Abstract
The larval distribution and feeding habits of Cynoglossus abbreviatus and Cynoglossus lighti were investigated around the Rokkaku estuary in Ariake Bay during October 2004 and March and April 2005. Cynoglossus abbreviatus started and completed metamorphosis at larger sizes than C. lighti. Developmental phases consisted primarily of individuals at metamorphosis and postmetamorphosis, which were mainly distributed inside and just outside the Rokkaku River in March–April for C. abbreviatus and October for C. lighti. Tidal changes in vertical distribution just outside the river mouth differed between the closely related species; C. abbreviatus was distributed in the surface and middle layers at flood tide, and aggregated near the bottom (where the current speed was lowest) at ebb tide. Cynoglossus lighti stayed mainly near the bottom during all tidal phases. Larvae of both Cynoglossus species selectively fed on copepods (Pseudobradya sp.), which were scarce in the waters. These facts suggest that the nursery grounds of the two Cynoglossus species were different in terms of their tidal vertical distribution patterns.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cynoglossus abbreviatus and Cynoglossus lighti (Cynoglossidae) are distributed from southern Japan to the South China Sea and to Ariake Bay, and from the Yellow Sea to the South China Sea, respectively (Yamada 2002). Cynoglossus abbreviatus is highly restricted to Ariake Bay in Japan, and it has been suggested by Menon (1977) that C. lighti is a junior synonym of C. joyneri.
In Ariake Bay, information on the spawning, distribution and early development of these species (Takita 1980; Fujita et al. 1986; Hayashi et al. 2000) is fragmented, and very little is known about their early life histories. Recently, Koshiishi et al. (2001) and Aoyama et al. (2007) observed that Cynoglossus juveniles settled around the inner estuary of the bay, and Aoyama et al. (2007) suggested that there may be a marginal spatial difference in the settlement of the two Cynoglossus species.
In this paper, the ontogenies of the two Cynoglossus species are described and compared in detail. Furthermore, we compared the early migration patterns and feeding habits of the two species to examine potential spatial and trophic differences in their use of nursery grounds.
Materials and methods
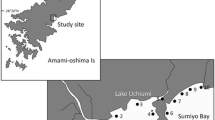
Cynoglossus abbreviatus and C. lighti larvae were collected around the Rokkaku estuary (Stns. R1–R10), located in the inner part of Ariake Bay, around the spring tides of March and April 2005 (for C. abbreviatus), and October 2004 (for C. lighti) (Fig. 1). Collections were made by oblique tows of a larva net (1.3 m mouth diameter, 0.5 mm mesh aperture) at sea stations (Stns. R8–R10), by discrete-depth horizontal tows at surface and middle layers with a larva net (1.3 m mouth diameter, 1 mm mesh aperture), and by a specialized beam trawl (width 0.25 × 1.5 m, 1.0 mm mesh aperture) of the near-bottom layer at estuary stations (Stns. R1–R7). The specialized beam trawl was modified after Kuipers (1975), and was designed to keep the lower beam of the mouth 5 cm off the bottom to collect pelagic larvae distributed near the bottom (Ebrahim et al. 2006). These collections were made in October 2004 and April 2005. To examine differences in the sizes and vertical distributions of the two species relative to the tidal activity, discrete-depth horizontal tows were carried out from flood to ebb tides at Stn. R7, located just outside the river mouth, in October 2004 and March 2005.
Diagram of Ariake Bay showing the stations where fish larvae were collected in October 2004 and March and April 2005. Discrete-depth horizontal tows with a larva net and near-bottom net were performed at Stns. R1–R7 (estuary stations); Stn. R7 was sampled for the tidal collections. Open circles (Stns. R8–R10) in the sea indicate stations for oblique tows with a larva net
Towing depths and filtered water volumes (m3) were checked and calculated using a divers watch (Log Memory 1473, Casio) and flow meter (2030R, General Oceanics). For the collection of zooplankton, vertical tows were made with a plankton net (0.225 m mouth diameter, 0.1 mm mesh aperture) at each fish sampling station.
All samples were fixed in 10% seawater formalin, and larvae and juveniles of the two Cynoglossus species were sorted and transferred to 80% ethanol and subsequently measured for size. Their developmental stages were determined according to the degree of migration of the right eye and as per the following: premetamorphosis stage, until the eye begins to migrate; early metamorphosis stage, until the migrating eye is visible from the opposite side; late metamorphosis stage, until the eye completes its migration; postmetamorphosis stage, from the completion of eye migration.
