according to the method of Bradford [20]. All spectrophotometric measurements were performed using a DU 530 spectrophotometer (Beckman Coulter, Brea, CA).
DNA isolation
Hepatic cellular DNA was isolated by proteinase K digestion and phenol–chloroform–isoamyl alcohol extraction from the homogenates [21]. Tissue homogenates were resuspended in 1 mM EDTA, 1 ml 1% (w/v) sodium dodecyl sulphate, and incubated for 1 h at 37°C in the presence of proteinase K. Then, 50 μl Tris–HCl (1 M, pH 7.5) was added to the lysates and the samples were extracted successively with 1 volume of phenol, 1 volume of phenol–chloroform–isoamyl alcohol (25:24:1) and 1 volume of chloroform–isoamyl alcohol (24:1). After the addition of 0.1 volume of 5 M NaCl, DNA was precipitated with 2 volumes of absolute ethanol. The samples were stored at −20°C for 24 h and then centrifuged at 2,000 g for 10 min. DNA samples were then washed three times with 70% ethanol and the DNA was dissolved in water [21].
Analysis of 8-OHdG by HPLC-EC
8-OHdG levels were measured by high-performance liquid chromatography with electrochemical detection (HPLC-EC) as previously described [21]. Briefly, DNA samples were heated in boiling water for 2 min and then quenched immediately in ice water. After the addition of 100 μl sodium acetate (30 mM, pH 3.5), DNA samples were digested to nucleosides with 10 μg nuclease P1 (1 mg/ml) at 37°C for 30 min. Then, the samples were treated with 10 μl 0.5 M Tris and alkaline phosphatase at 37°C for 1 h. The specimens were then filtered through a 0.20 μm pore filter and analysed by HPLC-EC (HP1100; Agilent Technologies, Waldbronn, Germany): column, M BondapakC18 3 μm (3.9 mm × 30 cm); eluent, methanol (90:10 v/v) containing 50 mM KH2PO4 buffer; flow rate, 1 ml/min; EC detector, oxidation potential +0.7 V.
Measurement of MDA levels
Tissue MDA levels were determined spectrophotometrically according to the method described by Ohkawa et al. [22]. In this method, MDA reacts with thiobarbituric acid to form a coloured complex that has maximum absorbance at 532 nm. MDA is the most abundant individual aldehyde resulting from lipid peroxidation, and its determination by thiobarbituric acid is the most common method of estimating lipid peroxidation. Therefore, MDA levels are expressed as nanomoles TBARS per gram of protein.
Statistical analysis
Results are expressed as mean ± SD. The Kruskal–Wallis one-way analysis of variance followed by the Mann–Whitney U test with Bonferroni correction for multiple comparisons were used to evaluate the significance of differences among the four groups. The Wilcoxon signed ranks test was used to analyse differences between the ischaemic and reperfusion phases. P values <0.05 were considered significant in the Kruskal–Wallis and Wilcoxon signed ranks tests, and P values <0.013 were considered statistically significant in the Mann–Whitney U test with Bonferroni correction. SPSS 11.5 for Windows (SSPS, Chicago, IL) was used for these calculations.
Results
The serum levels of AST, ALT and LDH of the study and sham-operated groups are shown in Table 1. Compared to the sham-operated group, the levels of AST, ALT and LDH were significantly greater in both the ischaemic groups and reperfusion groups; the changes were more pronounced in reperfusion groups than in ischaemic groups (Table 1).
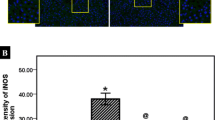
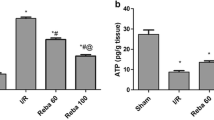
The tissue levels of 8-OHdG and TBARS in the study groups are shown in Figs. 1 and 2. IR caused a marked increase in tissue levels of 8-OHdG compared to the sham-operated group (Fig. 1). The increase in liver tissue 8-OHdG levels was significantly higher in the reperfusion phase compared to the ischaemic phase (P < 0.0001). However, pretreatment with NAC and with EGb761 reduced the tissue 8-OHdG levels in both groups 3 and 4 (Fig. 1). In group 2, IR caused a significant increase in liver tissue TBARS levels compared to the sham-operated group (P < 0.001). This increase in TBARS levels was partially prevented by pretreatment with NAC and with EGb761 (Fig. 2). Compared to the EGb761-pretreated rats, the tissue levels of 8-OHdG and TBARS were lower in the NAC-pretreated rats in both the ischaemia and reperfusion phases.
