Abstract
Female sexual strategies affect male strategies and can play an important role in shaping mating systems. We investigated female sexual behaviour within five groups of grey-cheeked mangabeys in Kibale National Park, Uganda, and tested the hypothesis that females exhibit mate choice using as indications the prevalence of (1) females soliciting matings by presenting to males and (2) females refusing to mate with approaching males. In addition, we describe how these behaviours as well as grooming and copulation calls are distributed over high-ranking, low-ranking and migrating males and discuss these patterns with regard to trade-offs that could play a roll in female mate choice in multi-male groups. Females were promiscuous and initiated almost half of the matings, with both resident and migrating males. More than half of male mating approaches were refused by peak females. Female mate choice in this species may depend on individual female preferences, oestrus phase and male tactic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Female mate choice is one of the main processes of sexual selection (Darwin 1859). Despite the fact that for humans (Buss 1994) and a wide variety of animals (Andersson 1994) the importance of female mate preferences and mate choice has been widely accepted, for non-human primates, evidence for female mate preferences remains largely anecdotal (Paul 2002). Gowaty (1997) postulated that this is a consequence of highly variable and often antagonistic sexual tactics, and therefore, in non-human primates, female mate choice can be constrained by female–female competition, male–male contests, reproductive suppression, male choice and male coercion. Thus, female preferences will remain speculative, but behavioural studies can produce data on female choice (Manson 1992).
There is evidence for female mate choice in non-human primates (Keddy-Hector 1992; Paul 2002; Pusey and Wolf 1996; Small 1989; Smuts 1987). However, in species where females stay in multi-male groups and females can mate with several males (as in grey-cheeked mangabeys), female choice can be especially important, but also cryptic (Smuts and Smuts 1993), due to male behaviour or female–female competition (Bercovitch 1991; Manson 1992; Soltis et al. 2001). In Papionines, there are several examples of active involvement of females in the mating process such as following, presentation and grooming between non-consort females and males, participation in ‘sneak’ matings with subordinate males (Nunn 1999; Smuts 1983) and female competition for mating partners (Bercovitch 1995).
Although mate preference criteria of females are usually unknown, skewed female mate choice has been demonstrated in some non-human primate species living in multi-male groups. Females may choose to mate with high-ranking males (Dixon et al. 1993); Macaca mulatta: (Bercovitch and Nurnberg 1997); Papio cynocephalus: (Altmann et al. 1996); Cercocebus torquatus atys: (Gust et al. 1998) or mid-ranking males [Macaca mulatta: (Manson 1992, 1994); Macaca fuscata: (Huffman 1991; Soltis et al. 2001)]. In baboons, females mate with multiple males but may try to develop a special relationship with at least one male (Smuts 1985), who also forms an affiliative bond with her offspring (Bercovitch 1991; Palombit et al. 1997). However, that relationship rarely has a positive influence on male mating success (Bercovitch 1991; Huffman 1991; Manson 1994). In contrast to these affiliative bonds, females may show preferences towards newcomer males (Bercovitch 1997; Small 1989). Several paternity studies provided evidence that such female behaviour towards newcomers can have a positive impact on the reproductive success of migrating males (Berard et al. 1994; Fietz et al. 2000; Gagneux et al. 1997; Launhardt et al. 2001).
One means whereby females can manipulate male sexual behaviour is signalling fertility. Sexual swellings are common signals among female primates that live in multi-male groups (Clutton-Brock and Harvey 1976). The function of exaggerated swelling is controversial and may vary among species; it may be a reliable indicator of timing of ovulation (Nunn 1999), or female quality (Pagel 1994), or it may be a within-female indicator of conception across cycles (Emery and Whitten 2003; Zinner et al. 2002). The timing of exaggerated sexual swellings in females is not always synchronised with ovulation (Dixon 1998; Nunn 1999), and therefore, females may have a large time window to exhibit sexual behaviour that does not lead to fertilisation, increasing the opportunity for female manipulation of male behaviours (Pagel 1994; van Schaik et al. 2000). The presence of sexual swellings can promote male competition which often concentrates paternity in one, dominant male (the best male hypothesis; Clutton-Brock and Harvey 1976). On the other hand, sexual swellings can confuse paternity among males to reduce aggression towards infants, when females mate with many males during the periods with sexual swellings (the many males hypothesis; Hrdy and Whitten 1987). During their ovarian cycle, females can also employ both strategies, depending on fertility (the graded signal hypothesis; Nunn 1999).