A maximum of 20 specimens of both species were randomly selected for diet analysis from each sampling station. Prey organisms were removed from the larval digestive tract (mouth to rectum) and zooplankton were collected from the waters. All were subsequently classified and enumerated using Chihara and Murano (1997) as a taxonomic guide for most prey, and Wells (1976) as a guide for the copepod genus Pseudobradya.
At each sampling station, water temperature (°C) and salinity (psu) were measured at 0.5 m depth intervals, from surface to bottom, using an STD (AST500-P, Alec Electronics), and turbidity (NTU) was measured at 1 m depth intervals, from surface to bottom, with a water quality checker (WQC-22A, TOA DDK). The current was measured with an ADCP (WHSZ12000-I-UG12, RD Instruments) at 0.5 m depth intervals, from a depth of 1 m to the bottom.
We followed Yamada (2002) in considering C. lighti and C. joyneri as distinct, and used C. lighti. A representative series of specimens used in this study was deposited in the Usa Institute of Marine Biology, Kochi University (UKU-502001001–502001007).
Results
Description of larvae. Identification. Four Cynoglossus species are present in Ariake Bay (Takita 1980). Cynoglossus abbreviatus and C. lighti (Figs. 2, 3) could be identified to species from early to postmetamorphosis stages on the basis of differences in adult meristic characters (counts of dorsal and anal fin rays, and vertebrae) (Yamada 2002). In C. abbreviatus, the identification of premetamorphosis stage larvae (Fig. 2a) was verified by melanophore patterns traced back from late metamorphosis stage larvae (Fig. 2c). In C. lighti, the pigment band was obscure in the premetamorphosis stage (Fig. 3a), but gradually became conspicuous during the early metamorphosis stage (Fig. 3b), and double pigment rows were distributed along the dorsal and ventral margins of the tail from pre- to early metamorphosis stages.
Morphology. Size-specific compositions of the developmental stages are shown in Fig. 4. Metamorphosis started at 10–11 mm body length (BL) in C. abbreviatus, and 4–5 mm BL in C. lighti, and was subsequently completed at 13–14 mm and 5–6 mm BL, respectively. Cynoglossus abbreviatus was predominantly observed in the late metamorphosis stage, whereas C. lighti was mostly observed in the postmetamorphosis stage.
There were 60–62 myomeres in C. abbreviatus and 50–53 myomeres in C. lighti. At all developmental stages, C. abbreviatus larvae were more elongate than C. lighti larvae (Figs. 2, 3). The gut was thick and coiled into a single large loop and protruded markedly from the ventral body margin until premetamorphosis (Figs. 2a, 3a) and early metamorphosis (Figs. 2b, 3b). The gut reached ca. 28% BL in premetamorphosis, and ca. 36% BL in postmetamorphosis in C. abbreviatus (Fig. 2a, d), and ca. 34% and 39%, respectively, in C. lighti (Fig. 3a, d). Metamorphosis size differed between the two species. Eye migration started at ca. 10 and 4 mm BL and finished at ca. 13 and 5 mm BL in C. abbreviatus and C. lighti, respectively (Figs. 2, 3). The dorsal and anal distal pterygiophores of C. abbreviatus and C. lighti appeared in early metamorphosis larvae at ca. 11 and 5 mm BL, respectively (Figs. 2b, 3b). The full complement of these rays was present in late and postmetamorphosis larvae at ca. 12 and 6 mm BL, respectively. A single, elongate, sail-like anterior ray in the dorsal fin was present in C. abbreviatus, and double ones, like filaments, were present in C. lighti. These persisted until late metamorphosis, and then each elongated ray disappeared in both species at the postmetamorphosis stage. In both species the relatively large pectoral fin was retained until late metamorphosis, but completely disappeared in the postmetamorphosis stage. In C. abbreviatus, the pelvic fin with rays appeared in early metamorphosis and remained during postmetamorphosis. The pelvic fin was not present in C. lighti of ca. 5 mm BL in early metamorphosis, but it did appear in the postmetamorphosis stage. In C. abbreviatus, the caudal anlagen began to develop with notochord flexion in ca. 12 mm BL late metamorphosis larvae, and no C. lighti larvae with developing caudal anlagen were collected. Thereafter, in both species, the caudal fin was complete in the postmetamorphosis stage.