Discussion
Reactive oxygen species such as HO−, HOO− and O2 − may attack almost any cellular structure, and DNA is a potential target for free radicals. Damage to DNA by oxygen free radicals results in the production of a large number of lesions which may be categorized as strand breaks and base modification products [23]. 8-OHdG, a DNA base-modified product, may be a good marker of oxidative DNA damage if it can be analysed with high sensitivity [14]. In the present study, we measured 8-OHdG levels by HPLC-EC, and clearly detected the appearance of 8-OHdG in the DNA of the reperfused ischaemic liver tissue. Howard et al. [24] determined 8-OHdG levels in the heart, liver and lung tissues of mice exposed to side-stream cigarette smoke by HPLC. Their data showed oxidative DNA damage-related increases in 8-OHdG levels in all three tissues. It has also been reported that the level of 8-OHdG is increased in several clinical situations such as chronic viral hepatitis, alcoholic liver disease, haemochromatosis, hepatocellular carcinoma and primary biliary cirrhosis [13, 25, 26]. However, Cordis et al. [23] determined the levels of 8-OHdG in the DNA from rat hearts that had undergone IR. They suggested that the formation of 8-OHdG may serve as a sensitive marker not only for the development of oxidative stress associated with IR but also for other oxidative stress-mediated heart diseases such as cardiomyopathy and congestive heart failure [23]. We found that there was a significant elevation of 8-OHdG in liver tissue from rats during both the ischaemia and reperfusion phases with the increase being more pronounced in the reperfusion groups than in ischaemic groups.
The thiol NAC, the acetylated variant of the l-cysteine, is converted in the body into metabolites capable of stimulating glutathione synthesis, which supports glutathione biosynthesis as a source of SH group. SH groups are essential for defence against reactive oxygen species [27]. It has been showed that NAC has beneficial effects in a number of pathological conditions caused by free radicals. Skrzydlewska and Farbiszewski [28] investigated the effects of NAC on glutathione and lipid peroxidation in the liver and on the activities of serum ALT and AST after acute methanol intoxication in rats. They found that the use of NAC after methanol ingestion apparently diminished lipid peroxidation, and elevated glutathione levels in the liver. They suggested that NAC exerts its protective effect by acting as a precursor for glutathione and as a free radical scavenger [28]. In another study [29], Bahcecioglu et al. found that pretreatment with NAC causes a decrease in the hepatic lipid peroxidation and the serum ALT elevation, and they suggested that NAC might be useful to prevent tissue damage in hepatic IR. In the present study, we observed that increases in liver TBARS and 8-OHdG levels during both the ischaemia and reperfusion phases were mitigated by administration of NAC. Yusof et al. [21] demonstrated that 5-aminolaevulinic acid causes a linear increase in 8-OHdG levels in Chinese hamster ovary cells, and in the presence of NAC, 8-OHdG levels returned to control levels, corroborating our results with respect to the effect of NAC treatment. However, in another study, it was found that NAC seems to be of limited value as a radioprotective agent against X-ray-induced DNA damage [30]. Pretreatment with NAC at a dose of 250 mg/kg (i.p.) in the present study had an inhibitory effect on 8-OHdG formation induced by IR in rat liver DNA, and we think that NAC may be used against liver IR injury, probably by inducing glutathione synthesis in hepatic tissue.
