Abstract
Two forms of muraenid leptocephali, collected from the western Pacific Ocean, were identified as Gymnothorax sagmacephalus Böhlke 1997 and Gymnothorax albimarginatus (Temminck and Schlegel 1846) on the basis of morphometric and genetic analyses. The leptocephali of each species were characterized, respectively, by counts of 172–175 and 186–191 myomeres, 43–44 and 47 predorsal myomeres, 109–113 and 127–134 preanal myomeres, and 100–104 and 118–119 last vertical blood vessel myomeres. Gymnothorax sagmacephalus leptocephali had minute melanophores over much of the head and body, closely resembling the condition in Gymnothorax minor (Temminck and Schlegel 1846), whereas those of G. albimarginatus not only had minute melanophores over much of the head and body, but also a pair of melanophore groups on the posteroventral and posterodorsal aspects of the head. Such groups are here considered to represent highly specific characters. Although a previous opinion postulated that G. sagmacephalus is a juvenile of G. albimarginatus, and the adult morphologies of the two species have a lot in common, they clearly differ in both leptocephalus morphology and genetic sequence. Therefore, G. sagmacephalus was concluded as being a valid species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Muraenidae (moray eels) is one of the most diverse families of anguilliform fishes, consisting of two subfamilies, 15 genera and ca. 200 species (Böhlke et al. 1989). Because of their very simplified morphology, including a lack of pectoral and pelvic fins, confluence of the dorsal, caudal and anal fins, and either the fusion or deletion of many bones (Böhlke et al. 1989), moray eels have remarkably few characters to distinguish species other than color, which is often variable within species. On the other hand, their leptocephalus larvae have many discernible characters (Tawa et al. 2013), for example fin and anus positions, and various pigmentation patterns. Leptocephali of the two subfamilies (Uropterygiinae and Muraeninae) can be distinguished by the dorsal- and anal-fin origin positions, being more anterior in the latter (Smith 1989b). However, generic level identification of muraenid leptocephali has proven difficult owing to the incomplete state of muraenid taxonomy. The most important character established thus far for identifying most type of leptocephali at the species level is total myomere number, which generally corresponds to the total vertebral number in adults (Smith 1989a). On this basis, a relatively large number of Atlantic muraenid leptocephali have been identified at species level (Blache 1977; Smith 1989b). Although some Indo-Pacific leptocephali have been identified at species level (D’Ancona 1928; Nair 1947; Jones and Pantalu 1952; Castle 1965), all such identifications require reconsideration due to their extremely weak evidentiary bases. In a recent study, the leptocephali of Gymnothorax minor (Temminck and Schlegel 1846) and Strophidon ui Tanaka 1918 were identified following observations of metamorphosis of live leptocephali (Tawa and Mochioka, 2009; Tawa et al. 2012a). Leptocephali of five further species [Scuticaria tigrina (Lesson 1828), Gymnothorax buroensis (Bleeker 1857), Gymnothorax meleagris (Shaw 1795), Gymnothorax eurostus (Abbott 1860) and Gymnothorax pseudothyrsoideus (Bleeker 1852)] have been recently identified on the bases of morphometric and genetic analysis (Tawa et al. 2012b, 2013). Indeed, genetic analysis has become a valuable tool for correctly identifying muraenid leptocephali, particularly ethanol-preserved specimens. The leptocephali of previously unassigned muraenid morphological types, collected from the western Pacific Ocean, were identified in this study as Gymnothorax sagmacephalus Böhlke 1997 and Gymnothorax albimarginatus (Temminck and Schlegel 1846), on the bases of morphometric characters and mitochondrial DNA sequence analyses, and their detailed morphology described.