Pigmentation. Throughout ontogeny, the rows of melanophores along the dorsal and ventral margins of the trunk and tail were irregularly distributed in C. abbreviatus, but were regularly distributed in C. lighti (Figs. 2, 3). Ventral abdomen melanophores were found in all specimens of both species, but were denser in C. abbreviatus than in C. lighti. Three bands were formed before the postmetamorphosis stage in C. abbreviatus, and only one band by the early metamorphosis stage in C. lighti. Thereafter, in the postmetamorphosis stage, these melanophores became more densely dispersed across the body in C. abbreviatus than in C. lighti. In the postmetamorphosis stages of both species, conspicuous rows of melanophores appeared over the dorsal, pelvic, anal and caudal membranes along the rays. Melanophores were densely distributed on the elongated dorsal ray in C. abbreviatus, but this was not observed in C. lighti.
Distribution of larvae in the bay. General physical condition. Based on vertical profiles of water temperature and salinity, the Rokkaku estuary waters at Stns. R1–R7 were well mixed vertically during the two seasons of this study (Fig. 5). Stable clines toward lower salinities were found from Stn. R10 to Stn. R1 in October, but in April the salinities were markedly lower around the river mouth (Stns. R7, R8). In both seasons, pronounced turbidity fronts were observed around the river mouth, with extremely turbid waters (over 1,200 NTU) occurring in layers deeper than 2 m inside the river mouth. Water temperatures varied little horizontally and vertically during both seasons.
Horizontal and vertical distributions. Cynoglossus abbreviatus larvae were collected mainly in March and April, and C. lighti larvae in October. No C. abbreviatus larvae and a few C. lighti occurred in the sea stations (Stns. R8–R10) (Fig. 6). Larvae aggregated inside (Stns. R5, R6) and just outside (Stn. R7) the river mouth at late metamorphosis to postmetamorphosis in C. abbreviatus, and mostly during the postmetamorphosis stage in C. lighti. Cynoglossus abbreviatus larvae composed mainly of individuals in late metamorphosis occurred as far as the uppermost river station, with their abundance gradually decreasing along the upper reach. In contrast, C. lighti larvae were seldom distributed in the river. Larvae of both species frequently occurred most abundantly near the bottom.
Comparison of the horizontal and vertical distributions of larvae and juveniles of the two Cynoglossus species collected in April and October. S, M and B along the vertical axes denote the surface, middle and near-bottom layers at each station, respectively. NC not collected; otherwise same as in Fig. 4
Tidal changes in the distribution of the larvae. Physical conditions. Strong tidal currents within the estuary drastically changed the vertical structure of the water column (Fig. 7). The current speed was marginally stronger in the surface than in the bottom layers during flood and ebb tides. Turbidities became higher when the current speed was >0.5 kt during both tides in both seasons. Based on water temperature and salinity gradients, the water column showed either strong mixing or weak stratification.
Tidal changes in the vertical distributions of the two species. Cynoglossus abbreviatus larvae were dispersed in the surface and middle layers at flood tide, and were aggregated near the bottom at ebb tide (Fig. 8). Cynoglossus lighti larvae over 10 mm BL were found predominantly near the bottom at both tides, but individuals <10 mm BL were found in the surface and bottom layers at flood tide, and moved mainly to the middle layer at ebb tide.
Feeding habits. Both species of Cynoglossus larvae largely fed on copepods of the genus Pseudobradya, which were present at low densities at all stations (Fig. 9). Conversely, Sinocalanus and its nauplius stages were dominant in the water column, but were never consumed by C. abbreviatus and C. lighti. Larvae of both Cynoglossus species consumed mainly Pseudobradya at Stn. R7 near the river mouth, where the copepod Oithona davisae was most abundant. In both Cynoglossus species, the feeding incidence was over 75% at all locations, and the number of feeding events per individual fluctuated irrespective of location.
We found ontogenetic changes in feeding preference. Oithona davisae were the main food for pre- and early metamorphic larvae of both Cynoglossus species, but they then shifted to Pseudobradya during later stages (Fig. 10). Feeding incidence and number of feeding events per individual increased with development in the two species.
