Abstract
Some fish species living in mudflats construct burrows for dwelling and hiding. The goby Parapocryptes serperaster is a burrowing fish in mudflats of many estuaries in South East Asia. This study was carried out in the Mekong Delta, Vietnam, to examine burrow morphology and usage by this species. Morphology of the burrows constructed by P. serperaster was investigated by resin castings in situ to obtain the physical structure and configuration of each burrow. Fish from the burrows were caught and measured before burrow casts were made. Fish burrows comprised several openings, a few branching tunnels and multi-bulbous chambers. The surface openings were circular, and the shapes of branching tunnels were nearly round. The burrows had interconnected tunnels and various short cul-de-sac side branches. The burrow structure differed between fish sizes, but burrow dimensions were positively correlated with fish size, indicating that larger fish can make larger and more sophisticated burrow. The burrow structure and dimensions were not different between the dry and wet seasons. Laboratory observations showed that P. serperaster used body movements to dig burrows in the sediment. Burrows could provide a low-tide retreat and protection from predators, but were not used for spawning and feeding for this goby species. This study indicates that the burrowing activity of gobies is an important adaptation for living in shallow and muddy habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Some fishes have the ability to construct burrows in habitats ranging from fresh to salt water and from mudflats to the deep sea (Atkinson et al. 1998; Clark et al. 2000; Gonzales et al. 2008; Jones et al. 1989; Takeda et al. 2012). Fish construct a burrow through twisting the body and ejecting mud pellets from the mouth (Atkinson and Taylor 1991). Burrow construction is an individual behaviour without cooperation of other organisms in most fish species such as the goby Pseudapocryptes elongatus (see Dinh 2008), air-breathing eel goby Odontamblyopus lacepedii (see Gonzales et al. 2008) and the goby Taenioides cirratus (see Itani and Uchino 2003). However, some other fishes create burrows together with other organisms living in the same habitat. For example, the goby Cryptocentrus cryptocentrus works together with the pistol shrimp Alpheus djiboutensis to dig burrows (Karpulus et al. 1972).
Burrows can be used for predator protection, feeding, spawning and egg incubation (Atkinson and Taylor 1991). For instance, the gobiid fish Signigobius biocellatus uses burrows for living and spawning (Hudson 1977), while the goby Taenioides cirratus uses burrows mainly for living (Itani and Uchino 2003). In addition, the mudskipper Periophthalmus modestus uses burrows for spawning and egg care (Ishimatsu et al. 2007). Moreover, burrows have also been used as a refuge to hide for predators and a foraging place in Boleophthalmus boddarti (see Clayton and Wright 1989). Furthermore, burrows can be used to store oxygen for survival of eggs, as found in some mudskippers species (Ishimatsu et al. 1998; Ishimatsu et al. 2007).
The burrow structures of gobies and mudskippers have been categorized as U-shape, J-shape, I-shape and Y-shape based on morphological configuration (Atkinson and Taylor 1991). For example, the mudskipper P. elongatus creates burrows in a Y-shape (Dinh 2008), while Periophthalmodon schlosseri creates J-shaped burrows (Ishimatsu et al. 1998) and B. boddarti makes U-shaped burrows (Clayton and Wright 1989). In contrast, burrows of some fishes such as the eel goby O. lacepedii have no clearly defined shapes (Gonzales et al. 2008). Although the shape, structure and configuration of burrows may have important implications for the life strategy of demersal fishes (Atkinson and Taylor 1991), little is known on structural variation in burrows of fishes. In the Mekong Delta region where dry and wet seasons are distinctive and fish habitats are severely affected by heavy floods (Le et al. 2007), adaptation of fish burrow to flood change is not known.
The goby Parapocryptes serperaster is an amphibious fish (Murdy 2011) with an elongated and round body living in estuaries (Khaironizam and Norma-Rashid 2000). In the past, research on this fish has been focused on its morphology and diet preference (Khaironizam and Norma-Rashid 2000), habitat use (Takita et al. 1999), taxonomic characteristics (Murdy 2011) and geographic distribution (Dinh et al. 2013; Kottelat et al. 1993; Rainboth 1996; Talwar and Jhingran 1991). However, little is known on the burrow morphology and utilization of this goby species and how a P. serperaster builds its burrows. Therefore, this study aimed to understand (1) the morphological structure and burrow utilization by P. serperaster at different sizes between rainy and dry seasons and (2) fish burrowing behaviour and burrow utilization. This study thus seeks to contribute to the understanding of fish adaption in muddy habitats and the function and structural diversity of burrows used by gobiid fish in tropical tidal estuary.