Post-copulation calls can be indicators of female choice although their ultimate function remains often a matter of speculation (O’Connell and Cowlishaw 1994). At least 15 different hypotheses have been proposed to explain the function of female copulation calls in primates (Maestripieri and Roney 2005). For example, (a) female copulation calls may be associated with orgasm [Japanese macaques: (Troisi and Carosi 1998)], (b) females call to synchronise orgasm with the male (Hamilton and Arrowood 1978) and therefore promote the sperm transfer to aid conception (Fox and Fox 1971), (c) female copulation calls can incite male sperm competition to ensure that sons will inherit the best sperm [‘sexy sons’ hypothesis; (O’Connell and Cowlishaw 1994] and (d) female copulation calls may also have a function of announcing paternity certainty to promote paternal investment (Henzi 1996).
Grey-cheeked mangabeys live in multi-male groups, and males often migrate between groups, while females tend to stay in their natal groups. Females exhibit sexual swellings that cover a relatively small area ventral to the base of the tail (Chalmers and Rowell 1971; Danjou 1972; Rowell and Chalmers 1970; Wallis 1983). Females with sexual swellings are present throughout the year, and matings are recorded only during the oestrus period (Wallis 1983). Females usually copulate with more than one male (Chalmers 1968; Wallis 1979). High-ranking males were about five times more aggressive towards females than migrating males, and low-ranking males behave almost three times more aggressively towards females than migrating males. However, high-ranking males are seven times less aggressive towards juveniles than migrating males, and in comparison to migrating males, low-ranking males are 14 times less aggressive towards juveniles (Arlet et al., in preparation). Dr. William Olupot (personal communication) observed two females carrying dead infants shortly after new males entered the group, which suggests that migrating male aggression towards infants can be lethal. Males can also care for offspring (Arlet et al., in preparation; Struhsaker and Leyland 1979; Wallis 1979).
We investigated female sexual behaviour within five groups of grey-cheeked mangabeys in Kibale National Park, Uganda, and tested the hypothesis that females exhibit mate choice using as indications the prevalence of (1) females soliciting matings by presenting to males and (2) females refusing to mate with approaching males. In addition, we describe how these behaviours as well as grooming and copulation calls are distributed over high-ranking, low-ranking and migrating males and discuss these patterns with regard to trade-offs that can be expected to play a roll in female mate choice in multi-female–multi-male groups. While much is known about female sexual behaviour in the closely related baboons, arboreal mangabeys can provide an interesting comparison because habitat can be an important factor in mating systems.
Materials and methods
Study site and subjects
After a pilot study in 1999, data were collected for 6 months in 2001 (January–June) and supplemented with observations in 2002 (April–June) in Kibale National Park (0°13′–0°41′N and 30°19′–30°32E). Kibale (795 km2) is a moist, evergreen medium altitude forest with a mosaic of swamp, grassland, thicket and colonizing forest (Chapman and Lambert 2000). Mangabeys in Kibale live in multi-male groups of on average 14 individuals (Olupot 1999; Wallis 1979; Waser 1977). In total, we observed 26 sub-adult and adult males and 35 females.
Fourteen males were recognised by the unique colour combination of their collars or attached radios placed on them in an earlier study (Olupot 1999). Males without a collar were classified as adult male (AM, N = 7) or sub-adult male (SAM, N = 5) and were recognisable on the basis of their relative sizes and other distinguishing features. In each of the groups, there were at least two individual males present in the same group between January–June 2001. These males were regarded as resident males (N = 16). Males that emigrated at least once from a group or that immigrated into a group and then dispersed during these 6 months were called migrating males (N = 10). This excluded visiting males that were sighted only briefly (<2 days) in groups.
