Abstract
The spatial and diurnal tidal variability of dissolved organic carbon (DOC) concentrations and the composition of dissolved organic matter (DOM), as evaluated by high-temperature catalytic oxidation and excitation–emission matrix combined with parallel factor analysis (EEM–PARAFAC), respectively, were determined in Liverpool Bay. EEM–PARAFAC modeling resulted in six fluorescent components characterized as terrestrial humic-like (two), microbial humic-like (two), and protein-like (two). The spatial distributions of DOC and the four humic-like components were negatively correlated with salinity in the high-salinity waters observed in this study (30.41–33.75), suggesting that terrestrial DOM was conservatively distributed. The spatial patterns of protein-like components were largely different from those of DOC, humic-like components, and chlorophyll a, suggesting that these distributions were the combined result of production and degradation in the bay in addition to river inputs. These findings suggest that the DOM dynamics in Liverpool Bay are strongly controlled by river-dominated allochthonous DOM inputs with some less significant contributions of autochthonous DOM within the bay. In addition, the temporal variations of DOM associated with the diurnal tidal cycles were determined at one inshore (31.34–32.24 salinity) and one offshore (33.64–33.75 salinity) station in the bay. Negative linear relationships between salinity and DOM characteristics, i.e., DOC, humic-like, and protein-like components, were observed at the inshore station. In contrast, no relationship was observed at the offshore station, suggesting that the export of DOM through rivers and possibly tidal flats have a noticeable influence on DOM concentration and composition up to a relatively elevated salinity of around 33 in Liverpool Bay.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Liverpool Bay is located in the eastern Irish Sea and is a region strongly influenced by the input of freshwater from rivers in North West England as well as North Wales, with an annual mean freshwater discharge of 400 m3 s−1 (Sharples and Simpson 1993). Fluvial loading to the region typically results in high winter nutrient levels (up to 51 μM DIN and 5 μM DIP; Greenwood et al., submitted for publication) with correspondingly high annual production and algal biomass (Gowen and Stewart 2005). In addition to the annual diatom-dominated spring bloom, there are frequent blooms of the nuisance microflagellate Phaeocystis spp. throughout the summer months. These factors combined have led to concerns that Liverpool Bay may be at risk of eutrophication and a subsequent recommendation that the biogeochemistry of the region be monitored closely (Gowen et al. 2008).
Dissolved organic matter (DOM) plays an important role in biogeochemical cycling and ecosystem function in coastal environments. For example, chromophoric DOM (CDOM), the light-absorbing fraction of DOM, is mainly derived from the terrestrial environment and is ecologically important due to its optical-absorbing properties (Blough et al. 1993). Thus, as with other coastal regions suffering a high degree of terrestrial impact, it is important to understand the source, nature, and fate of DOM (and hence organic carbon) discharged into and produced in Liverpool Bay. However, detailed research on environmental dynamics, such as the spatiotemporal variability of DOM in Liverpool Bay, has scarcely been carried out (Foster and Morris 1974), and thus its role in driving the biogeochemical processes in this region has not been defined.
The first step in evaluating DOM environmental dynamics in coastal environments is to determine its spatiotemporal variability, both from a quantitative and compositional perspective. Dissolved organic carbon (DOC) concentration is usually used as a quantitative parameter of DOM. Optical means of analysis are suitable for qualitative assessment due to the high sample throughput needed for large sampling grids and time series (e.g., Jaffé et al. 2008). In particular, excitation–emission matrix (EEM) fluorescence combined with parallel factor analysis (PARAFAC) has been applied to characterize DOM in coastal environments and successively evaluate the environmental dynamics of terrestrial DOM and autochthonous DOM separately (Stedmon et al. 2003; Yamashita et al. 2008; Fellman et al. 2010; Kowalczuk et al. 2009, 2010).
The purpose of the present study is to assess the spatiotemporal variability of DOM concentration and composition in Liverpool Bay through DOC and EEM–PARAFAC analysis. Special attention will be paid to determine the factors controlling the spatiotemporal variability of DOM concentrations and composition with regards to autochthonous and allochthonous sources.
2 Materials and methods
2.1 Sampling site and water collection
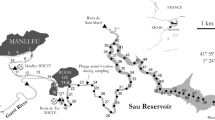
The surface distributions of DOC and CDOM in Liverpool Bay were surveyed on 5th and 6th February 2009 during an Irish Sea Observatory cruise on the R.V. Prince Madog (Fig. 1). A spatial grid surface water samples were obtained from the ship’s underway pumping system which samples water from approximately 3 m below the surface. The pipes of this pumping system are coated with Teflon. The ship’s pumping system is flushed prior to the cruise. It should be noted that the sampling locations for chlorophyll a were different from those of DOC and CDOM.