Materials and methods
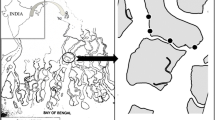
Study site. This study was carried out in intertidal mudflats in Kinh Ba River, Cu Lao Dung District, Soc Trang Province, Mekong Delta, Vietnam (9°26’3”N, 106°13’28”E), from June 2012 to May 2013 (Fig. 1). The intertidal flat was mainly characterized by mud and muddy sand sediments. Soc Trang is a typical province in the Mekong Delta with a long coastline and large areas of mudflats. The region has a typical dry (January to May) and wet (June to December) seasonal cycle with an average annual temperature of 27 °C (Soc Trang Statistical Office 2012).
The distance from the riverbank to riverbed of the mudflat was nearly 3 m at the lowest tide. Tides are semi-diurnal with a spring tidal range of ~0.7 m. Most field work was conducted on low tide in the early afternoon. An area of 10 m2 (5 m along the riverbank and 2 m from the riverbank to the riverbed) was chosen for monthly investigations of burrow morphology, activities and utilization by Parapocryptes serperaster. The study area included 17–18 burrows at each time of observation during the study period.
Burrow casting and analysis. The burrow structure of P. serperaster was studied monthly by casting burrow in the field and analysing burrow casts in the laboratory. Each month, two burrows were randomly selected from the 10 m2 sampling site. The burrows constructed by P. serperaster were characterized by no “footprints” made by fish pelvic fins around the burrow openings, while burrows of the sympatric mudskipper Boleophthalmus boddarti and crabs had “footprints” of their body parts (pelvic fins or legs). Burrow casts were made using polyester resin as described by Atkinson and Chapman (1984). The polyester resin (En Chuan Chemical industries Co., Ltd, Taiwan) was mixed with hardener (2 %, v/v) in a 500 ml bottle, before it was immediately poured in situ into the burrow openings for casting. Mud was piled around the burrow entrance to prevent resin from spilling over the mud surface. After 24 h, the hardened casts were carefully removed from the sediment by hand and then brought to the laboratory for analysis. In the laboratory, each burrow cast was measured for the number of openings (large and small), bulbous chambers and burrowing branches (Fig. 2). The casts were also measured for the maximum width and length dimensions, length of each tunnel, total burrow length, diameter of tunnel, diameter of opening and bulbous chamber, and displacement volume (Fig. 2). These data were used to compare the structural variation between burrows. The burrow structure was analysed based on the number of openings, interconnected chambers and burrowing branches using the method of Gonzales et al. (2008).
The relationship between fish size and burrow dimensions was tested by regression analysis between fish length and the diameter of burrow openings, bulbous chambers, tunnel diameter (i.e., the distance of tunnel cross section), burrow depth, burrow length and width, total burrow length and burrow volume to test if a goby uses the burrow constructed by itself or just uses any existing burrows made by other fishes regardless of burrow size for hiding. Fish were caught by hand as they moved out of the burrow during casting. If fish were trapped in resin or casts were broken, additional casts and fish samples were made in the next fortnightly field work. Seasonal differences in burrow structure were examined between 10 burrow casts in the dry season and 14 burrow casts in the wet season to quantify the number of burrow openings and bulbous chambers, burrow depth, width and length, and total length and volume of burrows as described by Gonzales et al. (2008). These data were used to test whether the rainy season can influence the burrow structure due to the increase of mud and sand contents in flood water.