Females with sexual swellings were present throughout the year. The swelling increases in size and colour gradually, deepening to pink (oestrous adult female inflating: OAFi) until the maximum stage of swelling is reached (oestrous adult female peak: OAFp). As oestrus passes, the swelling becomes less turgid and the colour changes to dark purple (oestrous adult female deflating: OAFd; Danjou 1972; Deputte 1991; Wallis 1983). The duration of sexual swellings average 17–31 days, with the phase from quiescence to peak swelling being 4–14 days long, the peak size of sexual swelling lasting 2–4 days and the deflating stage lasting 7–14 days (Danjou 1972; Deputte 1991; Rowell and Chalmers 1970; Wallis 1983).
Observation methods
From January 2001 to June 2001, two observers collected behavioural observations for 8–9 h/day, for 6 consecutive days per week, for a total of 2,036 h. To measure the rate of movement of males between groups, we censused the focal group every day during each observation period. Dominance interactions among males were recorded on the basis of approach–retreat interactions following de Waal (1987), which were scored for all nearby males during focal follows of oestrus females (Olupot and Waser 2001). Dominance relationships are ordinal within a group, and therefore, we simplified this classification to compare males across groups; the highest-ranking male was classified as high ranking and others as low ranking. In two groups, the ranks of the two highest-ranking males could not be distinguished and both males were classified as high ranking. Mating was defined as a mount involving intromission (Wallis 1983), and mating success of a male was measured as the number of observed matings.
We predominantly followed females with sexual swellings. Focal females were classified as ‘adult female’—without sexual swelling (AF), or female with sexual swelling—‘inflating female’ (OAFi), ‘peak female’ (OAFp) and ‘deflating female’ (OAFd). The behaviours of focal females were recorded all day, divided into 30-min sampling blocks with no more then 5 min between blocks. During these all-day follows we recorded female behaviour. During focal sampling of females with sexual swellings, we recorded all occurrences of interactions with males and other group members within 10 m of the focal female. We focussed on the following behaviours which have been used to study female mate choice in the socially similar baboons (Bercovitch 1995): (1) the direction and frequency of grooming during the oestrous period, (2) the direction of sexual presentation by females, (3) sexual refusals, (4) subsequent successful matings and (5) the presence of female copulation calls.
Data analysis
To compare the behaviour of different classes of females with sexual swellings and males, the observations were divided by the number of days the interaction could have occurred during the 6-month period (when a particular male and female were observed together in a group). For the grooming data, we excluded males that were guarding females. In our analysis, each day represented one data point for females and for males. These standardised values were then used for statistical comparisons and graphical representations. To simultaneously explore multiple factors and their interactions we used an Analysis of Multi-Factor Regression (GLM) approach on the number of events (such as sexual presentations, matings, copulation calls, mating refusals and grooming) per the number of days an interaction could have occurred, thus using a single data point for each male. The analyses were performed using Statgraphics 5.0. (two-tailed probability with alpha at 0.05).
Results
Sexual presentations
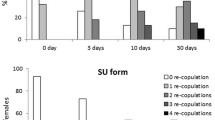
Grey-cheeked mangabey females mated only when they had a sexual swelling. Forty-eight percent of sexual presentations by females led to matings (N = 63 sexual presentations of 26 females). Most of the sexual presentations that resulted in mating were performed by females with peak swellings (66%), then inflating females (18%) and deflating (16%; GLM analysis: p = 0.01, df = 2, F = 4.84, R 2 = 32.67%).