The temporal changes in DOC and CDOM with tide were determined at two stations between 1st and 3rd May 2009. The R.V. Prince Madog was anchored for 25 h (17:14 on 1st to 18:00 on 2nd May 2009) at an offshore station (Fig. 1; 53°37.073′ N, 3°55.55′ W; 47.4 m) in a region presumably not influenced by riverine input. After completion of the offshore survey, a further 25-h anchored inshore station (53°32.32′ N, 3°20.27′ W; 19 m) was completed (from 22:00 on 2nd to 23:00 on 3rd May 2009) in an area known to be influenced by freshwater runoff from the two major rivers in the region, the Mersey and the Dee although Liverpool Bay also receives freshwater from the Ribble, Lune, Weaver, Clwyd and Conwy (Greenwood et al., submitted for publication). Hourly discrete water samples were taken from 1 m below the surface using 5-l Niskin bottles mounted on a rosette sampler.
For the DOC and CDOM samples of the spatial survey, 60 ml of seawater was filtered through pre-combusted GF/F filters using an acid-washed and sample-rinsed glass syringe. On the temporal survey, the filtering was performed through pre-combusted GF/F filters in an acid-washed glass filter assembly which had been rinsed with the sample three times prior to each sample collection. The samples were then stored in 125-ml translucent LDPE bottles (Nalgene) which had been prepared by soaking in 0.5 M HCl for 24 h followed by 0.1 M NaOH for a further 24 h prior to the cruise. The bottles containing the samples were subsequently wrapped in aluminum foil and refrigerated until analysis.
2.2 Salinity, chlorophyll a, and DOC
Profiles of salinity and temperature were collected using a Sea-Bird Electronics 911plus system mounted on a rosette frame, supporting eight 5-l Niskin bottles.
A volume of 100 ml of seawater was filtered onto a 0.2-μm polycarbonate filter which was subsequently placed in a capped glass tube and frozen at −20°C. When ready for analysis, 5 ml of 90% acetone was added to the tube, which was then sonicated for 10 min. The filter was removed from the glass tube and fluorescence was read before and after acidification with a few drops of 10% HCl using a Turner 10-AU fluorometer calibrated with a chlorophyll a standard from spinach (Sigma-Aldrich, C5753).
DOC concentrations were determined by high-temperature catalytic oxidation with a Shimadzu TOC-VCSH TOC analyzer (Kyoto, Japan).
2.3 Optical analysis
EEM fluorescence spectra were obtained using a Horiba Jovin Yvon SPEX Fluoromax-3 fluorometer according to Yamashita et al. (2010a) with some modifications. Forty-one emission scans from 290 to 600 nm at 2-nm intervals were acquired at excitation wavelengths between 250 and 450 nm at 5-nm intervals. Bandpass was set at 5 nm for excitation and emission. Fluorescence spectra were scanned with 0.25 s of integration time and acquired in S/R ratio mode. Several post-acquisition steps were involved in the correction of the fluorescence spectra. First, the UV–visible absorption spectra measured by a dual-beam spectrophotometer (Yamashita et al. 2010a) were used for inner filter corrections according to McKnight et al. (2001). After this procedure, the EEM of Milli-Q water was subtracted from sample EEMs. Secondly, the excitation and emission correction files obtained every month using rhodamine b and supplied by the manufacturers, respectively, were applied for the correction of the specific instrument’s components (Cory et al. 2010). Finally, fluorescence intensities were also corrected to the area under the water Raman peak (excitation = 350 nm) analyzed daily (Lawaetz and Stedmon 2009) and then were converted to quinine sulfate units using a calibration with quinine sulfate monohydrate in 0.1 N sulfuric acid.
Ninety-seven samples collected from Liverpool Bay were used for PARAFAC analysis. For PARAFAC modeling, EEM matrix of excitation wavelengths from 250 to 450 nm and emission wavelengths from 290 to 520 nm were used. The analysis was carried out in MATLAB (Mathworks, Natick, MA) with the DOMFluor toolbox, and split-half analysis and random initialization (Fig. 2) were used to validate the identified components (Stedmon and Bro 2008).