Laboratory experiment and burrowing behavioural observations. Although burrow casts in the field provided information on the burrows dug by fish of different sizes, the casts could not show the process of burrow building and utilization. The burrowing activity of P. serperaster was thus further investigated in both laboratory and field. The laboratory experiment was conducted at Can Tho University in three replicated aquaria (50 × 40 × 60 cm) from 4 to 25 June 2013 based on the method of Dou et al. (2007) using the sediment and associated biota from the field and 10 fish (12–16 cm TL) were placed in each aquarium. The environment of the aquarium mimicked that in the field with a 15° slope muddy flat and a water level covering half of the flat (Fig. 3). The temperature in the aquarium was 29.5 °C, pH ≈ 7.0 and salinity of 2, which were similar to the conditions in the field where fish were collected. A system with four cameras (QTC-203c, Questek Company, Taiwan) was set up to record burrowing activities of P. serperaster in the aquarium.
Field observations on fish burrowing behaviour were carried out twice a month and each lasted 2 days in the study area. The visual observation lasted 3 hours per day during the low tide, following the method of Bhatt et al. (2009). Inside the burrow, fish were observed using an endoscope camera (Video Borescope Inspection Camera, code: 177845, EXTECH Company) to check fish occupancy, movement, burrowing activities and the presence of eggs or embryos inside the burrow for one hour per day during the low tide on each field trip (Ishimatsu et al. 2007). In the field and laboratory, external observations were focused on feeding, predator avoidance and reproductive uses of each burrow. In the field, observations lasted 3 hours per day during the low tide, while in the laboratory, 8 hours per day were spent to observe fish behaviour as described by Tytler and Vaughan (1983). Fish entering a burrow with head first indicates that the fish may use the burrow for predator avoidance, but not for foraging prey. On the other hand, fish entering tail first shows that fish may use the burrow for foraging the prey (Able et al. 1982).
Data analysis. The 50 percentile fish body length at first maturation was considered the criterion to separate between a juvenile and an adult (Froese and Binohlan 2000). According to this criterion, the size of P. serperaster to reach maturation was predicted at 15.8 cm based on the fish collected in another study (unpublished data). In regression analysis, the independent variable was fish total length, and the dependent variables of burrow measurements included the number of openings, opening diameter, bulbous chambers, bulbous diameter, tunnel diameter, burrow depth, width and length, total burrow length and burrow volume of 24 burrow casts (two burrow casts per month). The differences of burrow depth, length and width, total burrow length and volume of burrows between dry and wet seasons were tested by MANOVA (Lawley–Hotelling test) at P < 0.05. The Minitab package software v16.0 was used for data analyses.
Results
Burrow structure and its relationships with fish size and season. A total of 24 goby burrows were analysed during this annual study. The burrows comprised interconnected segments with slight slopes on the bottom with 2–5 short cul-de-sac side branches, 2–4 burrow openings and 1–3 bulbous chambers (Fig. 2a). The burrow structure of Parapocryptes serperaster was in either U-shape (Fig. 2b) or W-shape (Fig. 2c). The burrow openings were usually flat and slightly circular without a mound and “footprint” (Fig. 2d). The main openings were usually larger than sub-openings, and the former was not constricted whereas the latter was usually constricted. The tunnel walls were roughly smooth and their cross sections were slightly round.
Fish in the burrows were 14–18 cm in total length (TL). Fish length had a strong relationship with burrow opening diameter (P < 0.01), bulbous chamber diameter (P < 0.01) and tunnel diameter (P < 0.01), resulting in significant regression coefficients (R 2, Fig. 4a–c). Similarly, fish length was correlated with burrow depth (P < 0.01), burrow width (P < 0.01), burrow length (P < 0.01) and total burrow length (P < 0.01, Fig. 5a–d). The volume of burrows ranged from 171 to 715 ml and had a strong relationship with fish length (P < 0.01, Fig. 6).
The burrow depth, width, length, and total burrow length, size of openings and volume, and the number of openings and bulbous chambers differed significantly between juvenile (<15.8 cm) and adult (15.8–18 cm) gobies (MANOVA, F = 5.181, P < 0.01). These values were significantly higher for burrows occupied by adults than those by juveniles, suggesting that the burrow size and complexity increased with fish size. The opening diameter, bulbous diameters, tunnel diameter, burrow depth, width, length, and total burrow length in the wet season were slightly greater than those in the dry season, but the difference was not significant (MANOVA, F = 1.002, P = 0.445). Similarly, the number of openings, number of bulbous chambers and volume of burrows were not significantly different between seasons (MANOVA, F = 0.902, P = 0.482, Fig. 7).