Materials and methods
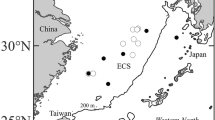
Specimens. A total of eight leptocephali representing the subfamily Muraeninae (type A: TM 172–175; type B: 186–191) collected from the western Pacific Ocean were examined during this study (Fig. 1; Table 1). Six leptocephali, collected by Rectangular Midwater Trawl (RMT) during a cruise of the R/V Kaiyo Maru (Japanese Fisheries Agency) in August and September 2000, and preserved in 99.5% ethanol just after collection, were examined morphologically after reconstitution of the specimens with distilled water in the laboratory. One leptocephalus, collected by Multiple Opening/Closing Net and Environmental Sensing System (MOCNESS) during a cruise of the R/V Hokko Maru (Fisheries Research Agency) in October 2007, was examined morphologically prior to fixing in 99.5 % ethanol, and a further specimen, collected by Isaac-Kidd Midwater Trawl (IKMT) during a cruise of the R/V Hakuho Maru in March 2010, was similarly examined prior to fixing in 99.5 % ethanol. The following measurements and counts were made according to Tawa et al. (2013): total length (TL), preanal length (PAL), predorsal-fin length (PDL), head length (HL), body depth (BD), total myomeres (TM), predorsal-fin myomeres (PDM), preanal myomeres (PAM), last vertical blood vessel myomeres (LVBV), myomeres between dorsal-fin origin and anus (ADM) and dental formula. Melanophores were observed by intense epi-illumination, following Tawa and Mochioka (2009) and Tawa et al. (2012a, 2013). All larvae were deposited in the collection of the Kyushu University Museum (KYUM).
Seven adult specimens of three species, Gymnothorax albimarginatus, G. sagmacephalus and G. minor, were collected from coastal areas around Japan between 1999 and 2009 (Table 1). Total vertebrae (TV) were counted from radiographs according to Böhlke (1989). Muscle tissue samples were taken from each specimen and preserved in 99.5 % ethanol for subsequent DNA analysis. All specimens were deposited in the collection of the Kyushu University Museum (KYUM).
DNA extraction, PCR amplification and analysis. Eight leptocephali and seven adult specimens (see above) were used for DNA analysis. Total genomic DNA was extracted from approximately 25 mg (leptocephali, < 25 mg) of ethanol-preserved muscle tissue using the DNeasy tissue kit (Qiagen), following the manufacturer’s protocol. A partial sequence of the mitochondrial DNA 16S rRNA region was amplified by the polymerase chain reaction (PCR) using A and B primers (5’–GGTCCWRCCTGCCCAGTGA–3’ and 5’–CCGGTCTGRACYAGATCACGT–3’, respectively). PCR amplifications were carried out in a Thermal Cycler PTC–100 (MJ Research, Inc) at 94 °C for 1 min, followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 50 °C for 30 s and 72 °C for 90 s, and extension at 72 °C for 6 min. PCR products were purified with Wizard SV Gel and PCR Clean-up System (Promega) following the manufacturer’s protocol. Sequencing reactions were analyzed on an ABI PRISM 3100 and 3730 Genetic Analyzer (Applied Biosystems) using the Big Dye Terminator version 3.1 Cycle Sequencing Kit (Applied Biosystems). All sequences obtained were submitted to the DDBJ, EMBL and GenBank nucleotide sequence databases under the accession numbers AB808682–AB808696. Eleven species examined in previous studies and registered with DDBJ, EMBL and GenBank (Tawa et al. 2012b, 2013) were included in the following genetic analysis (Table 1). A neighbor-joining (NJ) tree (Saitou and Nei 1987) and distance matrices were generated using MEGA5 (Tamura et al. 2011) in an analysis of Kimura two-parameter distances (Kimura 1980).
Results
Species identification. The holotype of Gymnothorax sagmacephalus collected from Japan (adult) has missing tail tip (Böhlke 1997), resulting in an incomplete vertebral column (TV = 172+). The adult G. sagmacephalus specimens examined in the present study had lower TV counts of 170–174 (n = 3), compared with TV counts in adult G. albimarginatus of 184–195 (n = 7) (Böhlke, 1997) and 183–189 (n = 3) (present study; Table 1). The two leptocephali types considered here, having TM counts of 172–175 (type A) and 186–191 (type B), therefore corresponded to the TV counts of adult G. sagmacephalus and G. albimarginatus (Table 1). However, TV counts of adult Gymnothorax verrilli (Jordan and Gilbert 1883) (170–171), Gymnothorax phasmatodes (Smith 1962) (160–174) and Gymnothorax prolatus Sasaki and Amaoka 1991 (182–187) (see Böhlke 1997) also resembled those of either G. sagmacephalus or G. albimarginatus.