3 Results and discussion
3.1 PARAFAC components
PARAFAC analysis using 97 surface water samples obtained from Liverpool Bay statistically separated EEMs into six components (Fig. 2). The spectral characteristics of the six components found in Liverpool Bay were similar to those of previous PARAFAC studies carried out from a wide range of aquatic environments, i.e., from streams to the open ocean (Cory and McKnight 2005; Stedmon and Markager 2005; Murphy et al. 2008; Williams et al. 2010; Yamashita et al. 2010b).
Component 1 (C1) was composed of two peaks with excitation maxima at <250 and 320 nm at 422-nm emission (Fig. 2). This component could be characterized as a mixture of terrestrial humic-like peaks A and C according to Coble (1996). The spectral characteristics of this peak were also similar to terrestrial humic-like PARAFAC component obtained in other coastal environments (C1; Kowalczuk et al. 2009). There were two distinct excitation maxima (265 and 370 nm) observed in the EEM of component 2 (C2). This component had emission maxima at 464 nm and could not be categorized using traditionally defined peaks (Coble 1996). However, the spectral features of C2 were similar to the PARAFAC components which are likely to be microbially derived humic-like components from the terrestrial aquatic environment (SQ2, Cory and McKnight 2005; C4, Yamashita et al. 2010a). This component was also similar to the terrestrial/autochthonous component (C4) defined in a temperate estuary and its catchment (Stedmon and Markager 2005).
The spectral characteristics showed a broad, continuous excitation with bumps around 300 and 410 nm for component 3 (C3). C3 had the longest emission maximum (510 nm) among the six components obtained in this study. This component could also not be categorized using traditional peak definitions (Coble 1996) but was similar to terrestrial humic-like components (SQ1, Cory and McKnight 2005; C2, Yamashita et al. 2010a). Similar PARAFAC components were also found in coastal environments and assigned as terrestrial humic-like components (C3, Yamashita et al. 2008; C4, Kowalczuk et al. 2009). Component 4 (C4) had two excitation maxima at <250 and 295 nm at 358-nm emission. This component might be categorized as similar to peak N which correlated with chlorophyll a in surface ocean or to marine humic-like peak M according to Coble (1996) and Coble et al. (1998). Similar PARAFAC components have also been found in oceanic environments (C2, Murphy et al. 2008; C2, Yamashita et al. 2010b) as well as coastal environments (C6, Yamashita et al. 2008; C4, Fellman et al. 2010) and assigned as microbial humic-like components.
Two components, component 5 (C5) and component 6 (C6), were found in the EEM region corresponding to protein-like fluorophores (Coble 1996; Mayer et al. 1999; Yamashita and Tanoue 2003). C5 had a peak at 280-nm excitation and 334-nm emission, and the peak of C6 was located at 275-nm excitation and 302-nm emission. The spectral patterns of C5 and C6 resembled those of tryptophan- and tyrosine-like fluorophores, respectively (Coble 1996; Mayer et al. 1999; Yamashita and Tanoue 2003). On the other hand, polyphenols such as tannin and lignin have also been considered to contribute to the fluorescence in this EEM region (Maie et al. 2007; Hernes et al. 2009).
3.2 Spatial variability of DOC and CDOM in Liverpool Bay
The spatial distributions of salinity, chlorophyll a, and DOC in February 2009 are shown in Fig. 3. The salinity observed in February 2009 ranged from 30.41 to 33.75. The spatial distribution of salinity showed an east–west gradient (Fig. 3a). This patter is due to freshwater input from the multiple rivers and the northward flow of the freshwater plume which primarily originates from the rivers Mersey and Dee in the southeast corner of the bay. The concentrations of chlorophyll a ranged from 0.09 to 1.12 mg m−3 and were relatively high in water with salinity around 32 (Fig. 3b). The chlorophyll a concentrations observed here were considerably low compared to those observed throughout the rest of the year in Liverpool Bay (Panton et al., submitted for publication). In addition, Gowen et al. (2000) reported a maximum spring bloom concentration of chlorophyll a of 43.9 mg m−3 and a mean summer biomass of 8.8 mg m−3 in Liverpool Bay. The DOC concentrations ranged from 85 to 132 μMC and were highest in the eastern part of the bay where salinity was lowest (Fig. 3c). The distributional pattern of DOC was a mirror image of the salinity distribution, and the two parameters were negatively correlated (R 2 = 0.86), suggesting that the distribution of DOC in Liverpool Bay is strongly controlled by the mixing of offshore water with freshwater from the rivers Ribble, Mersey, and Dee even in the high-salinity region. Concurrently, a strong negative correlation between salinity and DOC suggests that the contribution of autochthonous DOM produced in the bay represents a less important contribution to the bulk DOM pool in this salinity range. Foster and Morris (1974) found similar distributional patterns for ultraviolet absorption during winter and summer in the same area and suggested that CDOM mainly derives from the river Mersey during winter. The lack of spatial similarity in the distributional patterns of DOC and chlorophyll a concentrations (Fig. 3b, c) supports this argument.
