Abstract
The glycocalyx is the inner most layer of the endothelium that is in direct contact with the circulating blood. Shear stress affects its synthesis and reorganization. This study focuses on changes in the spatial distribution of the glycocalyx caused by shear stimulation and its recovery following the removal of the shear stress. Sialic acid components of the glycocalyx on human umbilical vain endothelial cells are observed using confocal microscopy. The percentage area of the cell membrane covered by the glycocalyx, as well as the average fluorescence intensity ratio between the apical and edge areas of the cell is used to assess the spatial distribution of the glycocalyx on the cell membrane. Our results show that following 24 h shear stimulation, the glycocalyx relocates near the edge of endothelial cells (i.e., cell–cell junction regions). Following the removal of the shear stress, the glycocalyx redistributes and gradually appears in the apical region of the cell membrane. This redistribution is faster in the early hours (\(<\)4 h) after shear stimulation than that in the later stage (e.g., between 8 and 24 h). We further investigate the recovery of the glycocalyx after its enzyme degradation under either static or shear flow conditions. Our results show that following 24 h recovery under shear flow, the glycocalyx reappears predominantly near the edge of endothelial cells. Static and shear flow conditions result in notable changes in the spatial recovery of the glycocalyx, but the difference is not statistically significant. We hypothesize that newly synthesized glycocalyx is not structurally well developed. Its weak interaction with flow results in less than significant redistribution, contrary to what has been observed for a well-developed glycocalyx layer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The endothelial glycocalyx contains negatively charged molecules such as proteoglycans, glycosaminoglycans (GA Gs), glycoprotein and plasma proteins. It forms a brush-like structure at the luminal surface of the vascular endothelium, with a thickness of between several hundreds of nanometers and a few microns (Luft 1966; Oohira et al. 1983; Jackson et al. 1991; Squire et al. 2001). As a mechanotransducer, the glycocalyx modifies the stress on endothelial cells and transforms it to the endothelial cytoskeleton (Damiano and Stace 2002; Mochizuki et al. 2003). On the other hand, shear stress results in the deformation of the glycocalyx and endothelial cytoskeleton, triggering a number of signaling events involved in cell proliferation and morphological changes (Davies 1997; Stamatas and McIntire 2001; Helmke and Davies 2002). Yao et al. (2007) reported that the degradation of the endothelial glycocalyx abolished not only the alignment of endothelial cells to shear flow, but also the relationship between the shear stress and cell proliferation rate. A number of studies have demonstrated that depletion of different GAGs components of the glycocalyx, e.g., heparan sulfate proteoglycan (HSPG), hyaluronic acid (HA), chondroitin sulfate (CS), is responsible for the significant drop of the flow-dependent NO production (Mochizuki et al. 2003; Lopez-Quintero et al. 2009). One of the earliest in vivo studies by Hecker et al. (1993) reported a 64 % deduction of the shear-dependent NO release when neuraminidase was used to remove sialic acids from the endothelial glycocalyx in rabbit femoral arteries. A similar phenomenon has also been observed in studies in vitro. Tarbell et al. exposed endothelial cells to different enzymes to selectively degrade various GAGs component: Hep III for HSPG, neuraminidase for sialic acid, chondroitinase for CS and hyaluronidase for HA. Their study showed that HS, HA and SA depleting caused NO production blockage, but not CS, and this might be related to glypican–caveolae–eNOS mechanism of the shear-induced NO transduction (Pahakis et al. 2007).
This study focuses on the effects of shear stress on the spatial distribution of the glycocalyx on endothelial cell membranes. A flow chamber similar to the one used in our earlier study is employed (Bai et al. 2010). The same immunofluorescent staining method has been applied to observe the endothelial glycocalyx after the shear exposure (Bai and Wang 2012). We calculate two different parameters to characterize the spatial distribution of the glycocalyx on the endothelial cell membrane: (a) the percentage area of the cell membrane covered by the glycocalyx layer and (b) the fluorescence intensity ratio between the apical and edge regions of endothelial cells. They give quantitative measures of the observed changes in the distribution of the glycocalyx following shear exposure. Comparisons are made at different time points after shear stimulation to study the redistribution of the glycocalyx on the cell membrane. Further studies are carried out to analyze the recovery of the glycocalyx layer following its enzyme degradation in either static or shear flow conditions.