Partial sequences (about 520 bp) of mitochondrial DNA 16S rRNA were obtained from eight leptocephali (representing the two morphological types) and seven adults (representing three species) in the present study. All genetic analyses were conducted on 26 sequences (497 bp) including the sequences of eleven species examined in previous studies (Tawa et al. 2012b, 2013) after alignment. A neighbor-joining tree using all sequences is shown in Fig. 2. Two species, G. sagmacephalus and G. albimarginatus, clearly formed separate clades that contained the two leptocephalus types (A and B, respectively). The average (and range) of interspecific variations (nucleotide substitution rate) between 14 species was 11.9 (5.2–17.8) % (Table 2). In comparison, the intraspecific variations of G. sagmacephalus and G. albimarginatus were much lower at 0.7 (0.4–1.0) % and 0.8 (0.6–1.2) %, respectively (Table 2). Furthermore, the nucleotide substitution rates between G. sagmacephalus and leptocephalus type A [0.8 (0.6–1.2) %], and G. albimarginatus and leptocephalus type B [0.8 (0–1.4) %] were well in accordance with intraspecific variations of G. sagmacephalus and G. albimarginatus, respectively (Table 2). Accordingly, leptocephalus types A and B were identified as G. sagmacephalus and G. albimarginatus, respectively, on the bases of their meristic counts and DNA sequences.
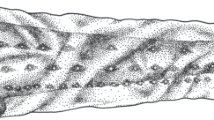
Description of leptocephalus larvae, Gymnothorax sagmacephalus Böhlke 1997 (Fig. 3). TL 30.2–85.1 mm, TM 172–175 (n = 4), PDM 43–44 (4), PAM 109–113 (4), ADM 64–68 (4), LVBV 100–104 (4). Proportion in percent of TL: PDL 27.0–33.1 % in 67.1–85.1 mm TL (3), ca. 43.0 % in 30.2 mm TL; PAL 72.0–80.2 % (3), 86.1 %; HL 4.7–4.9 % (3), 7.3 %; BD 14.0–17.6 % (3), 15.6 %. Dental formula: 1 + 5+6–7/ 1 + 7–8 + 3 (3), 1 + 2 + 4/1 + 1 + 4. Melanophores small, compacted into several clearly defined areas: scattered on ventral surface of rostrum (= palate) (VSR; Fig. 3c); scattered around nasal organ (NO; Fig. 3c); grouped on dorsal surface of head and brain (SHB; Fig. 3c); several on the ventral and lateral surface of the posterior part of the brain, continuous with a group on opercular region (PVB, OP; Fig. 3c); several on posterior margin of lower jaw (PLJ; Fig. 3c); numerous on ventral portion of throat (VT; Fig. 3c), continuous with ventral somatic row from throat to posterior margin of gallbladder, a single row in 30.2 mm TL specimen, a single row but scattered around gallbladder in three large specimens in 67.1–85.1 mm TL [TG; Fig. 3c, e, type “A” of Smith (1989b)]; a single splanchnic row above intestine (along pronephric ducts) from gallbladder to anus, spaced at zero to two per myomere (SPGA; Fig. 3e); a single somatic row along ventral midline under gut from gallbladder to anus, spaced at three or four per myomere (SOGA; Fig. 3e); a single somatic row from about ninth myomere to dorsal fin origin along dorsal midline, spaced at about two per myomere (DM; Fig. 3c, d); a row of minute melanophores along dorsal- and anal-fin bases, about one per ray (DAFB; Fig. 3f); a row along ventral spinal cord from about first myomere to caudal tip, zero to two per myomere from first to about 10th myomere and subsequently two to five per myomere until caudal tip (number increasing posteriorly, SC; Fig. 3f); a row along dorsal spinal cord from about first myomere to caudal tip, zero to two per myomere from first to about 10th myomere and subsequently one to five per myomere until caudal tip in 67.1–85.1 mm TL (DSC, Fig. 3f) (absent in 30.2 mm TL).