The spatial distributions of the four humic-like components were similar to that of DOC concentrations (Fig. 4a–d). In a similar manner to DOC, the fluorescence intensities of humic-like components were strongly negatively correlated with salinity (R 2 = 0.98 for C1, R 2 = 0.98 for C2, R 2 = 0.98 for C3, R 2 = 0.90 for C4). These relationships suggested that all of four humic-like components were of terrestrial origin and conservatively distributed in the bay, even though two of the four components (C2 and C4) showed spectral signatures characteristic of microbial origin or OM degradation products. The spectral characteristics of C4 are similar to the microbial (marine) humic-like “peak M” and “peak N” which have been reported to correlate with chlorophyll a in surface ocean as mentioned above. In the present study, however, C4 was distributed similarly to other humic-like components but not to chlorophyll a. This suggests that C4 is a microbially derived humic-like component of possibly riverine origin.
While the conservative behavior of terrestrial humic-like components has been reported for a wide range of salinities in coastal environments (Yamashita et al. 2008; Fellman et al. 2010), Yamashita et al. (2008) and Fellman et al. (2010) found increases in microbial humic-like components, corresponding to C4 in this study, at low- to mid-salinity waters in the estuaries of central Japan and southeastern Alaska, respectively. However, these studies were performed along a much larger salinity gradient than those observed in the present study and may, as such, reflect estuarine processing of terrestrial and/or autochthonous DOM.
The autochthonous production of microbial (marine) humic-like fluorophores during the microbial degradation of organic matter has been reported from in vitro experiments (Parlanti et al. 2000; Rochelle-Newall and Fisher 2002; Nieto-Cid et al. 2006; Lønborg et al. 2010) as well as from field observations (Nieto-Cid et al. 2005; Yamashita et al. 2008). On the other hand, it is known that the humic-like fluorophores can be reactive and degraded by sunlight (Moran et al. 2000; Del Vecchio and Blough 2002; Nieto-Cid et al. 2006; Clark et al. 2008). These findings suggested that the autochthonous production of microbial humic-like components and/or the photodegradation of humic-like components might occur at low- to mid-salinity waters, resulting in modified humic-like components being distributed conservatively in high-salinity waters in Liverpool Bay.
The spatial distributions of the protein-like C5 and C6 components were different to those of humic-like components (Fig. 4e, f). As expected from the distributional patterns, the fluorescence intensities of protein-like C5 and C6 did not correlate with salinity (R 2 = 0.17 for C5, R 2 = 0.02 for C6). The distributional patterns of protein-like components were also different from that of chlorophyll a.
Fellman et al. (2010) found a sudden decrease in the fluorescence intensities of the protein-like component at low salinities and suggested this to be the result of microbial decomposition processes in low-salinity waters. The rapid degradation of the tryptophan-like fluorophore was also observed during the degradation experiments using coastal water samples (Lønborg et al. 2010). On the other hand, increases in the fluorescence intensities of tryptophan-like component at mid-salinity waters have also been reported in coastal environments (Yamashita et al. 2008; Fellman et al. 2010), and the production of tryptophan-like fluorophores by phytoplankton has been observed for in vitro experiments (Romera-Castillo et al. 2010). Thus, the lack of similarity in the distribution of the fluorescence intensities of protein-like components with both salinity and chlorophyll a as observed in this study suggests that the levels of protein-like components are most likely the combined result of the production and degradation of aromatic amino acids and possibly an allochthonous contribution in the form of aromatic amino acids and/or polyphenols. The patterns are not simply a result of dilution by the mixing of high- and low-salinity waters.