2 Materials and methods
2.1 Cell culture and cell proliferation
HUVECs (primary pooled) are purchased from Lonza (Lonza Cologne AG, Germany). They are thawed and cultured in M199 medium (Gibco) in collagen type I-coated flasks (\(5\upmu \text{ g }/\text{ ml }\)) at \(37^{\circ }\text{ C }\) & 5 % \(\text{ CO }_{2}\). The culture medium contains 10 % fetal bovine serum, endothelial cell growth factor-\(\beta \) (1 ng/ml), endothelial cell growth supplement from bovine neural extract (\(3\,\upmu \text{ g }/\text{ ml }\)), thymidine (\(1.25\upmu \text{ g }/\text{ ml }\)), heparin (\(10\upmu \text{ g }/\text{ ml }\)), 100 U/ml penicillin, 100 mg/ml streptomycin (all supplements are from Sigma Aldrich). The medium is changed every 2 days. After reaching 80 % confluence, HUVECs are treated with 0.25 % trypsin containing 0.02 % EDTA (Sigma Aldrich) and cells are split and seeded on collagen type I-coated glass slides. Following different periods of culture (in this study, day 4, 7, 10, 14 and 21 are investigated), cells are ready for shear stress stimulation or neuraminidase treatment.
2.2 Shear stress stimulation
We use the same parallel plate flow chamber as reported in Bai et al. (2010). The sketch is shown in Fig. 1a, where hydrostatic pressure is provided by the height difference between the two reservoirs and a peristaltic pump completing the flow circuit. Details of the flow chamber are shown in Fig. 1b, where the HUVEC monolayer is surrounded by a silicon gasket and sandwiched between two transparent polymethylmethacrylate (PMMA) slides. For steady laminar flow between two parallel plates, the wall shear stress can be expressed as (e.g., Depaola et al. 1992):
where \(\tau \) is the wall shear stress \((\text{ dyn }/\text{ cm }^{2}), \mu \) is the viscosity of the culture medium, \(Q\) is the flow rate (ml/s), \(h\) and \(W\) are the height and width of the channel, respectively, (they are 0.25 mm, i.e., the thickness of the silicon gasket, and 20 mm, respectively). More details on the flow chamber can be found in Bai et al. (2010).
a Schematic drawing of the steady flow bioreactor; b Schematic drawing of the rectangular parallel plate flow chamber; c Stack images of the glycocalyx (top), cytoplasm (middle) and nucleus (bottom) of day 14 HUVECs after 24 h of shear stimulation. \(\text{ Bar } = 10\, \upmu \text{ m }\); d Corresponding images in black and white for the percentage area analysis
HUVECs are seeded on cover slips and after different days in culture, they are exposed to a steady shear stress of \(12\, \text{ dyn }/\text{ cm }^{2}\) for 24 h. The pH of the culture medium is kept by gassing the medium with a mixture of 95 % air and 5 % \(\text{ CO }_{2}\), and the temperature is maintained at \(37\,^{\circ }\text{ C }\). Cells are monitored during the shear stimulation. Control groups in static medium are concurrently performed. At the end of shear stress stimulation, HUVECs are kept in static culture medium for 0, 2, 4, 8 or 24 h before staining and observation using a confocal microscope.
2.3 Enzyme treatment using neuraminidase
Neuraminidase from clostridium perfringens (Sigma Aldrich) is used in the study. It cleaves N-acetyl neuraminic acid residues of glycoproteins and targets specifically the sialic acid component of the endothelial glycocalyx. Cultured HUVECs are treated with neuraminidase according to the protocol by Barker et al. (2004). Serum-free M199 medium is used to wash the sample gently twice before it is incubated with 5 U/ml neuraminidase for 30 min at \(37\,^{\circ }\text{ C }\). The control group remains in the HUVECs culture medium. The recovery process takes place in the culture medium with 10 % serum under two different conditions for 24 h: static medium or steady flow with a shear stress of \(12\, \text{ dyn }/\text{ cm }^{2}\). At the end of the recovery, HUVECs are stained and observed under the confocal microscope.
2.4 Immunofluorescence imaging and analysis
Wheat germ agglutinin (WGA) was used to bind to N-acetyl-D-glucosamine and sialic acid (SA) component of the glycocalyx (Barker et al. 2004; Megens et al. 2007). The endothelial cytoplasm was stained by Cell Tracker Red (Invitrogen). Endothelial cell nucleus was stained using Hoechst 33342 (Sigma Aldrich). Cultured HUVECs were briefly washed using serum-free M199. WGA-FITC and cell tracker red were applied to live cells for 15 min at \(37\,^{\circ }\text{ C }\) before Hoechst 33342 was applied for 5 min. Live cells were washed three times using serum-free M199 after staining and kept in 10 % serum culture medium, ready for confocal microscopy (Leica Microsystems, Wetzlar, Germany). The intensity of the SA was analyzed using the software Image J (National Institutes of Health, USA).