EGb761 is a standardized plant extract of dried Ginkgo biloba leaves, and recent studies have provided considerable support for the protective effects of EGb761 against IR injury [12, 31, 32]. In addition, EGb761 is now widely prescribed for the treatment of disorders such as cardiovascular diseases, Alzheimer’s disease and cerebral ischaemia, all of which have aetiologies associated with oxidative stress [33–35]. However, there are no reports of the effect of EGb761 on 8-OHdG formation induced by IR in rat liver. The protective effect of EGb761 against cellular damage has been associated rather with its high free radical scavenging ability. Schindowski et al. [36] analysed age-related increases in oxidative stress-induced levels of apoptotic lymphocytes in mice, and they found a statistically significant decrease in reactive oxygen species-induced apoptosis in mice receiving EGb761. Bahcecioglu et al. [29] reported that EGb761 reduces the increases in liver TBARS levels and serum ALT and AST activities caused by carbon tetrachloride in rats, and they suggested that its protective effect against carbon tetrachloride-induced liver damage may be due to its capacity to reduce lipid peroxidation. EGb761 has also been shown to exhibit an effective oxygen radical scavenging activity, which improves hepatic microcirculation in the rat liver after warm ischaemia [11]. In the present study, pretreatment with the EGb761 had a beneficial effect on tissue 8-OHdG levels in rats of groups 3 and 4 compared to control rats. In our experiment, EGb761 significantly decreased 8-OHdG formation in the hepatic tissue of rats that had undergone liver IR. In addition, rats that received EGb761 showed a lower increase in serum AST, ALT and LDH levels after hepatic IR when compared to rats in the control group. EGb761 might be a useful agent to attenuate postischaemic reperfusion injury, providing a variety of positive effects on hepatic microcirculation and liver regeneration [37].
In conclusion, oxidative DNA damage occurred widely in the hepatic tissue after 90 min of liver ischaemia and 90 min reperfusion, possibly because of an abnormal increase of reactive oxygen species. Also, antioxidant molecules, such as NAC and EGb761, may be useful in preventing postischaemic reperfusion injury to hepatic tissue. However, these findings require confirmation with a much larger sample size before they can be accepted as meaningful.
References
Jaeschke H (1998) Mechanisms of reperfusion injury after warm ischemia of the liver. J Hepatobiliary Pancreat Surg 5:402–408
Stein HJ, Oosthuizen MM, Hinder RA, Lamprechts H (1991) Oxygen free radicals and glutathione in hepatic ischemia/reperfusion injury. J Surg Res 50:398–402
Yildirim A, Gumus M, Dalga S et al (2003) Dehydroepiandrosterone improves hepatic antioxidant systems after renal ischemia-reperfusion injury in rabbits. Ann Clin Lab Sci 33:459–464
McCord JM (1985) Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med 312:159–163
Chavez-Cartaya R, Jamieson NV, Ramirez P et al (1999) Free radical scavengers to prevent reperfusion injury following experimental warm liver ischaemia. Is there a real physiological benefit? Transpl Int 12:213–221
Koeppel TA, Thies JC, Lehmann T et al (1996) Improvement of hepatic microhemodynamics by N-acetylcysteine after warm ischemia. Eur Surg Res 28:270–277
Koeppel TA, Lehmann TG, Thies JC et al (1996) Impact of N-acetylcysteine on the hepatic microcirculation after orthotopic liver transplantation. Transplantation 61:1397–1402
Dunne JB, Davenport M, Williams R, Tredger JM (1994) Evidence that S-adenosylmethionine and N-acetylcysteine reduce injury from sequential cold and warm ischemia in the isolated perfused rat liver. Transplantation 57:1161–1168
Walcher F, Marzi I, Flecks U, Larsen R (1995) N-acetylcysteine failed to improve early microcirculatory alterations of the rat liver after transplantation. Transpl Int 8:317–323
Steib A, Freys G, Collin F et al (1998) Does N-acetylcysteine improve hemodynamics and graft function in liver transplantation? Liver Transpl Surg 4:152–157
Topp S, Knoefel WT, Schutte A et al (2001) Ginkgo biloba (EGB 761) improves microcirculation after warm ischemia of the rat liver. Transplant Proc 33:979–981
Du G, Willet K, Mouithys-Mickalad A et al (1999) EGb 761 protects liver mitochondria against injury induced by in vitro anoxia/reoxygenation. Free Radic Biol Med 27:596–604