Among the six PARAFAC components, the different relationships between salinity and fluorescent components, i.e., negative relationships for humic-like components and no relationships for protein-like components, indicated that the contribution of autochthonous protein-like components in bulk DOM becomes greater with increases in salinity. Similar results have also been found in other costal environments (Yamashita et al. 2008; Kowalczuk et al. 2009; Fellman et al. 2010). Such a higher contribution of protein-like components in higher salinity might affect the lower R 2 values of the relationship between salinity and DOC compared to those between salinity and humic-like components. It should be noted that, in this study, the spatial distributions of DOC and its fluorescent components were measured in February, during which biological production is low; therefore, one may expect the relationship between DOC and salinity to vary between seasons in response to biological production. However, Foster and Morris (1974) found a linear relationship between salinity and ultraviolet absorbance during December, March, and July, but a non-linear relationship in September in Liverpool Bay. Thus, the relative importance of the riverine versus and biological sources of DOC and how these vary seasonally need to be further explored in Liverpool Bay.
3.3 Diurnal variability of DOC and CDOM along the tidal cycle in Liverpool Bay
At two stations in Liverpool Bay (Fig. 1), 25-h tidal cycle surveys were performed to determine the daily and tidal variations in DOM concentrations and composition. One station was located relatively close to the land (inshore station) and the other was located relatively far from the land (offshore station). The tidal ranges (difference between high and low waters) at the inshore and offshore stations were 5.2 and 5.5 m, respectively. Figures 5 and 6 show the changes in salinity, chlorophyll a, and DOM concentrations and fluorescent components. At the offshore station, salinity ranged from 33.64 to 33.75 and showed only a small change with tide during 25 h of monitoring (Fig. 5a). On the other hand, larger changes in salinity with tide, i.e., from 31.34 to 32.24, were observed at the inshore station (Fig. 5a). The levels of chlorophyll a at the offshore station (3.87–9.30 mg m−3) were higher than those at the inshore station (0.52–5.49 mg m−3) (Figs. 5b and 7a). For both inshore and offshore stations, the concentrations of chlorophyll a were higher during the day (5:00 to 15:00 h) compared to the night.
Relationships between salinity and chlorophyll a (a), DOC (b), C1 (c), C2 (d) C3 (e), C4 (f), C5 (g), and C6 (h) during 25 h of monitoring at inshore and offshore stations. The unexpectedly high values of DOC found in Fig. 5 were not included
The DOC concentrations at the inshore station were higher (121–151 μMC, excluding one outlier) than those at the offshore station (89–104 μMC, excluding two outliers) (Figs. 5c and 7b). At the inshore station, high concentrations of DOC were evident during the tidal ebbing that was characterized by lower salinity, and the DOC concentrations were negatively correlated with salinity (Fig. 7b; R 2 = 0.59, excluding an outlier shown as a closed square in Fig. 5c). However, a variation in DOC concentrations along with tidal variability was not evident at the offshore station (Fig. 7b; R 2 = 0.02, excluding two outliers shown as closed circles in Fig. 5c).
The temporal variations in the fluorescence intensities of the four humic-like components were similar to that of DOC (Figs. 6a–d and 7c–f). These fluorescence intensities were also negatively correlated with salinity at the inshore station (Fig. 7c–f; R 2 = 0.97 for C1, R 2 = 0.97 for C2, R 2 = 0.97 for C3, R 2 = 0.91 for C4) but not at the offshore station (Fig. 7c–f; R 2 = 0.25 for C1, R 2 = 0.05 for C2, R 2 = 0.15 for C3, R 2 = 0.17 for C4). In addition, in a similar manner with DOC, the four humic-like components at the inshore station were more abundant compared to the offshore station. The negative relationships found at the inshore station suggest that the humic-like components are associated to mid-salinity waters and are strongly controlled by tidal cycling and freshwater inputs. As part of the 25-h observation period at the inshore station, two samples were collected between the hours of 17:00 and 18:00 on two consecutive days. The fluorescence intensities of the four humic-like components were almost identical between these samples (Fig. 6a–d), supporting the idea that photo- and/or bio-degradation was minor during the time period of the observations, and there was no significant input of other DOM sources. On the other hand, the low and narrow range of concentrations of DOC and four humic-like components at the offshore station might mask the correlation with salinity and DOC as well as the fluorescence intensities and instead might be due to the larger contribution of more recalcitrant (more degraded) marine DOM above a salinity of around 33. It is interesting to point out that, in most estuaries, the dominance of allochthonous (i.e., terrestrial, river-dominated) DOM is replaced by autochthonous (marine)-derived DOM at salinities above 30 (De Souza-Sierra et al. 1997; Kowalczuk et al. 2003; Jaffé et al. 2004; Maie et al. 2006). This allochthonous influence can still be clearly observed in Liverpool Bay at salinities of up to 33.