2.5 Theoretical considerations
In order to assess the distribution of the glycocalyx layer on the endothelial cell membrane, two different methods of quantification are performed:
-
1)
Percentage area of the cell membrane that is covered by the glycocalyx, \(A\). In this analysis, stack images of each color are created, as shown in Fig. 1c. They are transformed to black and white images using the threshold function of the Image J software. The background pixels are set as black, and the colored pixels are set as white, regardless of the brightness of the different pixels (Fig. 1d). The percentage area of the cell membrane covered by the glycocalyx is calculated as the ratio of the green dye (WGA) area over the total area of the cell, i.e., the total area by WGA (SA), CTR (cytoplasm) and Hoechst (nucleus):
$$\begin{aligned} A\% =\frac{A_\mathrm{WGA}}{A_\mathrm{WGA} \cup A_\mathrm{CTR} \cup A_\mathrm{Hoechst}}\times 100\,\% \end{aligned}$$ -
2)
Ratio of WGA intensity between the apical and the edge regions of HUVECs, \(R\). As in our earlier study (Bai and Wang 2012), we divide the cell surface into three regions: the ‘apical’ region is the area of the cell membrane that covers the entire endothelial nucleus, and the remaining cell membrane is divided equally into the ‘middle’ and the ‘edge’ regions. This makes possible a spatial distinction of the cell membrane surface. The WGA intensity ratio between the apical and the edge regions of HUVECs is defined as
$$\begin{aligned} R=\frac{I_\mathrm{apical}}{I_\mathrm{edge}} \end{aligned}$$where \(I\) is the average fluorescence intensity of WGA in the region. \(R\) indicates the evenness of the glycocalyx distribution. Values of \(A\) and \(R\) are calculated at different time points, i.e., 0, 2, 4, 8 or 24 h, following the end of the shear stimulation to study the redistribution of the glycocalyx on HUVEC membrane.
3 Results
3.1 Distribution of the glycocalyx affected by the shear flow
In Fig. 2, HUVECs are cultured on glass slides for 13 days in static medium before they are subjected to 24 h of steady shear stress stimulation at \(12\, \text{ dyn }/\text{ cm }^{2}\). They are then kept in the static medium for different periods of time, i.e., 0, 2, 4, 8 and 24 h before staining and observation. As shown in Fig. 2a, HUVECs at the end of 24 h shear exposure are seen to elongate with their long axis aligned in the flow direction. After 24 h recovery in the static medium, as shown in Fig. 2b, HUVECs appear to have regained their original morphology. Figure 2c, d shows immunofluorescent images of HUVECs at 0 and 24 h after shear stimulation, respectively. Here the glycocalyx layer can be seen on the cell membrane in both \(x-y\) cross-sectional images. More information on the spatial distribution of the glycocalyx is seen in the reconstructed \(x-z\) cross-sectional images that cut across the nucleus, as shown in Fig. 2e. Here, comparison is made between the control group (i.e., cells in static medium for 14 days without shear stimulation) and groups either immediately after shear stress exposure (i.e., at \(t = 0\) h) or at different times (i.e., \(t = 2\), 4, 8 and 24 h) after shear stimulation. For the control group, WGA staining is evenly distributed on the entire cell membrane and the thickness of the glycocalyx layer is approximately \(1\upmu \text{ m }\). At \(t = 0\) h (i.e., immediately after 24 h of shear flow), the majority of the WGA staining is near the edge of the cell membrane, away from the nucleus. This pattern remains for \(t = 2\) h, however, at \(t = 4\) h, WGA staining starts to appear at the apical region of the cell membrane above the nucleus. As time progresses, more WGA dye is observed in the apical region of the cell membrane. After 24 h recovery, WGA staining appears to cover the entire cell membrane, indifferent to that of the control group.
