Abstract
Objective
To estimate the social/economic costs of fragile X syndrome (FXS) in Europe and to assess the health-related quality of life (HRQOL) of patients and caregivers.
Methods
A cross-sectional study was conducted in a sample of European countries. Patients were recruited through patients’ associations. Data on their resource use and absence from the labour market were retrospectively obtained from an online questionnaire. Costs were estimated by a bottom-up approach and the EuroQol-5 Domain (EQ-5D) questionnaire was used to measure patients’ and caregivers’ HRQOL.
Results
Five countries were included in the analysis. The mean annual cost of FXS per patient varied from €4951 in Hungary to €58,862 in Sweden. Direct non-healthcare costs represented the majority of costs in all countries but there were differences in the share incurred by formal and informal care among those costs. Costs were also shown to differ between children and adults. Mean EQ-5D utility score for adult patients varied from 0.52 in France (n = 42) to 0.73 in Hungary (n = 2), while for caregivers this score was consistently inferior to 0.87.
Conclusion
Our findings underline that, although its prevalence is low, FXS is costly from a societal perspective. They support the development of tailored policies to reduce the consequences of FXS on both patients and their relatives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fragile X syndrome (FXS) is the leading cause of inherited intellectual disability and results from mutations on the fragile mental retardation 1 (FMR1) gene of the X chromosome [1]. These mutations are associated with reduced levels of the FMR protein, triggering manifestations that vary widely from patient to patient [2] but often result in intellectual disability, as well as cognitive and behavioural impairments and, in particular, autism spectrum disorder for which FXS is the leading monogenic cause [3]. In addition, individuals affected by FXS often display identifiable features with a long narrow face, prominent ears and high-arched palate [4]. This disease affects males more often and more severely than females, with an estimated incidence of 1 in 5000 males and 1 in 9000 females [5]. In Europe, the overall prevalence of the disease is estimated at 20 per 100,000 inhabitants [6], with a prevalence reaching 22.6 per 100,000 habitants in the UK [7].
Despite the low incidence of FXS, previous research suggests that the societal impact of rare diseases deserves wider attention [8] and that FXS has significant consequences for both patients and caregivers. It is indeed estimated that only 10 % of adult male patients live on their own [9] and that one in three caregivers of FXS patients visit a health professional for anxiety and depressive symptoms each year. Moreover, 79 % of families of FXS patients report financial difficulties linked to the disease [10]. Apart from a French analysis [11], very little information is available on the cost incurred by FXS for health and social care systems. No study has assessed this cost on a large scale using data from several countries, nor has any study conjointly determined the impact of this disease on the health-related quality of life (HRQOL) of patients and caregivers through the use of standardised quantitative tools.
In this context, the objectives of this article were to estimate the social/economic costs (direct healthcare costs, direct non-healthcare costs and labour productivity losses) of FXS in European countries in 2012, to assess the HRQOL of patients with FXS and their caregivers and to describe variations between countries.
Methodology
Research design and subjects
Within the BURQOL-RD project, we conducted a cross-sectional survey of people diagnosed with FXS who received outpatient care and were living in the community. They were recruited from associations and registries specific to FXS in eight European countries (Bulgaria, France, Germany, Hungary, Italy, Spain, Sweden and the UK). All patients and caregivers were informed about the objectives of the study and the confidentiality of their data. Their agreement to participate was then collected through an online form or by post. Complete anonymity of the collected data was guaranteed as recruitment was carried out by patient organisations and no form of identification was present in the data sent to researchers.
Data were collected between September 2011 and April 2013. Questionnaires were administered by e-mail and postal surveys through patient organisations. Different questionnaires were available to patients depending on their age group: children (under 18) and adults. For children, their legal representative completed the questionnaire for them. In the case of FXS adult patients with intellectual disability, relatives could also fill in the questionnaire on the patient’s behalf. For patients with informal caregivers, the principal caregiver, defined as the person who spent the most hours helping the patient, was also asked to complete a separate questionnaire.
Information gathered at a specific point in time is commonly used in cost and HRQOL studies [12, 13]. This was the approach we selected, with a questionnaire that was detailed enough to reduce either exaggeration or underestimation. It covered a 6-month period prior to the study, with the exception of hospital admissions for which the annual frequency was collected. Data were then extrapolated over a 1-year period. The information compiled from patients included socio-demographic characteristics, use of healthcare services, formal and informal care as well as quantitative data regarding absence from the labour market (temporary and permanent sick leave or early retirement). Principal caregivers were asked to provide information regarding their demographic characteristics, relationship to the patient, number of hours spent on informal care and resulting work limitations.