The fluorescence intensities of protein-like components also showed changes with tidal variability at the inshore station but not at the offshore station (Fig. 6e, f). In addition, differences in the abundance of the protein-like components between daytime and nighttime, which were observed for chlorophyll a (Fig. 5b), were not detected (Fig. 6e, f). These observational results suggest that the mid- and high-salinity waters, which have high and low levels of protein-like components, respectively, are controlled by the tidal cycling. However, the relationships between salinity and protein-like components (Fig. 7g, h; R 2 = 0.53 for C5, R 2 = 0.48 for C6) at the inshore station were lower compared to the humic-like components. The temporal changes in protein-like components were more variable compared to the humic-like components (Fig. 6). Such variable distributions of the protein-like fluorophores compared to the humic-like fluorophores with changes in salinity have been observed in other coastal systems (Mayer et al. 1999). In addition, the differences in fluorescence intensities between the inshore station and the offshore station were smaller for protein-like components compared to humic-like components, particularly for C6 (Fig. 6). Such distributional characteristics of protein-like components suggested that their abundance are controlled not only by the combined effects of the tide and the river flow but also through additional autochthonous source inputs and diagenetic removal processes. As for the humic-like components, the fluorescence intensities of protein-like components were not correlated to salinity (R 2 = 0.04 for C5, R 2 = 0.03 for C6) at the offshore station.
These data agree with the idea that the protein-like components in Liverpool Bay may have a mixed allochthonous and autochthonous source and possibly different removal mechanisms (i.e., bio-degradation) compared to their humic-like (photodegradation) counterparts. In addition, phytoplankton may not be a main contributor to the protein-like fluorescence at observed salinity range, where this fluorescence may in fact have contributions of both amino acid- and polyphenol-like fluorescence (Yamashita and Tanoue 2003; Maie et al. 2007; Hernes et al. 2009).
4 Concluding remarks
The spatial and daily tidal variation of DOM concentration and composition in Liverpool Bay were studied for evaluating the environmental dynamics of DOM in the bay. The spatial distribution of the quantitative parameter of DOM, namely, DOC concentration, and humic-like components distributions obtained by EEM–PARAFAC were similarly distributed but were antipodal to salinity in the high-salinity waters observed in this study (30.41 to 33.75). These parameters were negatively linearly correlated with salinity within this salinity range. In addition, daily tidal surveys show that the DOC and humic-like components were negatively linearly correlated with salinity at the inshore (31.34–32.24 salinity) but not at the offshore (33.64–33.75 salinity) stations, suggesting that the export of DOM through rivers and possibly tidal flats have a noticeable influence on DOM concentration and composition up to a relatively elevated salinity of around 33 in Liverpool Bay. The R 2 values of the linear relationship between the humic-like components and salinity were, however, higher than those between DOC and salinity for the spatial survey and the daily tidal survey at the inshore station, suggesting some minor contribution of autochthonous DOM to the DOC–salinity relationships. In agreement with such differences in relationships, protein-like components were not correlated with salinity for the spatial survey, implying that river-derived inputs as well as autochthonous production and possibly degradation were all factors controlling the protein-like components. The different diurnal tidal variations between protein-like components and chlorophyll a suggest that a combination of allochthonous inputs and in situ production and degradation are important drivers controlling the dynamics of protein-like components in Liverpool Bay. While this study was limited due to a single spatial and temporal survey of DOM, the results suggest that long-term spatial monitoring of DOC and DOM quality using EEM–PARAFAC will lead to a better understanding of carbon dynamics in Liverpool Bay.