Kitada T, Seki S, Iwai S et al (2001) In situ detection of oxidative DNA damage, 8-hydroxydeoxyguanosine, in chronic human liver disease. J Hepatol 35:613–618
Kasai H (1997) Analysis of a form of oxidative DNA damage, 8-hydroxy-2′-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat Res 387:147–163
Maulik G, Cordis GA, Das DK (1996) Oxidative damage to myocardial proteins and DNA during ischemia and reperfusion. Ann N Y Acad Sci 793:431–436
Chen J, Jin K, Chen M et al (1997) Early detection of DNA strand breaks in the brain after transient focal ischemia: implications for the role of DNA damage in apoptosis and neuronal cell death. J Neurochem 69:232–245
Loft S, Larsen PN, Rasmussen A et al (1995) Oxidative DNA damage after transplantation of the liver and small intestine in pigs. Transplantation 59:16–20
Allameh A, Vansoun EY, Zarghi A (1997) Role of glutathione conjugation in protection of weanling rat liver against acetaminophen-induced hepatotoxicity. Mech Ageing Dev 95:71–79
Zeybek N, Gorgulu S, Yagci G et al (2003) The effects of Gingko biloba extract (EGb 761) on experimental acute pancreatitis. J Surg Res 115:286–293
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Yusof M, Yildiz D, Ercal N (1999) N-acetyl-l-cysteine protects against delta-aminolevulinic acid-induced 8-hydroxydeoxyguanosine formation. Toxicol Lett 106:41–47
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Cordis GA, Maulik G, Bagchi D et al (1998) Detection of oxidative DNA damage to ischemic reperfused rat hearts by 8-hydroxydeoxyguanosine formation. J Mol Cell Cardiol 30:1939–1944
Howard DJ, Briggs LA, Pritsos CA (1998) Oxidative DNA damage in mouse heart, liver, and lung tissue due to acute side-stream tobacco smoke exposure. Arch Biochem Biophys 352:293–297
Shimoda R, Nagashima M, Sakamoto M et al (1994) Increased formation of oxidative DNA damage, 8-hydroxydeoxyguanosine, in human livers with chronic hepatitis. Cancer Res 54:3171–3172
Cardin R, Saccoccio G, Masutti F et al (2001) DNA oxidative damage in leukocytes correlates with the severity of HCV-related liver disease: validation in an open population study. J Hepatol 34:587–592
Kelly GS (1998) Clinical applications of N-acetylcysteine. Altern Med Rev 3:114–127
Skrzydlewska E, Farbiszewski R (1999) Protective effect of N-acetylcysteine on reduced glutathione, reduced glutathione-related enzymes and lipid peroxidation in methanol intoxication. Drug Alcohol Depend 57:61–67
Bahcecioglu H, Ustundağ B, Ozercan I et al (1999) Protective effect of Ginkgo biloba extract on CCl4-induced liver damage. Hepatol Res 15:215–224
Abt G, Vaghef H, Gebhart E et al (1997) The role of N-acetylcysteine as a putative radioprotective agent on X-ray-induced DNA damage as evaluated by alkaline single-cell gel electrophoresis. Mutat Res 384:55–64
Tosaki A, Droy-Lefaix MT, Pali T, Das DK (1993) Effects of SOD, catalase, and a novel antiarrhythmic drug, EGB 761, on reperfusion-induced arrhythmias in isolated rat hearts. Free Radic Biol Med 14:361–370
Haramaki N, Aggarwal S, Kawabata T et al (1994) Effects of natural antioxidant Ginkgo biloba extract (EGB 761) on myocardial ischemia-reperfusion injury. Free Radic Biol Med 16:789–794
Luo Y (2006) Alzheimer’s disease, the nematode Caenorhabditis elegans, and Ginkgo biloba leaf extract. Life Sci 78:2066–2072
Chandrasekaran K, Mehrabian Z, Spinnewyn B et al (2001) Neuroprotective effects of bilobalide, a component of the Ginkgo biloba extract (EGb 761), in gerbil global brain ischaemia. Brain Res 922:282–292
Diamond BJ, Shiflett SC, Feiwel N et al (2000) Ginkgo biloba extract: mechanisms and clinical indications. Arch Phys Med Rehabil 81:668–678
Schindowski K, Leutner S, Kressmann S et al (2001) Age-related increase of oxidative stress-induced apoptosis in mice prevention by Ginkgo biloba extract (EGb761). J Neural Transm 108:969–978
Schutte A, Topp SA, Knoefel WT et al (2001) Influence of Ginkgo biloba extract (EGB 761) on expression of EGR-1 mRNA and HSP-70 mRNA after warm ischaemia in the rat liver. Transplant Proc 33:3724–3725
Conflict of interest statement
The authors declare that they have no commercial or financial interest in the publication of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keles, M.S., Demirci, N., Yildirim, A. et al. Protective effects of N-acetylcysteine and Ginkgo biloba extract on ischaemia-reperfusion-induced hepatic DNA damage in rats. Clin Exp Med 8, 193–198 (2008). https://doi.org/10.1007/s10238-008-0005-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-008-0005-1