Costing methodology
Costs were estimated from a societal perspective during a given year. We considered all healthcare resources used for prevention, treatment and rehabilitation, as well as additional resources used (through formal and informal care) or lost (through loss of labour productivity) within that year as a consequence of the illness considered [14]. Total and average annual costs were estimated through a bottom-up approach, which is the most accurate method for cost estimation and allows evidence-based decision-making [14, 15]. Three categories of costs were considered: direct healthcare costs (all goods and services directly linked to the diagnosis and treatment of the disease), direct non-healthcare costs including formal costs (professional caregivers, social services and non-healthcare transport) and informal costs (informal care provided by the patient’s relatives), and loss of labour productivity resulting from sick leave and early retirement from the labour market due to the illness.
Direct healthcare costs
Frequency of healthcare utilisation in terms of drugs, medical devices, medical tests, visits to health professionals, healthcare-related transportation, emergency visits and hospital admissions was derived from the questionnaire. Unit costs for each resource were obtained from healthcare cost databases at a national level and multiplied by the number of units of each resource used (see Supplementary Annex 2). Drug cost was calculated by determining the daily cost for each of the products used (based on the cost of each pack dispensed and the dose taken) and then multiplying by the duration of use. When no information concerning the number of units per pack was available, we assumed the largest pack size was dispensed.
Direct non-healthcare costs
Formal non-healthcare included care provided by social services at home or in institutions as well as non-healthcare transport. The number of hours of care by professional caregivers (defined as any home caregivers who are paid for their services) needed by patients was extracted from the questionnaire and valued using the mean wage of a professional home caregiver. Daily tariffs were used for services provided in institutions.
Informal non-healthcare is the provision of care by non-professional caregivers, such as a patient’s relative, friend or neighbour. Information about informal care was obtained from the items of the questionnaire concerning the time spent helping the patient with his/her basic activities of daily living (recall method). As a conservative criterion, and to prevent joint production, we censored the time of care to a maximum of 16 h per day (112 h per week) when the time of care reported exceeded this figure.
Costs were then computed through the proxy good method, which assumes that if these persons were not assisting the patient, a professional caregiver would have to be hired instead [16, 17]. We therefore computed the costs that would result from the substitution of an informal caregiver by a paid professional using the hourly wage of a health aide.
Loss of labour productivity
Costs linked to loss of productivity (including early retirement) were estimated by valuing the total number of hours not worked due to FXS using the human capital approach [18]. In this approach, the gross average earnings of a worker are used as a proxy for loss of labour productivity [19]. The total number of hours away from work due to the disease was extracted from the questionnaire and converted into monetary units. Our calculations were based on average gross wage figures from the National Wage Structure Surveys of the participating countries for the year 2012.
Patient and caregiver outcomes
Patient and caregiver outcomes were obtained by means of self-administered questionnaires: the EQ-5D, the Barthel Index and the Zarit Burden Interview. The EQ-5D is a simple generic instrument developed by a multidisciplinary group of researchers [20], which has been validated in many countries in Europe and is commonly used in economic evaluations and health technology assessments [21]. Previous studies carried out on developmental disorders have used this tool for both patients [22] and caregivers [23]. The questionnaire is divided into five items (mobility, self-care, daily activities, pain and discomfort, anxiety and depression) and it produces utility scores developed from general population-based valuation studies, reflecting societal preferences. Utility scores are on a scale of 0 (death) to 1 (perfect health), although negative scores are possible for states worse than death. A visual analogue scale (VAS) ranging from 0 (worst imaginable health) to 100 (best imaginable health) is also part of the EQ-5D and provides a quantitative measure of health outcome as judged by the individual respondents. Country-specific adult value sets were used when they were available. When they were not, other value sets were used (Danish value set for Sweden, Spanish for Italy, French for Hungary and British for Bulgaria) [24]. Because FXS results in intellectual disability, caregivers most often filled in the patient’s EQ-5D questionnaire. Despite the limitations of proxy assessment of a patient’s HRQOL by a relative [25, 26], previous research has shown that a caregiver can estimate the point of view of a patient with intellectual disability in a valid and reliable manner [27].