Gymnothorax sagmacephalus leptocephalus larvae. a Lateral view, 30.2 mm TL (KYUM–PI 4045), b lateral view, 85.1 mm TL (KYUM–PI 4124), c lateral view of head (KYUM–PI 4124), d lateral view of dorsal-fin origin (KYUM–PI 4124), e lateral view of gallbladder (KYUM–PI 4124), f lateral view of tip of caudal region (KYUM–PI 4124). Abbreviations of melanophore positions: VSR ventral surface of rostrum, NO nasal organ, SHB dorsal surface of head and brain, PVB posteroventral and lateral surface of brain, OP opercular region, PLJ posterior margin of lower jaw, VT ventral portion of throat, TG somatic row from throat to gallbladder, SPGA splanchnic row above intestine from gallbladder to anus, SOGA somatic row from gallbladder to anus, DM dorsal midline, DAFB dorsal- and anal-fin bases, SC ventral aspect of spinal cord, DSC dorsal aspect of spinal cord
Remarks. The leptocephali of this species reach a comparatively large size, with the largest specimen (85.1 mm TL) showing no sign of metamorphosis. The anus and last vertical blood vessel are posteriorly positioned, and the dorsal-fin origin is positioned anteriorly. This leptocephalus type has melanophores on many parts of the head and body. The melanophore patterns and positional relationship of the anus and dorsal-fin origin of the G. sagmacephalus leptocephali closely resembled those of G. minor (see Tawa and Mochioka 2009). However, the leptocephali were easily distinguished by TM counts (172–175 in the former; 135–142 in the latter). A few Indo-Pacific muraenid leptocephali (Muraeninae) with TM counts of 160–180 have been recorded (D’Ancona 1928; Castle 1965). D’Ancona (1928) described the leptocephali having TM counts of 158–162, thereby differing from those of G. sagmacephalus (TM 172–175). Castle (1965) described Leptocephalus Gymnothorax sp. (166 myomeres) on the basis of a single specimen (71.8 mm TL). Although meristic counts for this leptocephalus (TM 166, PAM 110, LVBV 103) closely resembled those of G. sagmacephalus, leptocephali of the latter could be distinguished by the presence of VSR, NO and SHB melanophores (absent in Leptocephalus Gymnothorax sp.).
Description of leptocephalus larvae, Gymnothorax albimarginatus (Temminck and Schlegel 1846 ) (Fig. 4). TL 10.2–93.0 mm, TM 186–191 (n = 3), PDM 47 (1), PAM 127–134 (3), ADM 86 (1), LVBV 118–119 (3). Proportion in percent of TL: PDL 31.0 % in 93.0 mm TL; PAL 82.8–85.8 % in 43.1–93.0 mm TL (2), 89.2 % in 10.2 mm TL; HL 4.9–6.5 % in 43.1–93.0 mm TL (3), 16.7 % in 10.2 mm TL; BD 12.1–14.9 % in 43.1–93.0 mm TL (3), 22.5 % in 10.2 mm TL. Dental formula: 1 + 4–7 + 4/1 + 4–9 + 2 in 43.1–93.0 mm TL (3), 1 + 3 + 0/1 + 3 + 0 in 10.2 mm TL. In 43.1–93.0 mm TL specimens, melanophores small, compacted into several clearly defined areas: scattered on ventral surface of rostrum (VSR; Fig. 4c); scattered around nasal organ (NO; Fig. 4c); grouped on dorsal surface of head and brain (SHB; Fig. 4c); several on posteroventral and lateral surface of brain, continuous with a group on opercular region (PVB, OP; Fig. 4c); a few on iris (IR; Fig. 4c), but usually rare (absent in 10.2–68.6 mm TL specimens); small number on lower jaw tip (TLJ; Fig. 4c); small number on posterior margin of lower jaw (PLJ; Fig. 4c); numerous on ventral portion of throat (VT; Fig. 4c), continuous with grouped melanophores on ventral body surface, six or seven somatic rows from heart to about eighth myomere (TGgroup; Fig. 4c) plus a single ventral somatic row from about 20th myomere to gallbladder (number increasing posteriorly, TG; Fig. 4e); a single splanchnic row above intestine (along pronephric ducts) from gallbladder to anus, spaced at six to nine per myomere (SPGA; Fig. 4e); grouped on dorsal-body surface, six or seven somatic rows from first to eighth myomeres (DMgroup; Fig. 4c) plus a single somatic row along dorsal midline, from about 39th myomere to dorsal-fin origin, spaced at about one per myomere (in 93.0 mm TL specimen, DM; Fig. 4d); a row of minute melanophores along dorsal- and anal-fin bases, about one per ray (DAFB; Fig. 4f); a row along ventral spinal cord from about first myomere to caudal tip, zero to two per myomere from first to about 10th myomere and subsequently about seven per myomere until caudal tip (SC; Fig. 4f); a row along dorsal spinal cord from about first myomere to caudal tip, zero to one per myomere from first to about 10th myomere and subsequently one to two per myomere until caudal tip (DSC; Fig. 4f). In 10.2 mm TL specimen, melanophores small and compact within a single region: a ventral row along spinal cord from about first myomere to caudal tip, zero to one per myomere (number increasing posteriorly, SC; Fig. 4a).