Morphological changes of HUVECs and their glycocalyx distribution. Cells are cultured in static medium for 13 days before they are exposed to 24 h exposure to shear stress of \(12\,\text{ dyn }/\text{ cm }^{2}\). Green—sialic acid component of the glycocalyx, Red—endothelial cytoplasm, Blue—cell nucleus. Arrow in a indicates the flow direction. \({Bar} = 10\,\upmu \text{ m }\). a Cell morphology immediately after shear stimulation; b Cell morphology after a further 24 h recovery in a static medium; c Confocal images of HUVECs immediately after shear stimulation; d Confocal images of HUVECs after a further 24 h recovery in a static medium; e \(x-z\) cross-sectional images of HUVECs during their recovery in a static medium at \(t = 0\), 2, 4, 8 and 24 h. The control group has HUVECs cultured in static medium for 14 days
3.2 Percentage area of the cell membrane covered by the glycocalyx, A
In Fig. 3, the percentage area of the cell membrane that is covered by the glycocalyx is calculated at \(t = 0\) and 24 h after the end of shear stimulation and compared to the control. As we reported in an earlier study (Bai and Wang 2012), it takes approximately 10 days for HUVECs in the culture medium to develop a glycocalyx layer that covers the entire cell membrane. Early development of the glycocalyx is predominately near the cell–cell junction regions. In the current study, we have used HUVECs that are cultured for different days in static medium before shear flow studies. The 4D, 7D, 10D, 14D and 21D groups represent HUVECs that have been cultured in static medium for 3, 6, 9, 13 and 20 days, respectively, and are then subjected to 24 h shear flow at \(12\, \text{ dyne }/\text{ cm }^{2}\). As seen in the figure, the control groups show increases in the glycocalyx coverage of the cell membrane surface from approximately 46.8 % (\(\pm \)7.0 %, \({n} = 6\)) at day 4 to 80.4 % (\(\pm \)7.0 %, \({n} = 6\)) at day 21. This is consistent with results reported in our earlier study on the spatiotemporal development of the glycocalyx (Bai and Wang 2012). Immediately after shear stimulation, i.e., with 0 hour recovery, the percentage area of the cell membrane covered by the glycocalyx decreases significantly (\({p} < 0.05\), denoted by *) from the control in all groups except for the 4D group. Following 24 h recovery in the static medium, the percentage area increases in all groups and the values exceed those of their controls.
Effects of the shear flow on the percentage area of the cell membrane that is covered by the glycocalyx layer. 4D, 7D, 10D, 14D & 21D groups represent HUVECs cultured for 3, 6, 9, 13 and 20 days in the static medium (for development of the glycocalyx) before they are subjected to 24 h shear stress at \(12\, \text{ dyn }/\text{ cm }^{2}\). Corresponding control groups are HUVECs not being exposed to shear stress at all. Red bars are values immediately after shear stimulation. Blue bars are values after a further 24 h recovery in a static medium. Asterisk (*) denotes statistical significance, \({p} < 0.05\)
In Fig. 4, we plot a more detailed time-recovery course of the glycocalyx layer on the cell membrane. Only HUVECs of the 7D, 14D and 21D groups are shown. The percentage areas increase with the recovery time in all groups. There seems to be a rapid recovery during the early period, i.e., between 0 and 4 h, which is followed by a much slower recovery between 8 and 24 h. Linear curve fitting of data within these two periods shows slopes of 5.04 %/h (7D group), 4.24 %/h (14D group) and 2.85 %/h (21D group) between 0 and 4 h, and 0.17 %/h (7D group), 0.64 %/h (14D group) and 0.38 %/h (21D group) between 8 and 24 h, respectively. They indicate a rapid recovery of the glycocalyx layer on the cell membrane within 4 h after the shear stimulation. On the other hand, comparison of the recovery speed between the 7D, 14D and 21D groups shows that the 7D group has the fastest recovery between 0 and 4 h, whereas the 21D group has the slowest.
3.3 WGA intensity ratio, R
The intensity ratio (\(R\)) of WGA between the apical and the edge regions of the endothelial cell membrane indicates the relative distribution of the glycocalyx between the two areas. In Fig. 5, we evaluate changes in \(R\) during the 24h recovery period after shear flow. As in Fig. 3, five groups of HUVECs are used, which have been cultured for different periods in static medium before the shear stress study. The control groups show the \(R\) value increases from 0.47 (\(\pm 0.09, n = 6\)) for 4D to 0.87 (\(\pm 0.08, n = 6\)) for 21D. This increase represents a more evenly distributed glycocalyx layer on the HUVEC membrane with time and is in agreement to our earlier results (Bai and Wang 2012). Following 24 h exposure to the shear flow, \(R\) decreases in all groups in comparison with their controls, and the decrease is significant for 10D, 14D and 21D groups. This indicates a redistribution of the glycocalyx from the apical region to the edge region of the cell membrane. After 24 h recovery in static medium, \(R\) increases to their control values. For 4D and 7D groups, \(R\) increases to above their control. The change in \(R\) value within the 24h recovery period is significant except for the 4D group. This is due to the fact that for the 4D group, there is not much glycocalyx on the apical region of the cell membrane; hence, the redistribution is less than significant.