To obtain a quantitative estimate of a patient’s degree of dependence, we used the Barthel Index, which is widely used to assess the level of functioning through ten items related to activities of daily living [28, 29]. The scores for each item are generally summed to give a score between 0 (total dependence) and 100 (independence) [28]. This method was used in all countries except Sweden and the UK, where the Barthel Index was assessed on a scale of 0–20 [30].
Caregivers also completed the Zarit Burden Interview (22-item version), which assesses the impact of a patient’s disabilities on his/her caregiver’s physical and emotional health as well as the repercussions on social and financial aspects. Each item is a statement that the caregiver is asked to respond to using a 5-point scale, indicating how often he/she has felt the suggested feeling, with options ranging from 0 (never) to 4 (nearly always). The total score is obtained by adding the responses of the individual items and ranges from 0 to 88, with a higher score indicating a higher perceived burden [31].
Results
Patients’ characteristics
Among the eight countries participating in the BURQOL-RD project, only five (France, Hungary, Italy, Spain and Sweden) provided enough valid questionnaires to be included in the analysis. Our sample was comprised of 241 patients with valid questionnaires, with 101 adults and 140 children under 18. Mean age was 18.1 and mean age at diagnosis was 6.5. The large majority of our population (88 %) was male and 66 % required a caregiver. Detailed patient characteristics by country are presented in Table 1. Consistently across countries, most adult patients were single (90.5–100 %) and less than 20 % worked regularly. Depending on the country, the majority of children either attended specialised schools (France) or regular schools with personalised support (Italy and Spain). Only data from countries where there were at least ten respondents were included in the overall analysis to ensure the representativity of the results. Results from all participating countries can be seen in the tables.
Costs
The mean total annual cost per FXS patient was €4951 in Hungary, €21,586 in Italy, €31,008 in Spain, €35,737 in France and €58,862 in Sweden (Table 2). Healthcare costs ranged from €110 in Hungary to €2675 in France. The main items were medical visits, followed by hospitalisations and drugs. Direct non-healthcare costs ranged from €4841 in Hungary to €57,909 in Sweden, with informal care being the most important item except in France and Sweden. Loss of labour productivity was very low with values close to zero except in France and Italy where they nonetheless still represented a small part of the total costs of FXS.
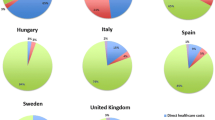
When looking specifically at the costs incurred by adults, the data of only four countries were included in the analysis based on the sample size (France, Italy, Spain and Sweden). Mean annual costs ranged from €13,596 per patient in Italy to €64,005 in Sweden (Table 3). In all considered countries, direct healthcare costs represented less than 7 % of total costs. They resulted mainly from medical visits, except in France where hospitalisation costs were the highest. Loss of labour productivity similarly contributed to a minimal part of total costs (less than 11 %). For all countries, the main costs were direct non-healthcare costs. For France and Sweden, non-healthcare formal costs represented the majority of costs, accounting for 58 and 85 % respectively of total costs while, for Italy and Spain, the majority of costs resulted from informal care (51 and 63 % of total costs, respectively) (Fig. 1).
For the paediatric patients, four countries were included in the analysis based on the sample size (France, Hungary, Italy and Spain). Mean annual costs ranged from €5295 per patient in Hungary to €38,368 in France (Table 4). In all countries, direct healthcare costs consistently represented less than 13 % of total costs (Fig. 2). The majority of those direct costs resulted from medical visits (Table 4). In Hungary, Italy and Spain, direct non-healthcare informal costs represented the majority of costs (96, 73 and 93 %, respectively) while in France direct non-healthcare formal costs were the most significant (Fig. 2), similar to what was observed for adult patients.
Outcomes
Patients
Mean utility scores for adult patients varied from 0.52 in France to 0.73 in Spain, while mean VAS scores varied from 66.5 in Sweden to 76.7 in Italy (Table 3).
Regarding the level of functioning, the mean Barthel Index varied from 81.5 in France (corresponding to moderate dependency) to 93.9 in Italy (slight dependency) in adult patients (Table 3), and from 73.9 in Italy to 84.0 in Hungary in paediatric patients, both corresponding to a moderate level of dependency (Table 4). In Sweden, where the Barthel Index was assessed on a scale of 0–20, the mean Barthel Index was 14.9 for adult patients and 14.3 for paediatric patients, corresponding to slight dependency (Tables 3 and 4).