References
Blough NV, Zafirou OC, Bonilla J (1993) Optical absorption spectra of water from the Orinoco River outflow: terrestrial input of colored organic matter to the Caribbean. J Geophys Res 98:2271–2278
Clark CD, Litz LP, Grant SB (2008) Salt marshes as a source of chromophoric dissolved organic matter (CDOM) to Southern California coastal waters. Limnol Oceanogr 53:1923–1933
Coble PG (1996) Characterization of marine and terrestrial DOM in seawater using excitation–emission matrix spectroscopy. Mar Chem 51:325–346
Coble PG, Del Castillo CE, Avril B (1998) Distribution and optical properties of CDOM in the Arabian Sea during the 1995 southwest monsoon. Deep Sea Res II 45:2195–2223
Cory RM, McKnight DM (2005) Fluorescence spectroscopy reveals ubiquitous presence of oxidized and reduced quinones in dissolved organic matter. Environ Sci Technol 39:8142–8149
Cory RM, Miller MP, McKnight DM, Guerard JJ, Miller PL (2010) Effect of instrument-specific response on the analysis of fulvic acid fluorescence spectra. Limnol Oceanogr Meth 8:67–78
De Souza-Sierra M, Donard OFX, Lamotte M (1997) Spectral identification and behavior of dissolved organic fluorescent material during estuarine mixing processes. Mar Chem 58:51–58
Del Vecchio R, Blough NV (2002) Photobleaching of chromophoric dissolved organic matter in natural waters: kinetics and modeling. Mar Chem 78:231–253
Fellman JB, Spencer RGM, Hernes PJ, Edwards RT, D’Amore DV, Hood E (2010) The impact of glacier runoff on the biodegradability and biochemical composition of terrigenous dissolved organic matter in near-shore marine ecosystems. Mar Chem 121:112–122
Foster P, Morris AW (1974) Seasonal distribution of ultraviolet absorption in the surface waters of Liverpool Bay. Estuar Coast Mar Sci 2:283–290
Gowen RJ, Stewart BM (2005) The Irish Sea: nutrient status and phytoplankton. J Sea Res 54:36–50
Gowen RJ, Mills DK, Trimmer M, Nedwell DB (2000) Production and its fate in two coastal regions of the Irish Sea: the influence of anthropogenic nutrients. Mar Ecol Prog Ser 208:51–64
Gowen RJ, Tett P, Kennington K, Mills DK, Shammon TM, Stewart BM, Greenwood N, Flanagan C, Devlin M, Wither A (2008) The Irish Sea: is it eutrophic? Estuar Coast Shelf Sci 76:239–254
Hernes PJ, Bergamaschi BA, Eckard RS, Spencer RGM (2009) Fluorescence-based proxies for lignin in freshwater dissolved organic matter. J Geophys Res 114:G00F03
Jaffé R, Boyer NJ, Lu X, Maie N, Yang C, Scully NM, Mock S (2004) Source characterization of dissolved organic matter in a subtropical mangrove-dominated estuary by fluorescence analysis. Mar Chem 84:195–210
Jaffé R, McKnight D, Maie N, Cory R, McDowell WH, Campbell JL (2008) Spatial and temporal variations in DOM composition in ecosystems: the importance of long-term monitoring of optical properties. J Geophys Res 113:G04032
Kowalczuk P, Cooper WJ, Whitehead MJ, Durako MJ, Sheldon W (2003) Characterization of CDOM in an organic-rich river and surrounding coastal ocean in the South Atlantic Bight. Aquat Sci 65:384–401
Kowalczuk P, Durako MJ, Young H, Kahn AE, Cooper WJ, Gonsior M (2009) Characterization of dissolved organic matter fluorescence in the South Atlantic Bight with use of PARAFAC model: interannual variability. Mar Chem 113:182–196
Kowalczuk P, Cooper WJ, Durako MJ, Kahn AE, Gonsior M, Young H (2010) Characterization of dissolved organic matter fluorescence in the South Atlantic Bight with use of PARAFAC model: relationships between fluorescence and its components, absorption coefficients and organic carbon concentrations. Mar Chem 118:22–36
Lawaetz AJ, Stedmon CA (2009) Fluorescence intensity calibration using the Raman scatter peak of water. Appl Sectrosc 63:936–940
Lønborg C, Álvarez-Salgado XA, Davidson K, Martínez-García S, Teira E (2010) Assessing the microbial bioavailability and degradation rate constants of dissolved organic matter by fluorescence spectroscopy in the coastal upwelling system of the Ría de Vigo. Mar Chem 119:121–129
Maie N, Boyer JN, Yang C, Jaffé R (2006) Spatial, geomorphological, and seasonal variability CDOM in estuaries of the Florida coastal Everglades. Hydrobiologia 569:135–150
Maie N, Scully NM, Pisani O, Jaffé R (2007) Composition of a protein-like fluorophore of dissolved organic matter in coastal wetlands and estuarine ecosystems. Water Res 41:563–570