Discussion
Although Menon (1977) suggested that Cynoglossus lighti was the synonym of C. joyneri, our C. lighti larvae were very different from C. joyneri larvae (see Minami 1983). Sizes at initiation and completion of metamorphosis are considerably smaller in the former than in the latter, and the row of melanophores is absent along the lateral midline of the tail in the former but is markedly present in the latter throughout ontogeny. Size at metamorphosis was negatively correlated with water temperature in Paralichthys olivaceus (see Seikai et al. 1986). Nevertheless, Minami’s (1983) study on C. joyneri larvae was conducted in Wakasa Bay, facing the Japan Sea, mainly during July and August when water temperatures (Munekiyo and Kuwahara 1977) are higher rather than those of Ariake Bay in October, so we believe that these morphological differences most likely reveal interspecies differentiation rather than phenotypic modification. Accordingly, C. lighti should be considered a more restricted species than C. abbreviatus to Ariake Bay in Japan.
Cynoglossus abbreviatus and C. lighti larvae bear an elongated dorsal ray. The dorsal ray is sail-like in C. abbreviatus and filament-like in C. lighti (Figs. 2, 3). The sail-like elongated dorsal ray is found in larvae of C. robustus (see Fujita and Uchida 1957) and the filament-like dorsal ray in the larvae of species in two genera: C. joyneri (see Minami 1983) and Paraplagusia japonica (see Minami 1982). The most notable difference between the two genera in adult specimens is the existence of fringed lips, which are present in the genus Paraplagusia and absent in the genus Cynoglossus (see Weber and Beaufort 1929). Accordingly, the characteristic of an elongated dorsal ray is not necessarily common to each of the two genera.
The two Cynoglossus fishes spawn in the central open area of Ariake Bay (Takita 1980; Hayashi et al. 2000; Koshiishi et al. 2001). Cynoglossus abbreviatus and C. lighti larvae were distributed mainly inside and just outside the river mouth, respectively, with few occurring in the open area of the bay (Fig. 6). This indicates that both Cynoglossus larvae aggregate in the estuary, using it as a nursery ground. However, their aggregated developmental stages differed between the two species; i.e., chiefly late metamorphosis stage in C. abbreviatus, vs. postmetamorphosis stage in C. lighti (Fig. 4). This difference in development probably reflects a difference between the two species in their transport distance and the time of pelagic dispersal from their spawning ground. However, immature and adult C. lighti are abundant around inter- and subtidal areas, whereas very few C. abbreviatus were observed in these habitats (Takita et al. 2003; Aoyama et al. 2007). Further research into spawning time and larval duration and dispersal are needed in order to understand the reason for this difference between the two species.
Cynoglossus abbreviatus larvae were distributed from the river mouth to the upper reaches of the river, but most C. lighti larvae remained just outside the river mouth, regardless of their developmental stage (Fig. 6). A similar pattern has also been observed in settled larvae and juveniles of the closely related species Paralichthys lethostigma and P. dentatus in a North Carolina inlet (Burke et al. 1991), where settlement patterns can be related to tidal shifts and their corresponding changes in vertical distribution. In this study, C. abbreviatus larvae were mainly distributed at surface and middle layer depths at flood tide, and aggregated near the bottom at ebb tide, where the current speed was lowest. On the other hand, more C. lighti larvae occurred near the bottom during both tides (Fig. 8). The extension to the upper reach in C. abbreviatus and the distribution just outside the river mouth in C. lighti can probably be explained by the process of selective tidal stream transport (Jager 1999; Forward et al. 2001). So why does C. abbreviatus extend its distribution upstream, while C. lighti remains at the river mouth? Both Cynoglossus larvae depend on Pseudobradya, which was scarce at all stations (Fig. 9). This result indicates that the two Cynoglossus larvae selectively fed on this copepod. However, the conscious differences in horizontal distribution cannot be attributed to food demands. Lastly, there is no doubt that the turbid estuary area is an important nursery ground for C. abbreviatus and C. lighti in Ariake Bay.