Caregivers
Mean age of caregivers ranged from 36.9 in Italy to 47.9 in France. The vast majority were female (66.7–100 % of all caregivers). In all countries considered, more than 50 % were still working. Their mean utility score ranged from 0.734 in Sweden to 0.874 in Hungary, while the mean VAS score ranged from 74.6 in Spain to 87.5 in Hungary. The mean Zarit burden measure was available in only three countries. Its value was 34.5 in Italy, 36.0 in Sweden and 39.9 in France, corresponding to a mild-to-moderate burden (Table 1).
Discussion
The economic burden of FXS in Europe is significant, with a mean annual cost per patient reaching up to €58,862. It represented 39 % of the gross domestic product per capita in Hungary in 2012, 64 % in Italy, 90 % in France, 107 % in Sweden and 110 % in Spain [32]. In each of the countries considered, the main contributors to the economic burden for adult patients were direct non-healthcare costs, either formal in France and Sweden or informal in Italy and Spain. Direct non-healthcare costs also represented the predominant share of costs for children. In addition, the impact of FXS on health-related quality of life was considerable for both patients and caregivers.
Distribution of FXS direct non-healthcare costs varied from country to country. For adult patients in the most Northern countries (France and Sweden), main costs were attributable to formal care, while main costs in Southern countries (Italy and Spain) were attributable to informal care. These differences have been highlighted in other studies and may be explained by stronger family ties in Southern European countries [33, 34]. In addition, France and Sweden had the highest gross domestic product among the study countries [35] and may therefore have been able to invest more in the development of social services than the others. Previous studies have also underlined that richer households were more likely to delegate care of dependent relatives than less wealthy households [36]. The same situation was observed for paediatric patients. Both French and Swedish paediatric patients mainly attended specialised schools, which may support the hypothesis that those two countries have developed more specialised services for FXS patients, even if the sample size was particularly small for children in Sweden.
There is currently very little literature on the cost of FXS. One study estimated the lifetime costs of FXS at USD 958,000 for men and USD 534,000 for women [37] (€1,113,000 and €620,000, respectively, after conversion to 2012 euros using the purchasing power parities [38, 39]). When dividing those costs by the life expectancy of patients [40, 41], they amounted to €14,089 annually for men and €7470 for women, which is lower than in all the countries we considered, except Hungary. However, the study dates back to 1998, and reported costs referred only to residential institutions. The most recent study, a cost-effectiveness analysis of a prenatal population-based fragile X carrier screening programme, was based on a lifetime cost estimate of USD 615,000 in 2004 (€632,000 in 2012, corresponding to an annual cost per patient of €8208), which was derived from a population of patients suffering from Down’s syndrome [42]. However, this study likely underestimates the real lifetime costs of FXS if one assumes that FXS patients have a life expectancy similar to the general population [43]. A recent publication reported the cost to society of patients with intellectual disability in Australia and found an annual cost per patient of AUD 60,000 (€43,000 in 2012) [44]. When subtracting allowances and costs resulting from education to increase comparability with our study, the remaining amount represented AUD 53,000 (€38,000 in 2012), which is within the range reported here. This would seem to confirm the validity of our findings, although differences in methods and populations limit this comparison.
To our knowledge, the HRQOL of FXS patients and their caregivers has never been measured. In our study, mean EQ-5D utility scores for FXS adult patients were lower than the mean values observed in the general population in both France and Hungary, where references are available [45, 46]. In Italy, mean VAS score for adult FXS patients was lower than that measured in a sample representative of the Italian general population [47] and lower than the mean value for patients affected by HIV [48], similar to what was observed in Spain [49]. In Sweden, the mean utility score for adult FXS patients was lower than for patients suffering from a broad range of disorders, including diabetes, asthma, mental distress, hypertension, angina pectoris and neck/shoulder pain [50]. There have also been no studies on the burden borne by caregivers of FXS patients. However, our findings are consistent with the mean utility score of 0.81 found in female caregivers of children with autism [51]. As with HRQOL, our study is the first to report the burden for caregivers of FXS patients through the Zarit Burden Interview, and we found the score to be significantly higher than any Zarit burden measure reported for other diseases with relatively comparable functional impairment in the three countries for which we had data. The highest score reported in the literature was 35.6 and was measured in caregivers of patients with Alzheimer’s disease in Brazil [52], while a recent study found a mean score of 29.3 in Japanese mothers of children with intellectual disability [53]. In addition, the substantial burden documented here is consistent with a study reporting that a considerable proportion of caregivers were injured in the past year by their child with FXS [10]. It might also be linked to a depressive disposition in some women carrying the genetic permutation [54, 55].