There was no difference between male categories in receiving sexual presentations, but peak females presented mainly to high-ranking males, and deflating females to migrating males (GLM analysis: R 2 = 32.67%, male category p = 0.1 df = 2 F = 2.29, male category × female category p = 0.002, df = 4, F = 4.85; Fig. 1)
Mating
Fifty-three percent of matings were initiated by females (N = 56 matings). Females mated with between one and three different partners (N = 28 oestrus females) during one cycle. Usually, one of the partners was a high-ranking male who also followed the females during this period, and in addition, she mated with one of the low-ranking or migrating males. During days with maximum swelling, females mated with two to three males, but during the deflating stage, females mated with one to two males only. Some matings with migrating males occurred outside the group (sneaky copulations). Most matings were recorded with high-ranking males (GLM analysis: p = 0.0002, df = 8, F = 9.67, R 2 = 60.5%). Peak females mated mainly with high-ranking males but also migrating males and low-ranking males (GLM analysis: p = 0.00001, df = 4, F = 7.86, R 2 = 60.5%; Fig. 2).
Copulation calls
We recorded 25 matings that were accompanied by a female call (grunt), which represents 46% of all recorded matings. The presence of female copulation calls depended on male rank (GLM: F = 8.72, df = 25, R 2 = 0.66, p = 0.0003); most female copulation calls occurred during matings with high-ranking males, except for matings with one migrating male, who elicited many copulation calls.
Mating refusals
One third of all mating approaches by males were refused (N = 87 approaches), and 59% was refused by peak females. High-ranking males approached more and received more refusals than other males (Poisson Regression, p = 0.0005), hence there was no difference between the male categories in proportion of approaches refused.
Grooming
For this analysis we used data of males that were not guarding females. Receptive females groomed males more than non-oestrus females (GLM: F = 8.09, df = 1, R 2 = 57%, p = 0.007). Most of the grooming by oestrus females was observed with high-ranking males (GLM: F = 8.19, df = 1, R 2 = 57%, p = 0.0001).
Discussion
This study revealed that females actively participate in mate choice; females are not just accepting mating with eager males but refuse as well as actively approach and solicit copulations by males. The observed behaviour can be seen as the range of solutions for the female’s dilemma—on one hand, she could choose to mate the best male, capable to protect her and her offspring, but she may also mate with many males to avoid inbreeding or reduce aggression or risk of infanticide (van Schaik et al. 2000). The evolution of prolonged follicular phases and unpredictable ovulations (van Schaik et al. 2000) as well as sexual swelling that advertises female reproductive state (Domb and Pagel 2001; Nunn 1999) and also copulation calls (Semple and McComb 2000) may give females opportunity to express their preferences within the social context.
In grey-cheeked mangabeys, 48% of the observed sexual presentations initiated by females resulted in matings. On average, resident and migrating males received similar presentation rates from females but females at the peak of sexual swelling presented mainly to high-ranking males and deflating females to migrating males. Oestrus females were also more active in grooming males than non-oestrus females, with preferences toward high-ranking males and then towards migrating males. Females tended to mate with more males during the peak of sexual swelling than during the deflating stage.
Mangabey females at the peak of sexual swelling refuse almost 60% of male mating approaches. High-ranking males are the ones who approach the most and receive more refusals than other males. That the proportion of mating refusals received by the male categories did not differ significantly conceals that particular females did refuse to mate more or less with particular males or male classes. The high incidence of refusals and variation among females indicates that they are not mating with any approaching male but are choosy. Moreover, mangabey females initiated more than half of the observed copulations and mated with more than one partner. This result corroborates the findings of Wallis (1983), where 42% of copulations were initiated by females. In many social species, females show physiological and behavioural adaptations that lead to copulation with more males and over a longer period than is necessary to ensure fertilisation (Smuts 1985). It seems that, similar to rhesus macaques (Manson 1992, 1994), mangabey females can mate with males of various ranks, but in some groups, they can be monopolised by high-ranking males which are able to prevent them from mating with other males as high-ranking males often attacked females that mated with other males.
That females mainly gave copulation calls when mating high-ranking males can be explained in two different ways. Firstly, it is possible that females of mangabeys give copulation calls to promote paternity of these mails by advertising their interest in mating with high-ranking males or to synchronise orgasm with the preferred male (Hamilton and Arrowood 1978) and, therefore, promote the sperm transfer up the reproductive track which aids conception (Fox and Fox 1971). Secondly, when a high-ranking male was in the proximity of a female mating with a migrating male or low-ranking male, he would attack them, and this risk may, therefore, explain why females only rarely gave copulation calls when mating these males.