References
Aoyama D, Kinoshita I, Fujita S (2007) The function of the inner estuary as nursery grounds for fishes in Ariake Bay. Aquabiology 29:16–25
Burke JS, Miller JM, Hoss DE (1991) Immigration and settlement pattern of Paralichthys dentatus and P. lethostigma in an estuarine nursery ground, North Carolina, U.S.A. Neth J Sea Res 27:393–405
Chihara M, Murano M (1997) An illustrated guide to marine plankton in Japan. Tokai University, Tokyo
Ebrahim A, Kinoshita I, Sashida M, Hashimoto T, Nunobe J (2006) Do the ayu (Plecoglossus altivelis altivelis) born in the river with an inlet or large estuary in its mouth perform a homing? La Mer 44:145–155
Forward RB Jr, Tankersley RA, Reinsel KA (2001) Selective tidal-stream transport of marine animals. Oceanogr Mar Biol Ann Rev 39:305–353
Fujita S, Uchida K (1957) On the development of the egg and prelarval stages of a sole, Cynoglossus robustus Günther. Sci Bull Fac Agric Kyushu Univ 16:319–323
Fujita S, Kitajima C, Hayashida G (1986) Induction of ovarian maturation and development of eggs, larvae and juveniles of the tonguefish, Cynoglossus abbreviatus, reared in the laboratory. Jpn J Ichthyol 33:304–315
Hayashi M, Ishida Y, Ueda T (2000) The spawning season of the tonguefish, Cynoglossus abbreviatus, in the Ariake Sea. Bull Fukuoka Fish Mar Technol Res Cent 10:19–22
Jager Z (1999) Selective tidal stream transport of flounder larvae (Platichthys flesus L.) in the Dollard (Ems estuary). Estuar Coast Shelf Sci 49:347–362
Koshiishi Y, Ohsaka Y, Hayashi M, Sano M, Murai T (2001) Distribution and growth of 0-age-group tonguefish (Cynoglossus lighti) in northeast Ariake Sound. Bull Fish Res Agency 1:23–34
Kuipers N (1975) On the efficiency of a two-metre beam trawl for juvenile place (Pleuronectes platessa). Neth J Sea Res 9:69–85
Menon AGK (1977) A systematic monograph of the tongue soles of the genus Cynoglossus Hamilton-Bchanan (Pisces: Cynoglossidae). Smithon Contrib Zool 238:i–vi + 1–129, pls 1–21
Minami T (1982) The early life history of a tongue fish Paraplagusia japonica. Bull Jpn Soc Sci Fish 48:1041–1046
Minami T (1983) The early life history of a tongue fish Cynoglossus joyneri. Bull Jpn Soc Sci Fish 49:719–724
Munekiyo M, Kuwahara A (1977) Eggs and larvae distribution of ribbon fish in western Wakasa Bay. Bull Jpn Soc Sci Fish 52:805–810
Seikai T, Tanangona JB, Tanaka M (1986) Temperature influence on larval growth and metamorphosis of the Japanese flounder, Paralichthys olivaceus in the laboratory. Bull Jpn Soc Sci Fish 52:977–982
Takita T (1980) Fish in Ariake Bay. Mar Sci Month 124:105–115
Takita T, Komura D, Kawahara I, Mori Y, Nakashima N, Ito S (2003) Distribution of fishes in the innermost area of Ariake Sound. Bull Saga Pref Ariake Fish Res Dev Cent 21:81–98
Weber M, Beaufort LF (1929) The fishes of Indo-Australian archipelago. V, Anacanthini, Allotriognathi, Heterosomata, Berycomorphi, Percomorphi: Families: Kuhliidae, Apogonidae, Plesiopidae, Pseudoplesiopidae, Priacanthidae, Centropomidae. EJ Brill, Leiden
Wells JBJ (1976) Keys to aid in the identification of marine harpacticoid copepods. University of Aberdeen, Aberdeen
Yamada U (2002) Cynoglossidae. In: Nakabo T (ed) Fishes of Japan with pictorial keys to the species, English edition. Tokai University, Tokyo, pp 1388–1392
Acknowledgments
We are grateful to T. Noguchi, K. Kuno and Y. Kawamura, Saga Prefectural Government, for their assistance with this study. Thanks are due to H. Katafuchi, A. Ebrahim and S. Kimata, who helped in our field investigations. We wish to acknowledge T.W. Miller and M. Wood for correcting the English of the manuscript. This work was partially supported by the Ministry of Education, Science and Culture, Japan (no. 20580203).
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s10228-009-0127-9
About this article
Cite this article
Yagi, Y., Kinoshita, I., Fujita, S. et al. Comparison of the early life histories of two Cynoglossus species in the inner estuary of Ariake Bay, Japan. Ichthyol Res 56, 363–371 (2009). https://doi.org/10.1007/s10228-009-0109-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-009-0109-y