Burrowing activities and utilization. Burrowing activity of P. serperaster was not recorded during field observations at a spring tide because of deep and turbid water. The goby rarely moved out of its burrow at low tide until approaching the burrow for making casts or catching fish. Yet, the goby jumped out of its burrow when mixed polyester resin was poured into the burrow to make a cast. No other organisms were seen in the goby burrows when an endoscope camera was used to explore the burrow, or during the period of burrow casting. Neither endoscopic nor external examinations showed any indication of juvenile fish inside the burrows.
In the laboratory experiment, one of 10 fish in the aquarium excavated a burrow in the sediment at the corner of the aquarium two days after introduction into the aquarium (Fig. 3). The period of burrow-digging activity coincided with the time of high tide in the field. The lengths of fish-created burrows were 16 cm in the first and second trials and 15 cm in the third trial, and each burrow was dug through fish body movement. The fish started using their head to probe the mud and then the pelvic fins and body twisting movement to make the hole deeper and wider. After 4 hours, the fish stopped digging and stayed in the hole for one day. The burrow was a simple shaft, 14 cm deep and with 4.1 cm opening diameter in the first and second trials, 13.5 cm deep and with 4.1 cm opening diameter in the third trial. The burrowing activity of P. serperaster was similar in the three trials in the laboratory.
In field observations, no evidence was found that gobies used burrows for feeding and predator avoidance, as the gobies were not found moving in and out their burrows during the period of low-tide observation. However, in the laboratory trials, P. serperaster entered burrows with head first and exited with their tail as observed in the video camera recording. No feeding activity was observed through the glass wall when the goby was hiding in the burrow.
Discussion
Burrowing morphology. The burrows of Parapocryptes serperaster had a few branches with 2–4 openings, and fewer openings and branches than those of Odontamblyopus lacepedii (see Gonzales et al. 2008) and Taenioides cirratus (see Itani and Uchino 2003) living in mudflats, but slightly more than those of the related species Pseudapocryptes elongatus inhabiting the mudflat of the Mekong Delta (Dinh 2008) (Table 1). The burrow depth of P. serperaster is about half the depth of P. elongatus (see Dinh 2008) and O. lacepedii (see Gonzales et al. 2008), but similar to that of T. cirratus (see Itani and Uchino 2003) (Table 1). The burrow width and length of P. serperaster were less than that of O. lacepedii (see Gonzales et al. 2008), T. cirratus (see Itani and Uchino 2003) and P. elongatus (see Dinh 2008) (Table 1). The total burrow length of the burrows of P. serperaster was, however, similar to the related species P. elongatus (see Dinh 2008) and much lower than that of O. lacepedii (see Gonzales et al. 2008) and T. cirratus (see Itani and Uchino 2003). With regard to the burrow volume, P. serperaster had a smaller burrow than O. lacepedii (see Gonzales et al. 2008). These comparisons for several burrow-building fish illustrate that the burrow dimensions are species specific.
Burrow openings of P. serperaster were usually flat and slightly circular, similar to those of T. cirratus (see Itani and Uchino 2003), O. lacepedii (see Gonzales et al. 2008) and P. elongatus (see Dinh 2008). The burrows of P. serperaster had roughly a vertical entry to the tunnels, followed by a gentle slope on the bottom with a few short cul-de-sac side branches. No “footprints” was observed at the burrow openings of P. serperaster, whereas a lot of “footprints” surrounded the burrow openings of a neighbouring mudskipper Boleophthalmus boddarti. The burrows of P. serperaster also lacked sediment mounds on the openings, whereas there was a dead coral fragment mound on the burrow of Valenciennea longipinnis (see Takegaki and Nakazono 2000) and a sediment mound on the burrow opening in O. lacepedii (see Gonzales et al. 2008), Taenioides rubicundus (see Itani and Uchino 2003) and Periophthalmodon scholosseri (see Ishimatsu et al. 1998). The goby P. serperaster may not use a mound for storing air around the opening surface, as found in P. elongatus (see Dinh 2008), whereas the mudskipper P. scholosseri uses mounds to store air (Ishimatsu et al. 1998). However, it is not clear how P. serperaster obtains oxygen supply inside the burrows during low tide. In this study, there was no significant difference in burrow dimensions of this goby species in the dry and wet seasons. It is possible because the burrow structure of P. serperaster does not depend on precipitation, though mud is softer in the wet season than in the dry season. In contrast, the burrow dimensions of O. lacepedii in the summer and winter seasons were slightly different (Gonzales et al. 2008).