Resident males are more aggressive to females than migrating males who, at the other hand, attack juveniles more often, which could probably lead to infanticide (Dr. W. Olupot, personal communication). Therefore, sexual interaction with more than one male may reduce aggression from migrating males to her juveniles, as a result of confused paternity (van Noordwijk and van Schaik 2000), but may increase aggression from high-ranking males.
It is also interesting that mainly high-ranking males provided infant care, and those males were most successful at obtaining matings. Similarly, female baboons may preferentially mate with males who invest most in parental care (Bercovitch 1991). It is possible that caretakers are more likely to be fathers based on their general mating success (as high-ranking males) and, although females mate with many partners, males may even recognise their own infants (Buchan et al. 2003), and thus this care could be regarded as paternal investment in offspring rather than a tactic to obtain more matings.
In mangabeys in Kibale, mate choice was mainly indicated by more than half of the matings being initiated by females and almost 60% of male approaches being refused by peak females. Female sexual behaviour depended on oestrus phase (number of partners, proportion of male categories presented to and mated with) and male tactic in the group.
Females mated mainly with high-ranking males, and that can be explained by monopolisation by these males (mate guarding), their aggression towards females, female preference based on ‘the best male’ or infant care. However, females also mated with migrating males and lower ranking males despite efforts of the high-ranking male to monopolise matings. This can be explained by attractiveness towards novel males (possibly to avoid inbreeding) or as a tactic to avoid their aggression towards juveniles. Female sexual behaviour in grey-cheeked mangabeys changes with oestrus phase and may therefore serve different functions at different times of the female reproductive cycle. These results indicate that female choice has an important role in shaping mating systems in multi-male and multi-female groups.
References
Altmann J, Alberts SC, Haines SA, Dubach J, Muruthi P, Coote T, Geffen E, Cheesman DJ, Mututua RS, Saiyalel SN, Wayne RK, Lacy RC, Bruford MW (1996) Behavior predicts genetic structure in a wild primate group. Proc Natl Acad Sci U S A 93:5797–5801
Andersson M (1994) Sexual selection. Princeton University Press, Princeton, NJ
Berard JD, Nurnberg P, Epplen JT, Schmidtke J (1994) Alternative reproductive tactics and reproductive success in male rhesus macaques. Behaviour 129:177–201
Bercovitch FB (1991) Mate selection, consortship formation and reproductive tactics in adult savanna baboons. Primates 32:437–452
Bercovitch FB (1995) Female cooperation, consortship maintenance, and male mating success in savanna baboons. anim behav 50:137–149
Bercovitch FB (1997) Reproductive strategies of rhesus macaques. Primates 38:247–263
Bercovitch FB, Nurnberg P (1997) Genetic determination of paternity and variation in male reproductive success in two populations of rhesus macaques. Electrophoresis 18:1701–1705
Buchan JC, Alberts SC, Silk JB, Altman J (2003) True paternal care in a multi-male primate society. Nature 425:179–181
Buss D (1994) The evolution of desire. Strategies of human mating. Basic Books, New York
Chalmers NR (1968) The social behavior of free living mangabeys in Uganda. Folia Primatol 8:263–281
Chalmers NR, Rowell TE (1971) Behavior and female reproductive cycles in a captive group of mangabeys. Folia Primatol 14:1–14
Chapman CA, Lambert JE (2000) Habitat alteration and the conservation of African primates: a case study of Kibale National Park, Uganda. Am J Primatol 50:169–186