The strengths of this study are based on its bottom-up approach, which enabled inclusion of all types of costs associated with FXS, including some less visible, such as informal costs and loss of labour productivity. A top-down approach would not have been as informative as it can only allocate a portion of a known expenditure to the disease [14]. Furthermore, to our knowledge, this study is the first to estimate both the cost incurred by FXS and the impact on HRQOL for FXS patients and caregivers. This study is also unique in taking a European perspective and in including countries from Northern, Central, Southern and Eastern Europe.
However, our findings should be interpreted in light of the following limitations. The intangible costs [56] linked to the loss of quality of life were not included in the cost analysis, leading to an underestimation of the economic burden of FXS. Further research should be carried out in that regard. In addition, there is a high degree of variability from country to country in our sample, as reflected by the standard deviation for the total annual cost per patient, which ranged from €8849 in Hungary to €61,357 in Sweden. Moreover, in three countries involved in the BURQOL-RD project (Bulgaria, Germany and the UK) data were insufficient for analysis.
The main shortcoming of using a retrospective online survey is the risk of recall bias. Indeed patients may not remember their use of healthcare resources accurately and may simply guess, leading to an under- or overestimation of costs [14]. It should also be noted that some of the cost estimations may have been more sensitive than others to recall bias as patients are more likely to remember large or recurrent expenses than small or rare ones [57]. The use of an online questionnaire may also skew the patient or caregiver population towards younger, better educated, healthier and wealthier individuals with easy access to the Internet, and we cannot therefore rule out a degree of selection bias in our study. Moreover, we only considered the loss of labour productivity for patients who had once worked, while some patients who had never worked may not have done so as a result of their disease. It was not possible to identify those patients based on the information collected in the questionnaire and it is therefore possible that we underestimated the share of loss of labour productivity in the total costs. Finally, costs of schooling were not considered, while they potentially could be significant in France and Sweden, where children predominantly attended specialised schools.
Despite the limitations we acknowledge here, and based on the available data, we believe that our study represents a reasonable estimate of costs in the range of true FXS patient costs in Europe. It is the first study to provide a framework to determine such costs using standardised quantitative tools and it underlines the high cost of the disease from a societal perspective, despite its low prevalence. Our description of the previously undocumented burden of FXS supports the development of tailored policies to reduce the consequences of FXS on both patients and their caregivers. Services developed for FXS patients should in particular account for the high burden on caregivers by offering them social support alongside patient care.
References
Bassell, G.J., Warren, S.T.: Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron 60, 201–214 (2008)
Jacquemont, S., Berry-Kravis, E., Hagerman, R., von Raison, F., Gasparini, F., Apostol, G., Ufer, M., Des Portes, V., Gomez-Mancilla, B.: The challenges of clinical trials in fragile X syndrome. Psychopharmacology (Berl.) 231, 1237–1250 (2014)
Hagerman, R., Hoem, G., Hagerman, P.: Fragile X and autism: intertwined at the molecular level leading to targeted treatments. Mol. Autism. 1, 12 (2010)