The U-shaped P. serperaster burrows is similar to the burrow of B. boddarti (see Clayton and Wright 1989), but the W-shaped P. serperaster burrow is different from the B. boddarti burrow. The U- and W-shaped P. serperaster burrows are different from those of the related P. elongatus (Y-shaped) (Dinh 2008), T. cirratus (see Itani and Uchino 2003) and O. lacepedii (see Gonzales et al. 2008). The goby P. serperaster seems to effectively use the sedimentary habitat with various burrow shapes, tunnels and openings to escape from predators, as fish can use the interconnected chambers to change directions when it moves in or out of the burrow. The burrow dimensions were strongly correlated with the size of P. serperaster, and this also occurred in the air-breathing eel goby, O. lacepedii (see Gonzales et al. 2008).
Burrowing activities and utilization. In the laboratory experiment, P. serperaster constructed burrows during the time coinciding with the occurrence of high tide in the field, whereas P. schlosseri built their shelters at low tide (Ishimatsu et al. 1998). No co-occurring macro-organism was found inside the burrows of P. serperaster, which is similar to other burrowing fishes such as mudskipper Boleophthalmus pectinirostris (see Chen et al. 2007), goby P. elongatus (see Dinh 2008), twostripe goby Valenciennea helsdingenii (see Clark et al. 2000), air-breathing eel goby O. lacepedii (see Gonzales et al. 2008) and the goby T. cirratus (see Itani and Uchino 2003). However, the activity of P. serperaster is different from the goby Cryptocentrus cryptocentrus that works together with the pistol shrimp Alpheus djiboutensis (see Karpulus et al. 1972). Based on laboratory tests, P. serperaster constructed shelters through body movement at the time coincident with high tide, as was observed on anguilliform fish (Herrel et al. 2011). However, since the configuration of burrow casts is complex, this gobiid fish may use various combinations of body parts to dig a burrow including mouth excavation and body twisting as observed in other gobies; Periophthalmodon septemradiatus (see Bhatt et al. 2009), V. longipinnis (see Takegaki and Nakazono 1999a), B. boddarti (see Clayton and Wright 1989) and yellowhead jawfish Opistognathus aurifrons (see Colin 1973) excavate their burrows by ejecting mud pellets or sands from their mouth.
During the field observation, P. serperaster rarely moved out of the burrow. The field burrow of this goby species had many openings, but the burrow in the glass aquarium was a straight tunnel. Burrow casts were in various shapes and had a few side branches. However, on laboratory observation, the P. serperaster entered the burrow with head first, which implies that this fish may have used the burrow as a place for living and as refuge to escape from predators, but maybe not for foraging, which is similar to burrow utilizations in other goby species such as goby T. cirratus (see Itani and Uchino 2003) and eel goby O. lacepedii (see Gonzales et al. 2008). In addition, this species could stay in the burrow for 18 hours and adapt to the condition inside the burrow. It is likely that P. serperaster obtains food from outside the burrow at high tide, as we did not find any evidence that this gobiid fish feeds inside the burrow.
This study provided no evidence that P. serperaster used the burrow for spawning or hatching, whereas the monogamous goby V. longipinnis (see Takegaki 2001; Takegaki and Nakazono 1999b), goby Zosterisessor ophiocephalus (see Mazzoldi et al. 2000), P. schlosseri (see Ishimatsu et al. 2009) and mudskipper P. modestus (see Ishimatsu et al. 2007) can use burrows for reproductive activities. However, Dinh (2008) reported that another goby species P. elongatus can lay eggs in the burrow located offshore in the Mekong Delta from June to November (wet season) with two spawning peaks in July and October. In this study, although no eggs or larvae were found in the P. serperaster burrows, the collection of larvae and juveniles of this goby species outside the burrow may provide a hint that burrows may not be used for reproductive activities in this species in the Mekong Delta.