Mayer LM, Schick LL, Loder TC III (1999) Dissolved protein fluorescence in two Maine estuaries. Mar Chem 64:171–179
McKnight DM, Boyer EW, Westerhoff PK, Doran PT, Kulbe T, Andersen DT (2001) Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol Oceanogr 46:38–48
Moran MA, Sheldon WM Jr, Zepp RG (2000) Carbon loss and optical property changes during long-term photochemical and biological degradation of estuarine dissolved organic matter. Limnol Oceanogr 45:1254–1264
Murphy KR, Stedmon CA, Waite TD, Ruiz GM (2008) Distinguishing between terrestrial and autochthonous organic matter sources in marine environments using fluorescence spectroscopy. Mar Chem 108:40–58
Nieto-Cid M, Álvarez-Salgado XA, Gago J, Pérez FF (2005) DOM fluorescence, a tracer for biogeochemical processes in a coastal upwelling system (NW Iberian Peninsula). Mar Ecol Prog Ser 297:33–50
Nieto-Cid M, Álvarez-Salgado XA, Pérez FF (2006) Microbial and photochemical reactivity of fluorescent dissolved organic matter in a coastal upwelling system. Limnol Oceanogr 51:1391–1400
Parlanti E, Wörz K, Geoffroy L, Lamotte M (2000) Dissolved organic matter fluorescence spectroscopy as a tool to estimate biological activity in a coastal zone submitted to anthropogenic inputs. Org Geochem 31:1765–1781
Rochelle-Newall EJ, Fisher TR (2002) Production of chromophoric dissolved organic matter fluorescence in marine and estuarine environments: an investigation into the role of phytoplankton. Mar Chem 77:7–21
Romera-Castillo C, Sarmento H, Álvarez-Salgado XA, Gasol JM, Marrasé C (2010) Production of chromophoric dissolved organic matter by marine phytoplankton. Limnol Oceanogr 55:446–454
Sharples J, Simpson JH (1993) Periodic frontogenesis in a region of freshwater influence. Estuaries 16:74–82
Stedmon CA, Bro R (2008) Characterizing dissolved organic matter fluorescence with parallel factor analysis: a tutorial. Limnol Oceanogr Meth 6:572–579
Stedmon CA, Markager S (2005) Resolving the variability in dissolved organic matter fluorescence in a temperate estuary and its catchment using PARAFAC analysis. Limnol Oceanogr 50:686–697
Stedmon CA, Markager S, Bro R (2003) Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy. Mar Chem 82:239–254
Williams CJ, Yamashita Y, Wilson HF, Jaffé R, Xenopoulos MA (2010) Unraveling the role of land use and microbial activity in shaping dissolved organic matter characteristics in stream ecosystems. Limnol Oceanogr 55:1159–1171
Yamashita Y, Tanoue E (2003) Chemical characterization of protein-like fluorophores in DOM in relation to aromatic amino acids. Mar Chem 82:255–271
Yamashita Y, Jaffé R, Maie N, Tanoue E (2008) Assessing the dynamics of dissolved organic matter (DOM) in coastal environments by excitation emission matrix fluorescence and parallel factor analysis (EEM–PARAFAC). Limnol Oceanogr 53:1900–1908
Yamashita Y, Maie N, Bricenõ H, Jaffé R (2010a) Optical characterization of dissolved organic matter in tropical rivers of the Guayana Shield, Venezuela. J Geophys Res 115:G00F10
Yamashita Y, Cory RM, Nishioka J, Kuma K, Tanoue E, Jaffé R (2010b) Fluorescence characteristics of dissolved organic matter in the deep waters of the Okhotsk Sea and the northwestern North Pacific Ocean. Deep Sea Res II 57:1478–1485
Acknowledgements
We would like to thank the Captain and crew of the R.V. Prince Madog and Matthew Palmer for coordinating the cruises and two anonymous reviewers for the valuable comments and suggestions that helped improve the quality of this manuscript. YY thanks the FIU College of Arts and Sciences for financial support during this study. AP was supported by a NERC SOFI studentship (NE/F012632/1). This is contribution number 500 from the Southeast Environmental Research Center.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Alejandro Jose Souza
This article is part of the Topical Collection on the UK National Oceanography Centre’s Irish Sea Coastal Observatory
Rights and permissions
About this article
Cite this article
Yamashita, Y., Panton, A., Mahaffey, C. et al. Assessing the spatial and temporal variability of dissolved organic matter in Liverpool Bay using excitation–emission matrix fluorescence and parallel factor analysis. Ocean Dynamics 61, 569–579 (2011). https://doi.org/10.1007/s10236-010-0365-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10236-010-0365-4