Gymnothorax albimarginatus leptocephalus larvae. a Lateral view, 10.2 mm TL (KYUM–PI 4430), b lateral view, 93.0 mm TL (KYUM–PI 4096), c lateral view of head (KYUM–PI 4096), d lateral view of dorsal-fin origin (KYUM–PI 4096), e lateral view of gallbladder (KYUM–PI 4096), f lateral view of tip of caudal region (KYUM–PI 4096). Abbreviations of melanophore positions: VSR ventral surface of rostrum, NO nasal organ, SHB dorsal surface of head and brain, PVB posteroventral and lateral surface of brain, OP opercular region, IR iris, TLJ lower jaw tip, PLJ posterior margin of lower jaw, VT ventral portion of throat, TGgroup ventral somatic 6 or 7 rows, TG somatic row from 20th myomeres to gallbladder, SPGA splanchnic row above intestine from gallbladder to anus, DMgroup dorsal, somatic 6 or 7 rows, DM dorsal midline, DAFB dorsal- and anal-fin bases, SC ventral aspect of spinal cord, DSC dorsal aspect of spinal cord
Remarks. Like leptocephali of G. sagmacephalus, those of G. albimarginatus reach a comparatively large size, with the largest specimen (93.0 mm TL) showing no sign of metamorphosis. The anus and last vertical blood vessel are posteriorly positioned, and the dorsal-fin origin is positioned anteriorly. The 10.2 mm TL specimen, thought to represent immediate post-hatching, had markedly different body proportions to the >40 mm TL specimens. Also, the posterior edge of the gut protruded slightly from the body. It is likely that the external gut represented an artifact resulting from body shrinkage after death, rather than a morphological character of small larvae. The melanophore pattern was incomplete. However, the meristic counts (TM, PAM and LVBV) were almost identical to those of the larger conspecific specimens. The 43.1 mm TL specimen possessed all of the melanophores described above for G. albimarginatus, including all over the head and body. Notably, however, a pair of melanophores in the DM and TG groups was unique to this species. Leptocephali with morphology similar to that of G. albimarginatus have not been previously described, although Miller and Tsukamoto (2004) provided a photograph (without comment) of a leptocephalus that now seems likely to be G. albimarginatus (Chapter 5, Family Muraenidae, figure 5.41).
Discussion
The genus Gymnothorax Bloch 1795 includes 124 valid species, of which 105 are distributed in the Indo-Pacific Ocean (Froese and Pauly 2013). For the first time, the leptocephali of two species, G. sagmacephalus and G. albimarginatus, from the western Pacific region were identified and described in this study. Overall, seven species of the leptocephali of Gymnothorax from Indo-Pacific had been described, including our previous findings (Tawa and Mochioka 2009; Tawa et al. 2013). Both leptocephali were characterized by many myomeres (172–175 in G. sagmacephalus and 186–191 in G. albimarginatus), the anterior position of the dorsal-fin origin (PDM 43–44 and 47) and posterior position of the anus (PAM 109–113 and 127–134), and melanophore patterns (see each description). Although leptocephali of Strophidon ui, described from the western North Pacific, also have many myomeres 184–196 (Tawa et al. 2012a), they differ from the aforementioned two species in both dorsal-fin origin location (PDM 84–90 in S. ui) and the absence of SHB, TG and SPGA melanophores.
The three species, G. sagmacephalus, G. albimarginatus and G. minor, clearly comprise one clade in the NJ tree based on 14 muraenid species (Fig. 2). Moreover, genetically G. sagmacephalus is closely related to G. minor (more than G. albimarginatus). Although adults of G. sagmacephalus and G. albimarginatus share similar morphologies, including many vertebrae (over 170), slender body proportions, dark brown body coloration and dental row formulae (Böhlke 1997), adult G. minor [with TV counts of 135–143 and 15–22 dark brown bars on a pale yellow body (Böhlke and McCosker 1997)] clearly differ. On the other hand, leptocephalus of G. minor (see Tawa and Mochioka 2009) closely resemble that of G. sagmacephalus in having the VSR, NO, SHB, PVB, PLJ, VT, OP, TG, DM, TG, SPGA, SPGA, SC and DAFB melanophores and similar anal position (percentage of PAL to TL: 72.0–80.2 % in the former vs. 70.2–80.1 % in the latter). Within the species group, the genetic relationship is in close accordance with leptocephalus morphology, rather than adult. In fact, our previous report (Tawa et al. 2013) suggested that the melanophore patterns of leptocephali representing four species groups, including G. buroensis, G. eurostus, G. meleagris and Gymnothorax milliaris (Kaup 1856), reflected moray phylogeny. The present study supports this general pattern.