References
Able KW, Grimes CB, Cooper RA, Uzmann JR (1982) Burrow construction and behavior of tilefish, Lopholatilus chamaeleonticeps, in Hudson Submarine Canyon. Environ Biol Fish 7:199–205
Atkinson R, Froglia C, Arneri E, Antolini B (1998) Observations on the burrows and burrowing behaviour of Brachynotus gemmellari and on the burrows of several other species occurring on Squilla grounds off Ancona, Central Adriatic. Sci Mar 62:91–100
Atkinson RJA, Chapman CJ (1984) Resin casting: a technique for investigating burrows in sublittoral sediments. Prog Underwat Sci 9:15–25
Atkinson RJA, Taylor AC (1991) Burrows and burrowing behaviour of fish. In: Meadows PS, Meadows A (eds) The environmental impact of burrowing animals and animal burrows. Zoological Society of London, Clarendon Press-Oxford, pp 133–155
Bhatt NY, Patel SJ, Patel DA, Patel HP (2009) Burrowing activities of goby fish in the recent intertidal mud flats along the Navinal coast, Kachchh, Western India. J Geol Soc India 74:515–530
Chen S, Hong W, Zhang Q, Su Y (2007) Why does the mudskipper Boleophthalmus pectinirostris form territories in farming ponds? J Mar Biol Assoc UK 87:615
Clark E, Stoll M, Alburn T, Petzold R (2000) Mound-building and feeding behavior of the twostripe goby, Valenciennea helsdingenii, in the South Red Sea. Environ Biol Fish 57:131–141
Clayton D, Wright J (1989) Mud-walled territories and feeding behaviour of Boleophthalmus boddarti (Pisces: Gobiidae) on the Mudflats of Kuwait. J Ethol 7:91–95
Colin PL (1973) Burrowing behavior of the yellowhead jawfish, Opistognathus aurifrons. Copeia 1973:84–90
Dinh TD (2008) Some aspects of biology and population dynamics of the goby Pseudapocryptes elongatus (Cuvier, 1816) in the Mekong Delta. PhD, Universiti Malaysia Teregganu, Malaysia
Dinh TD, Koichi S, Phuong NT, Hung HP, Loi TX, Hieu MV, Kenzo U (2013) Fishes of Mekong Delta, Vietnam. Can Tho University publisher, Can Tho
Dou SZ, Yamada Y, Okamura A, Tanaka S, Shinoda A, Tsukamoto K (2007) Observations on the spawning behavior of artificially matured Japanese eels Anguilla japonica in captivity. Aquaculture 266 (1–4):117–129
Froese R, Binohlan C (2000) Empirical relationships to estimate asymptotic length, length at first maturity and length at maximum yield per recruit in fishes, with a simple method to evaluate length frequency data. J Fish Biol 56:758–773
Gonzales TT, Katoh M, Ishimatsu A (2008) Intertidal burrows of the air-breathing eel goby, Odontamblyopus lacepedii (Gobiidae: Amblyopinae). Ichthyol Res 55:303–306
Herrel A, Choi HF, Dumont E, De Schepper N, Vanhooydonck B, Aerts P, Adriaens D (2011) Burrowing and subsurface locomotion in anguilliform fish: behavioral specializations and mechanical constraints. J Exp Biol 214:1379–1385
Hudson R (1977) Preliminary observations on the behaviour of the gobiid fish Signigobius biocellatus Hoese and Allen, with particular reference to its burrowing behaviour. Z Tierpsychol 43:214–220
Ishimatsu A, Hishida Y, Takita T, Kanda T, Oikawa S, Takeda T, Huat KK (1998) Mudskippers store air in their burrows. Nature 391 (6664):237–238
Ishimatsu A, Takeda T, Tsuhako Y, Gonzales TT, Khoo KH (2009) Direct evidence for aerial egg deposition in the burrows of the Malaysian mudskipper, Periophthalmodon schlosseri. Ichthyol Res 56:417–420
Ishimatsu A, Yoshida Y, Itoki N, Takeda T, Lee HJ, Graham JB (2007) Mudskippers brood their eggs in air but submerge them for hatching. J Exp Biol 210:3946–3954