3.4 Recovery of the glycocalyx following its depletion by neuraminidase
We further studied the recovery of the glycocalyx layer on HUVECs after enzyme treatment under two different conditions: either in a static medium or in a flow chamber where HUVECs are subjected to a shear stress of \(12\, \text{ dyn }/\text{ cm }^{2}\).
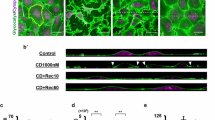
Figure 6 shows confocal images of HUVECs in the \(x-y\) (main panels), \(x-z\) (bottom panels) and \(y-z\) (side panels) cross-sections. In the figure, results of the 14D group HUVECs are presented, i.e., cells are cultured for 14 days to ensure the glycocalyx layer covers the entire cell membrane before the experiments. As seen in Fig. 6a, immediately after neuraminidase treatment, sialic acid staining of HUVECs shows little expression of the glycocalyx layer on cell membranes. After 24 h recovery in a static medium, as shown in Fig. 6b, the glycocalyx layer reappears and can be seen on the entire surface of the HUVEC membrane. In comparison, 24 h recovery under shear flow condition results in elongated HUVECs in the flow direction, as seen in Fig. 6c. Sialic acid staining appears on most areas of the cell membrane with patchy staining also being observed in the apical region of the membrane above the nucleus.
Recovery of the glycocalyx on 14 days old HUVECs after neuraminidase treatment. Main panels are the \(x-y\) cross-section images, \({bar} = 10\,\upmu \text{ m }\). Bottom and side panels are the \(x-z\) and \(y-z\) cross-section images, respectively. Arrow in c indicates the flow direction. a HUVECs treated with neuraminidase; b after 24 h recovery in a static medium; c after 24 h recovery in shear flow of \(12\,\text{ dyn }/\text{ cm }^{2}\)
Results on the percentage area (\(A\)) of the cell membrane that is covered by the glycocalyx layer are shown in Fig. 7. Five HUVEC groups, 4D, 7D, 10D, 14D and 21D, are studied. In all groups, following neuraminidase treatment, nearly 80 % of the cell membrane is free of WGA staining. Twenty hours recovery in a static medium shows significant increase in the area covered by the glycocalyx in all cell groups. Interestingly, 10D, 14D & 21D groups (i.e., HUVECs with a well-developed glycocalyx layer before neuraminidase treatment) show greater coverage of the cell membrane by the glycocalyx layer (i.e., \(>\)85 %) than that of the 4D and 7D groups (where the glycocalyx is predominantly in the edge areas of the cell membrane before neuraminidase treatment). In comparison, 24 h recovery in \(12\, \text{ dyn }/\text{ cm }^{2}\) shear flow also results in significant increases in \(A\) in all groups. The value, however, is consistently lower than that of the corresponding group in the static medium. For HUVECs with a well-developed glycocalyx layer before enzyme treatment, i.e., 10D, 14D and 21D groups, the difference in the percentage area between the static and shear flow conditions is not statistically significant (\({p} \approx 0.11 - 0.17\)). We will discuss this point later.
The percentage area of cell membrane covered by the glycocalyx after neuraminidase treatment (control) and 24 h recovery in either a static medium (red bars) or in a flow chamber (blue bars). 4D, 7D, 10D, 14D and 21D groups are HUVECs cultured for different days in medium (for development of the glycocalyx) before experiments
The ratio of WGA intensity (\(R\)) between the apical and edge areas of HUVECs is shown in Fig. 8. For all groups of HUVECs, neuraminidase treatment cleaves most of the glycocalyx on the cell membrane. WGA-FITC intensity level drops after enzyme degradation, but it is still detectable. Take 14 days HUVECs for example, before neuraminidase treatment, the glycocalyx layer appears on the entire cell surface and the average WGA-FITC intensities in the apical and edge regions are \(92 \pm 2.6\) and \(96 \pm 2.3\), respectively; after neuraminidase degradation, the average intensities drop to \(17.9 \pm 1.2\) and \(18.6 \pm 0.9\). The close to unity value of \(R (=\!\!\!0.95\pm 0.09, {n} = 30)\) indicates that neuraminidase degrades SA compound evenly all over the cell membrane. Twenty hours recovery in a static medium results in a decrease in the intensity ratio in all HUVEC groups. The significant decrease in 4D and 7D groups indicates a more rapid recovery of the glycocalyx near the cell–cell junction areas than that in the apical areas of the membrane. For 10D, 14D and 21D groups, the decrease in the \(R\) values is not statistically significant (\(p = 0.14\), 0.44 and 0.49, respectively). Twenty hours recovery under shear stress results in even smaller values of \(R\) for all HUVEC groups. The difference in \(R\) between the control and 24 h shear stress groups is statistically significant (except for 14D group). This indicates a predominant recovery of the glycocalyx in the edge region of the cell membrane. The difference in \(R\) between the static and shear flow conditions is notable but not statistically significant (\(p = 0.39\), 0.46 and 0.49 for 10D, 14D and 21D groups, respectively). This is an interesting finding, which we will discuss later. It has also been noted that the intensity ratio is bigger for HUVEC groups that have been cultured longer (e.g., 21D vs. 7D), indicating a more even reappearance of the glycocalyx for longer cultured HUVECs.