Clutton-Brock TH, Harvey PH (1976) Evolutionary rules and primate societies. In: Bateson PPG, Hinde RA (eds) Growing points in ethology. Cambridge University Press, Cambridge, pp 195–237
Danjou MC (1972) Contribution a l’Etude du Comportement Sexuel de Cercocebus albigena. Unpublished thesis, Universite de Rennes, France
Darwin C (1859) On the origin of species by means of natural selection. Murray, London
De Waal FBM (1987) Dynamics of social relationships. In: Hinde RA (ed) Primate social relationships. Blackwell, Oxford, pp 112–116
Deputte B (1991) Reproductive parameters of captive grey-cheeked mangabeys. Folia Primatol 57:57–69
Dixon AF (1998) Primate sexuality. Comparative studies of the prosimians, monkeys, apes and human beings. Oxford University Press, Oxford
Dixon AF, Bossi T, Wickings EJ (1993) Male dominance and genetically determined reproductive success in the mandrill. Primates 34:525–532
Domb LG, Pagel M (2001) Sexual swellings advertise female quality in baboons. Nature 410:204–206
Emery MA, Whitten PL (2003) Size of sexual swellings reflects ovarian function in chimpanzees (Pan troglodytes). Behav Ecol Sociobiol 54:340–351
Fietz J, Zischler H, Schwiegk C, Tomiuk J, Dausmann KH, Ganzhorn JU (2000) High rates of extra-pair young in the pair-living fat-tailed dwarf lemur, Cheirogaleus medius. Behav Ecol Sociobiol 49:8–17
Fox CA, Fox B (1971) A comparative study of coital physiology with special reference to the sexual climax. J Reprod Fertil 24:319–336
Gagneux P, Woodruff DS, Boesch C (1997) Furtive mating in female chimpanzees. Nature 387:358–359
Gowaty PA (1997) Sexual dialectics, sexual selection and variation in mating behavior. In: Gowaty PA (ed) Feminism and evolutionary biology: boundaries, intersections, and frontiers. Chapman and Hall, New York, pp 351–384
Gust DA, McCaster T, Gordon, TP, Gergits W, Casna N, McLure HM (1998) Paternity in sooty mangabeys. Int J Primatol 19:83–94
Hamilton WJ III, Arrowood PC (1978) Copulation vocalisations of chacma baboons (Papio ursinus), gibbons (Hylobates hoolock) and humans. Science 200:1405–1409
Henzi SP (1996) Copulation calls and paternity in chacma baboons. Anim Behav 51:233–234
Hrdy SB, Whitten PL (1987) Patterning of sexual activity. In: Smuts BB, Cheney DL Seyfarth RM, Wrangham RW, (eds). Primate societies. Chicago University Press, Chicago, pp 370–384
Huffman MA (1991) Mate selection and partner preferences in female Japanese macaques. In: Fedigan LM, Asquith PJ (eds) The monkeys of Arashiyama. thirty-five years of research in Japan and in the West. SUNY, New York, pp 101–116
Keddy-Hector AC (1992) Mate choice in non-human primates. Am Zool 32:62–70
Launhardt K, Borries C, Hardt C, Epplen JT, Winkler P (2001) Paternity analysis of alternative male reproductive routes among the langurs (Semnpithecus entellus) of Ramnagar. Anim Behav 61:53–64
Maestripieri D, Roney JR (2005) Primate copulation calls and postcopulatory female choice. Behav Ecol 16:106–113
Manson JH (1992) Measuring female mate choice in Cajo Santiago rhesus macaques. Anim Behav 44:405–416
Manson JH (1994) Male aggression: a cost of female mate choice in Cajo Santiago rhesus macaques. Anim Behav 48:473–475
Nunn CL (1999) The evolution of exaggerated sexual swellings in primates and the graded-signal hypothesis. Anim Behav 58:229–246
O’Connell SM, Cowlishaw G (1994) Infanticide avoidance, sperm competition and mate choice: the function of copulation calls in female baboons. Anim Behav 48:687–694
Olupot W (1999) Mangabey dispersal and conservation in Kibale Forest National Park, Uganda. Ph.D. thesis, Purdue University, USA
Olupot W, Waser, PM (2001) Correlates of intra-group transfer in male grey-cheeked mangabeys. Int J Primatol 22:169–187
Pagel M (1994) The evolution of conspicuous oestrus advertisement in Old World monkeys. Anim Behav 47:1333–1341