Itani G, Uchino T (2003) Burrow morphology of the goby Taenioides cirratus. J Mar Biol Assoc UK 83:881–882
Jones R, Gutherz E, Nelson W, Matlock G (1989) Burrow utilization by yellowedge grouper, Epinephelus flavolimbatus, in the northwestern Gulf of Mexico. Environ Biol Fish 26:277–284
Karpulus I, Szlep R, Tsurnamal M (1972) Associative behavior of the fish Cryptocentrus cryptocentrus (Gobiidae) and the pistol shrimp Alpheus djiboutensis (Alpheidae) in artificial burrows. Mar Biol 15:95–104
Khaironizam MZ, Norma-Rashid Y (2000) A new record of the Mudskipper Parapocryptes serperaster (Oxudercinae: Gobiidae) from peninsular Malaysia. Malays J Sci 19:101–104
Kottelat M, Whitten T, Kartikasari SN, Wirjoatmodjo S (1993) Freshwater fishes of western Indonesia and Sulawesi. Periplus Editions, Indonesia
Le TVH, Nguyen HN, Wolanski E, Tran TC, Haruyama S (2007) The combined impact on the flooding in Vietnam’s Mekong River delta of local man-made structures, sea level rise, and dams upstream in the river catchment. Estuar Coast Shelf S 71 (1–2):110–116
Mazzoldi C, Scaggiante M, Ambrosin E, Rasotto MB (2000) Mating system and alternative male mating tactics in the grass goby Zosterisessor ophiocephalus (Teleostei: Gobiidae). Mar Biol 137:1041–1048
Murdy E (2011) Systematics of Oxudercinae. In: Patzner RA, Tassell JLV, Kovacic M, Kapoor BG (eds) The biology of gobies. Science Publishers, pp 99–106
Rainboth WJ (1996) Fishes of the Cambodian Mekong (FAO Species Identification Field Guides). FAO, Rome
Soc Trang Statistical Office (2012) Soc Trang Ater 20 Years Estambishing - A Development Way. Soc Trang Statistical Office, Soc Trang
Takeda T, Hayashi M, Toba A, Soyano K, Ishimatsu A (2012) Ecology of the Australian mudskipper Periophthalmus minutus, an amphibious fish inhabiting a mudflat in the highest intertidal zone. Aust J Zool 59:312–320
Takegaki T (2001) Environmental factors affecting the spawning burrow selection by the gobiid Valenciennea longipinnis. J Fish Biol 58:222–229
Takegaki T, Nakazono A (1999a) Division of labor in the monogamous goby, Valenciennea longipinnis, in relation to burrowing behavior. Ichthyol Res 46:125–129
Takegaki T, Nakazono A (1999b) Reproductive behavior and mate fidelity in the monogamous goby, Valenciennea longipinnis. Ichthyol Res 46:115–123
Takegaki T, Nakazono A (2000) The role of mounds in promoting water-exchange in the egg-tending burrows of monogamous goby, Valenciennea longipinnis (Lay et Bennett). J Exp Mar Biol Ecol 253:149–163
Takita T, Agusnimar, Ali A (1999) Distribution and habitat requirements of oxudercine gobies (Gobiidae: Oxudercinae) along the Straits of Malacca. Ichthyol Res 46:131–138
Talwar PK, Jhingran AG (1991) Inland fishes of India and adjacent countries, vol 2. Balkema, Rotterdam
Tytler P, Vaughan T (1983) Thermal ecology of the mudskippers, Periophthalmus koelreuteri (Pallas) and Boleophthalmus boddarti (Pallas) of Kuwait Bay. J Fish Biol 23:327–337
Acknowledgments
We are grateful to Mr. Mien and Mr. Giang in Cu Lao Dung, Soc Trang, staffs and students at the Department of Biology, School of Education, Can Tho University for helping us to complete this study and to AusAID for funding of this PhD project.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Dinh, Q.M., Qin, J.G., Dittmann, S. et al. Burrow morphology and utilization of the goby (Parapocryptes serperaster) in the Mekong Delta, Vietnam. Ichthyol Res 61, 332–340 (2014). https://doi.org/10.1007/s10228-014-0402-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-014-0402-2