Ratio of WGA intensity between the apical and edge regions of cell membrane. Controls are results immediately after neuraminidase treatment. They are compared to results after 24 h recovery in either a static medium (red bars) or under \(12\, \text{ dyn }/\text{ cm }^{2}\) shear stress (blue bars). Results for 4D, 7D, 10D, 14D and 21D HUVECs groups are presented
4 Discussion
In this study, effects of shear stress on the spatial distribution of the endothelial glycocalyx are examined in vitro. The redistribution of the glycocalyx under shear flow reveals an adaptive mechanism, in which the glycocalyx reorganizes itself toward the cell–cell junction areas (Yao et al. 2007). This pattern can be observed in Fig. 2, where SA staining occurs predominantly near the edge of the cell membrane following 24 h shear flow stimulation at \(12\, \text{ dyn }/\text{ cm }^{2}\). The ratio of WGA intensity, \(R\), between the apical and edge areas of the cell membrane shows a similar pattern of change (see Fig. 5).
It has been reported that the shear stress simulates the synthesis of glycocalyx. For example, Arisaka et al. (1994) studied GAGs synthesis on porcine aortic ECs, and Elhadj et al. (2002) studied the proteoglycan production by bovine aortic endothelial cells. Similar results have been observed on SA staining in this study, as shown in Fig. 3. The percentage area of the cell membrane covered by the glycocalyx is consistently greater for the 24 h recovery groups than controls. It needs to be noted that the total time of HUVECs in culture for the 24 h recovery groups is 1 day longer than their controls, so strictly speaking, they are not directly comparable. However, comparing the 24 h recovery result in 4D group to the control of 7D group, we note similar values in the percentage area (i.e., 64.1 vs. 62.2 %) despite the fact that the shear stimulated 4D group HUVECs have had 2 days less in culture medium for glycocalyx development.
As reported in our earlier study (Bai and Wang 2012), a fully developed glycocalyx layer requires 10 days or more to develop in culture. The finding is consistent with results shown in Fig. 4: (a) the percentage area of the cell membrane covered by the glycocalyx at day 7 is notably smaller than those at day 14 and day 21; (b) in comparison, there is much less difference between day 14 and day 21; and furthermore, (c) the slope of recovery between 0 and 4 h for 7D group is much bigger than those of 14D or 21D groups.
Degradation of the endothelial glycocalyx is observed in diseases such as inflammation and atherosclerosis. Kang et al. (2011) reported that heparan sulfate proteoglycans recovered from Heparinase III degradation swiftly. Six hours after the Heparinase III treatment, there were 20 % recovery of the glycocalyx as indicated in their normalized HSPG intensity. Our results agree to Kang et al.’s observation, although different component of the glycocalyx layer, e.g., SA in our study rather than HSPG in Kang’s study, has been targeted by different enzyme (i.e., neuraminidase in our study rather than Heparinase III in theirs).
One interesting result from this study is that 24 h shear stimulation has statistically insignificant effect on the spatial distribution and recovery of the glycocalyx following its neuraminidase degradation. One possible explanation could be that the newly synthesized glycocalyx is not structurally well developed. This leads to its much weaker interaction with the flow (Wang 2007). As a result, the spatial distribution of the newly synthesized glycocalyx is largely unaffected by the shear flow, unlike the well-developed glycocalyx layer on HUVECs that have been cultured for 10 days or more.
The ratio of WGA intensity, \(R\), between the apical and edge areas of the cell membrane shows a similar pattern of change (as detailed in Fig. 8). It decreases following 24 h recovery in static medium, indicating a faster recovery of the glycocalyx layer near the cell–cell junction region than that in the apical region of the cell membrane. Twenty hours recovery under shear stress results in an even smaller value of \(R\) (i.e., even more glycocalyx near the cell–cell junctions). This is physiologically beneficial since the primary function of the vascular endothelium is to act as a semi-selective barrier between the blood and surrounding tissues. Discontinuities of the tight junction in intercellular clefts between adjacent cells provide a leakage route for macromolecules (Adamson et al. 1993). Molecular sieving by the glycoclayx at cell–cell junctions is very important to the permeability control. Following enzyme degradation, recovery of the glycocalyx predominantly near the edge of the cell membrane is the most effective way for the endothelium to regain its function of permeability control. This could be influenced by the flow condition.