Palombit RA, Seyfarth RM, Cheney DL (1997) The adaptive value of ‘friendships’ to female baboons: experimental and observational evidence. Anim Behav 54:599–614
Paul A (2002) Sexual selection and mate choice. Int J Primatol 23:877–904
Pusey A, Wolf M (1996) Inbreeding avoidance in animals. TREE 11:201–206
Rowell TE, Chalmers NR (1970) Reproductive cycles of the mangabey Cercocebus albigena. Folia Primatol 12:264–272
Semple S, McComb K (2000) Perception of female reproductive stale from vocal cues in a mammal species. Proc R Soc Lond Ser Biol Scie 267(1444):707–712
Small MF (1989) Female choice in nonhuman primates. Yearb Phys Anthropol 32:103–127
Smuts BB (1983) Dynamics of ‘special relationships’ between adult male and female olive baboons. In: Hinde RA (ed) Primate social relationships. Blackwell, Oxford, pp 112–116
Smuts BB (1985) Sex and friendship in baboons. Aldine, New York
Smuts BB (1987) Sexual competition and mate choice. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT (eds) Primate societies. University of Chicago Press, Chicago, pp 385–399
Smuts BB, Smuts R (1993) Male aggression and sexual coercion of females in nonhuman primates and other mammals: evidence and theoretical implications. Adv Study Behav 22:1–63
Soltis J, Thomsen R, Takenaka O (2001) The interaction of male and female reproductive strategies and paternity in wild Japanese macaques, Macaca fuscata. Anim Behav 62:485–494
Struhsaker TT, Leyland L (1979) Socioecology of five sympatric monkey species in the Kibale Forest, Uganda. Adv Study Behav 9:159–228
Troisi A, Carosi M (1998) Female orgasm rate increases with male dominance in Japanese macaques. Anim Behav 56:1261–1266
Van Noordwijk MA, van Schaik CP (2000) Reproductive patterns in eutherian mammals: Adaptations against infanticide? In: Van Schaik CP, Janson CH (eds) Infanticide by males and its consequences. Cambridge University Press, Cambridge, pp 332–360
Van Schaik CP, Hodges JK, Nunn CL (2000) Paternity confusion and the ovarian cycles of female primates. In: Van Schaik CP, Janson CH (eds) Infanticide by males and its consequences. Cambridge University Press, Cambridge, pp 361–387
Wallis SJ (1979) The sociology of Cercocebus albigena johnstonii (Lydekker). An arboreal, rain forest monkey. Ph.D. thesis, University of London, London
Wallis SJ (1983) Sexual behavior and reproduction of Cercocebus albigena johnstoni in Kibale Forest, western Uganda. Int J Primatol 4:153–166
Waser PM (1977) Feeding, ranging and group size in the mangabey Cercocebus albigena johnstoni in Kibale Forest, Western Uganda. Int J Primatol 4:153–166
Zinner D, Alberts SC, Nunn CL, Altmann J (2002) Significance of primate sexual swelling. Nature 420:142
Acknowledgements
We thank Prof Lynne Isbell and Dr Mark Grote for their valuable comments on the manuscript and statistical advices. We are grateful to William Olupot for allowing us to build on his work and use his telemetry system. We thank the Uganda National Council of Science and Technology, Uganda Wildlife Authority and Makerere University Biological Field Station for permission to conduct the research. Many thanks to John Rusoke, Richard Kaserengenyu, Clovis Kaganzi and Michał Ścinski for their invaluable help in the field. The research was made possible by financial support from the Polish Committee of Science, KBN Grant 6 PO4C 014 19 PB1125/PO4/2000/19 to Prof. K. Kaczanowski. The research complies with the current laws of Uganda.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R.F. Oliveira
Rights and permissions
About this article
Cite this article
Arlet, M.E., Molleman, F. & Chapman, C. Indications for female mate choice in grey-cheeked mangabeys Lophocebus albigena johnstoni in Kibale National Park, Uganda. acta ethol 10, 89–95 (2007). https://doi.org/10.1007/s10211-007-0034-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10211-007-0034-x