It has been suggested that G. sagmacephalus is a juvenile of G. albimarginatus (see Hatooka 2002). Gymnothorax sagmacephalus was described by Böhlke (1997) based on one specimen (534 mm TL) from Japan and was characterized by having the TV counts 172+, and two unique colorations—a dusky saddle on top of the head just behind the eyes and a large prominent dark triangle just before the dorsal-fin origin. Although G. albimarginatus are distinguished from G. sagmacephalus by having TV count of over 180 and lacking two unique colorations, Hatooka (2002) thought the two unique colorations of G. sagmacephalus are larval forms of G. albimarginatus. Also, both species could not be distinguished by TV counts due to damage in the holotype of G. sagmacephalus (see above) (Böhlke 1997). The TV counts of G. sagmacephalus (170–174) based on the three specimens newly corrected in this study tended to be lower than those of G. albimarginatus (183–189) (Table 1). Furthermore, the genetic analysis also separated the two species (Fig. 2). The final character clearly separating the two species are those leptocephalus morphologies including a pair of melanophore TG and DM groups in G. albimarginatus (absent in G. sagmacephalus) (Figs. 3c, 4c), and the meristic counts of TM, PAM, LVBV and ADM (for details, see descriptions of the two leptocephali). Accordingly, G. sagmacephalus is considered to be a valid species, along with G. albimarginatus.
Reference
Abbott CC (1860) Description of new species of apodal fishes in the museum of the Academy of Natural Sciences of Philadelphia. Proc Acad Nat Sci Philadelphia 12:475–479
Blache J (1977) Leptocéphales des poissons Anguilliformes dans la zone sud du Golfe De Guinée. Faune Tropicale. ORSTOM, Paris
Bleeker P (1852) Derde bijdrage tot de kennis der ichthyologische fauna van Celebes. Natuurkundig Tijdschrift voor Nederlandsch Indië 3:739–782
Bleeker P (1857) Tweede bijdrage tot de kennis der ichthyologische fauna van Boero. Natuurkundig Tijdschrift voor Nederlandsch Indië 13:55–82
Bloch ME (1795) Naturgeschichte der ausländischen Fische, vol 9. J. Morino, Berlin
Böhlke EB (1989) Methods and Terminology. In: Böhlke EB (ed). Fishes of the western North Atlantic. Sears Foundation for Marine Research, memoir 1, part 9, vol. 1, Anguilliformes and Saccopharyngiformes, pp 1–7
Böhlke EB, McCosker JE, Böhlke JE (1989) Family Muraenidae. In: Böhlke EB (ed). Fishes of the western North Atlantic. Sears Foundation for Marine Research, memoir 1, part 9, vol. 1, Anguilliformes and Saccopharyngiformes, pp 104–206
Böhlke EB (1997) Note on the identity of elongate unpatterned Indo-Pacific moray, with description of a new species (Muraenidae, subfamily Muraeninae). Proc Acad Nat Sci Philadelphia 147:89–109
Böhlke EB, McCosker JE (1997) Review of the moray eel genus Scuticaria and included species(Pisces: Anguilliformes: Muraenidae: Uropterygiinae). Proc Acad Nat Sci Philadelphia 148:171–176
Castle PHJ (1965) Muraenid leptocephali in Australasian waters. Trans Roy Soc NZ Zool 7:57–84
D’Ancona U (1928) Muraenoidi (Apodes) del Mar Rosso e del Golf di Aden. Memoria, Comitato Talassografico Italiano
Froese R, Pauly D (2013) FishBase. Updated April 2013. http://www.fishbase.org. Accessed 10 June 2013
Hatooka K (2002) Family Muraenidae. In: Nakabo T (ed). Fishes of Japan with pictorial keys to the species, English edition. Tokai University Press, Tokyo, pp 196–211
Jones S, Pantalu VR (1952) On the metamorphosing stages of the talabon eel, Muraenesox talabon (Cantor) with description of some leptocephali from the estuaries of Bengal and Orissa. J Asiat Soc Beng 18:129–140