There are a number of limitations in the current study that need to be addressed in future work. They include the spatial resolution of conventional confocal images (i.e., \(\sim \)250 nm in the \(x-y\) direction and \(\sim \)400 nm in the z direction). A new super resolution confocal system (Huang et al. 2008) is planned in our laboratory to significantly improve the quality of images. The dye used in the study, WGA, mainly stains the sialic acid component of the GAGs and may not represent the entire glycocalyx layer. The steady shear stress does not represent the physiological flow environment for endothelial cells. Endothelial cells are known to respond very differently to different flow regimes (e.g., steady, oscillatory, pulsatile and/or disturbed flows). Maintenance of a physiological laminar flow is considered to help normal vascular functioning, e.g., inhibition of thrombosis and inflammation of the vessel wall. On the contrary, disturbed or oscillatory flows near arterial bifurcations, branch ostia and curvatures are associated with enhanced macromolecular permeability and atheroma formation (e.g., Davies 1997; Chiu et al. 1998; Fisher et al. 2001; Cunningham and Gotlieb 2005). There is limited work on how different flows alter the distribution of the endothelial glycocalyx. Savery and Damiano (2008) studied the glycocalyx in arterioles throughout the cardiac cycle and reported statistically insignificant changes in its mean thickness despite the inherent unsteadiness of the flow regimes. It is noted that the method they used (i.e., based on hydrodynamic velocity profiles and slip velocity at the vessel wall) is very difficult to provide detailed information on the glycocalyx. We speculate that oscillatory or disturbed flows will result in a less edge-prone distribution of the glycoclayx layer on the cell membrane. This will weaken the molecular sieving effects of the glycocalyx near cell–cell junctions and lead to enhanced macromolecular permeability and atheroma formation. Current work in our laboratory is probing into this interesting problem. Despite all these limitations, the study provides a number of useful insights into shear stress-induced redistribution of the glycocalyx layer on endothelial cells.
Abbreviations
- \(\mathrm{CTR}\) :
-
Cell Tracker Red CMTPX
- \(\mathrm{CS}\) :
-
Chondroitin sulfate
- \(\mathrm{GAGs}\) :
-
Glycosaminoglycans
- \(\mathrm{HSPG}\) :
-
Heparan sulfate proteoglycans
- \(\mathrm{Hep III}\) :
-
Heparinase III
- \(\mathrm{HUVECs}\) :
-
Human umbilical vain endothelial cells
- \(\mathrm{HA}\) :
-
Hyaluronic acid
- \(\mathrm{NO}\) :
-
Nitric oxide
- \(\mathrm{SS}\) :
-
Shear stress
- \(\mathrm{WGA-FITC}\) :
-
Wheat germ agglutinin
References
Adamson RH, Michel CC, Parker KH, Phillips CG, Wang W (1993) Pathways through the intercellular clefts of frog mesenteric capillaries. J Physiol 466:303–327
Arisaka T, Mitsumata M, Kawasumi M, Tohjima T, Hirose S, Yoshida Y (1994) Effects of shear stress on glycosaminoglycan synthesis in vascular endothelial cellsa. Ann NY Acad Sci 748:543–554
Bai K, Huang Y, Jia XL, Fan YB, Wang W (2010) Endothelium oriented differentiation of bone marrow mesenchymal stem cells under chemical and mechanical stimulations. J Biomech 43:1176–1181
Bai K, Wang W (2012) Spatio-temporal development of the endothelial glycocalyx layer and its mechanical property in vitro. J R Soc Interface 9(74):2290–2298
Barker AL, Konopatskaya O, Neal CR, Macpherson JV, Whatmore JL, Winlove CP, Unwin PR, Shore AC (2004) Observation and characterisation of the glycocalyx of viable human endothelial cells using confocal laser scanning microscopy. Phys Chem Chem Phys 6:1006–1011
Chiu JJ, Wang DL, Chien S, Skalak R, Usami S (1998) Effects of disturbed flow on endothelial cells. J Biomech Eng 120:2–8
Cunningham KS, Gotlieb AI (2005) The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest 85(1):9–23
Damiano ER, Stace TM (2002) A mechano-electrochemical model of radial deformation of the capillary glycocalyx. Biophys J 82:1153–1175
Davies PF (1997) Overview: temporal and spatial relationships in shear stress-mediated endothelial signalling. J Vasc Res 34:208–211
Depaola N, Gimbrone MA, Davies PF, Dewey CF (1992) Vascular endothelium responds to fluid shear-stress gradients. Arter Thromb 12:1254–1257
Elhadj S, Mousa SA, Forsten-Williams K (2002) Chronic pulsatile shear stress impacts synthesis of proteoglycans by endothelial cells: effect on platelet aggregation and coagulation. J Cell Biochem 86:239–250
Fisher AB, Chien S, Barakat AI, Nerem RM (2001) Endothelial cellular response to altered shear stress. Am J Physiol Lung Physiol 281(3):L529–L533
Helmke BP, Davies PF (2002) The cytoskeleton under external fluid mechanical forces: hemodynamic forces acting on the endothelium. Ann Biomed Eng 30:284–296
Hecker M, Luckhoff A, Busse R (1993) Modulation of endothelial autacoid release by protein kinase C: feedback inhibition or nonspecific attenuation of receptor-dependent cell activation? J Cell Physiol 156:571–578
Huang B, Wang W, Bates M, Zhuang X (2008) Three-dimensional super-resolution imaging by stochastic optical reconstruction microscopy. Science 319:810–813
Jackson RL, Busch SJ, Cardin AD (1991) Glycosaminoglycans: molecular properties, protein interactions, and role in physiological processes. Physiol Rev 71(2):481–539
Kang H, Fan Y, Deng X (2011) Vascular smooth muscle cell glycocalyx modulates shear-induced proliferation, migration, and NO production responses. Am J Physiol Heart Circ Physiol 300:H76–H83
Lopez-Quintero SV, Amaya R, Pahakis M, Tarbell JM (2009) The endothelial glycocalyx mediates shear-induced changes in hydraulic conductivity. Am J Physiol Heart Circ Physiol 296:H1451–H1456
Luft JH (1966) Fine structure of capillary and endocapillary layer as revealed by ruthenium red. Fed Proc 25(6):1773–1783
Megens RTA, Reitsma S, Schiffers PHM, Hilgers RHP, De Mey JGR, Slaaf DW, Oudeegbrink MGA, Van Zandvoort MAMJ (2007) Two-photon microscopy of vital murine elastic and muscular arteries : combined structural and functional imaging with subcellular resolution. J Vasc Res 44:87–98
Mochizuki S, Vink H, Hiramatsu O, Kajita T, Shigeto F, Spaan JAE, Kajiya F (2003) Role of hyaluronic acid glycosaminoglycans in shear-induced endothelium-derived nitric oxide release. Am J Physiol Heart Circ Physiol 285:H722–H726
Oohira A, Wight TN, Bornstein P (1983) Sulfated proteoglycans synthesized by vascular endothelial cells in culture. J Biol Chem 258(3):2014–2021
Pahakis MY, Kosky JR, Dull RO, Tarbell JM (2007) The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem Biophys Res Commun 355:228–233
Savery MD, Damiano ER (2008) The endothelial glycocalyx is hydrodynamically relevant in arterioles throughout the cardiac cycle. Biophys J 95(3):1439–1447
Squire JM, Chew M, Nneji G, Neal C, Barry J, Michel C (2001) Quasi-periodic substructure in the microvessel endothelial glycocalyx: a possible explanation for molecular filtering? J Struct Biol 136(3):239–255
Stamatas GN, McIntire LV (2001) Rapid flow-induced responses in endothelial cells. Biotechnol Progr 17:383–402
Wang W (2007) Change in properties of the glycocalyx affects the shear rate and stress distribution on endothelial cells. J Biomech Eng 129:324–329
Yao Y, Rabodzey A, Dewey CF Jr (2007) Glycocalyx modulates the motility and proliferative response of vascular endothelium to fluid shear stress. Am J Physiol Heart Circ Physiol 293(2):H1023–1030
Acknowledgments
W.W. thanks the Royal Academy of Engineering for a Global Research Fellowship which supports his secondment at Harvard University. K.B. is funded by a College PhD studentship from Queen Mary, University of London. The project was supported in part by a MRC/EPSRC Discipline Bridging Initiative grant G0502256-77947.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bai, K., Wang, W. Shear stress-induced redistribution of the glycocalyx on endothelial cells in vitro. Biomech Model Mechanobiol 13, 303–311 (2014). https://doi.org/10.1007/s10237-013-0502-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-013-0502-3