Jordan DS, Gilbert CH (1883) List of fishes now in the museum of Yele College, collected by Prof. Frank H. Bradley, at Panama, with descriptions of three new species. Proc US Nat Mus 5:320–632
Kaup JJ (1856) Uebersicht der Aale. Archiv für Naturgeschichte 22:41–77
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Lesson RP (1828) Description du nouveau genre Ichthyophis et de plusieurs espèces inédites ou peu connues de poissons, recueillis dans le voyage autour du monde de la Corvette “La Coquille.” Mem Soc d’Hist Nat Paris 4:397–412
Miller MJ, Tsukamoto K (2004) An introduction to leptocephali: biology and identification. Ocean Research Institute, University of Tokyo, Tokyo
Nair RV (1947) On the metamorphosis of two leptocephali from the Madras plankton. Proc Indian Acad Sci Sec B 25:1–14
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sasaki K, Amaoka K (1991) Gymnothorax prolatus, a new moray from Taiwan. Jpn J Ichthyol 38:7–10
Shaw G, Nodder FP (1789–1813) The Naturalist’s Miscellany, or coloured figures of natural objects; drawn and described from nature. London
Smith DG (1989a) Introduction to leptocephali. In: Böhlke EB (ed). Fishes of the western North Atlantic. Sears Foundation for Marine Research, memoir 1, part 9, vol. 2, pp 657–668
Smith DG (1989b) Family Muraenidae: Leptocephali. In: Böhlke EB (ed). Fishes of the western North Atlantic. Sears Foundation for Marine Research, memoir 1, part 9, vol. 2, pp 900–916
Smith JLB (1962) The moray eels of the western Indian Ocean and the Red Sea. Ichthyol Bull JLB Smith Inst Ichthyol (23):419–444
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tanaka S (1918) Two new species of Japanese fishes. Zool Mag 30:51–52
Tawa A, Mochioka N (2009) Identification of aquarium-raised muraenid leptocephali, Gymnothorax minor. Ichthyol Res 56:340–345
Tawa A, Kishimoto H, Mochioka N (2012a) Larval identification following metamorphosis in the slender brown moray Strophidon ui from the western North Pacific. Ichthyol Res 59:8–13
Tawa A, Kobayakawa M, Yoshimura T, Mochioka N (2012b) Identification of leptocephalus larvae of the tiger moray Scuticaria tigrina (Anguilliformes; Muraenidae) based on morphometric and genetic evidence. Ichthyol Res 59:378–383
Tawa A, Kobayakawa M, Yoshimura T, and Mochioka N (2013) Identification of leptocephali representing four muraenid species from the western North Pacific, based on morphometric and mitochondrial DNA sequence analyses. Bull Mar Sci 89:461–481
Temminck CJ, Schlegel H (1846) Pisces. Parts 10–14. In: von Siebold PF (ed) Fauna Japonica. Lugduni, Batavorum, pp 173–269
Acknowledgments
We thank H Ikeda and R Utsumi (Wakayama prefecture) for their significant contributions to this work, and the captains and crews of the R/V Kaiyo Maru (Japanese Fisheries Agency) and the R/V Hokko Maru (Fisheries Research Agency) for their efforts in collecting specimens. Thanks are due also to GS Hardy (Ngunguru, New Zealand) for his critical comments on the manuscript and great help with English.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article was registered in the Official Register of Zoological Nomenclature (ZooBank) as 91A6F487-2357-4181-8773-4FD6F1F9A029.
This article was published as an Online First article on the online publication date shown on this page. The article should be cited by using the doi number.
About this article
Cite this article
Tawa, A., Aoyama, J., Yoshimura, T. et al. Leptocephalus larvae of two moray eels (Anguilliformes; Muraenidae), Gymnothorax sagmacephalus and Gymnothorax albimarginatus, identified from morphometric and genetic evidence. Ichthyol Res 61, 32–41 (2014). https://doi.org/10.1007/s10228-013-0369-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-013-0369-4