Heulens, I., Suttie, M., Postnov, A., De Clerck, N., Perrotta, C.S., Mattina, T., Faravelli, F., Forzano, F., Kooy, R.F., Hammond, P.: Craniofacial characteristics of fragile X syndrome in mouse and man. Eur. J. Hum. Genet. EJHG. 21, 816–823 (2013)
Crawford, D.C., Acuña, J.M., Sherman, S.L.: FMR1 and the fragile X syndrome: human genome epidemiology review. Genet. Med. Off. J. Am. Coll. Med. Genet. 3, 359–371 (2001)
Orphanet Report Series—Prevalence of rare diseases: http://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_decreasing_prevalence_or_cases.pdf (2013). Accessed 20 Nov 2015
Song, F.J., Barton, P., Sleightholme, V., Yao, G.L., Fry-Smith, A.: Screening for fragile X syndrome: a literature review and modelling study. Health Technol. Assess. Winch. Engl. 7, 1–106 (2003)
López-Bastida, J., Oliva-Moreno, J.: Cost of illness and economic evaluation in rare diseases. Adv. Exp. Med. Biol. 686, 273–282 (2010)
Hartley, S.L., Seltzer, M.M., Raspa, M., Olmstead, M., Bishop, E., Bailey, D.B.: Exploring the adult life of men and women with fragile X syndrome: results from a national survey. Am. J. Intellect. Dev. Disabil. 116, 16–35 (2011)
Bailey Jr, D.B., Raspa, M., Bishop, E., Mitra, D., Martin, S., Wheeler, A., Sacco, P.: Health and economic consequences of fragile X syndrome for caregivers. J. Dev. Behav. Pediatr. JDBP. 33, 705–712 (2012)
Chevreul, K., Berg Brigham, K., Brunn, M., des Portes, V., BURQOL-RD Research Network: Fragile X syndrome: economic burden and health-related quality of life of patients and caregivers in France. J. Intellect. Disabil. Res. JIDR. 59(12), 1108–1120 (2015)
Lorgelly, P.K., Joshi, D., Iturriza Gómara, M., Flood, C., Hughes, C.A., Dalrymple, J., Gray, J., Mugford, M.: Infantile gastroenteritis in the community: a cost-of-illness study. Epidemiol. Infect. 136, 34–43 (2008)
Lee, T.-J., Park, B.-H., Kim, J.W., Shin, K., Lee, E.B., Song, Y.-W.: Cost-of-illness and quality of life in patients with ankylosing spondylitis at a tertiary hospital in Korea. J. Korean Med. Sci. 29, 190 (2014)
Tarricone, R.: Cost-of-illness analysis: what room in health economics? Health Policy 77, 51–63 (2006)
Drummond, M., O’Brien, B., Stoddart, G., Torrance, G.: Methods for the economic evaluation of health care programmes, 2nd edn. Oxford University Press, Oxford (1997)
McDaid, D.: Estimating the costs of informal care for people with Alzheimer’s disease: methodological and practical challenges. Int. J. Geriatr. Psychiatry 16, 400–405 (2001)
Van den Berg, B., Brouwer, W.B.F., Koopmanschap, M.A.: Economic valuation of informal care. An overview of methods and applications. Eur. J. Health Econ. 5, 36–45 (2004)
Tranmer, J.E., Guerriere, D.N., Ungar, W.J., Coyte, P.C.: Valuing patient and caregiver time: a review of the literature. PharmacoEconomics. 23, 449–459 (2005)
Hodgson, T.A., Meiners, M.R.: Cost-of-illness methodology: a guide to current practices and procedures. Milbank Mem. Fund Q. Health Soc. 60(3), 429–462 (1982)
Brooks, R.: EuroQol: the current state of play. Health Policy Amst. Neth. 37, 53–72 (1996)
Dolan, P.: Modeling valuations for EuroQol health states. Med. Care 35, 1095–1108 (1997)
Van Steensel, F.J.A., Bögels, S.M., Dirksen, C.D.: Anxiety and quality of life: clinically anxious children with and without autism spectrum disorders compared. J. Clin. Child Adolesc. Psychol. Off. J. Soc. Clin. Child Adolesc. Psychol. Am. Psychol. Assoc. Div. 53(41), 731–738 (2012)
Khanna, R., Jariwala, K., Bentley, J.P.: Health utility assessment using EQ-5D among caregivers of children with autism. Value Health J. Int. Soc. Pharmacoecon. Outcomes Res. 16, 778–788 (2013)
EuroQol—EQ-5D-Y. http://www.euroqol.org/eq-5d-products/eq-5d-y.html. Accessed 20 Nov 2015
Pickard, A.S., Johnson, J.A., Feeny, D.H., Shuaib, A., Carriere, K.C., Nasser, A.M.: Agreement between patient and proxy assessments of health-related quality of life after stroke using the EQ-5D and health utilities index. Stroke 35, 607–612 (2004)
Diaz-Redondo, A., Rodriguez-Blazquez, C., Ayala, A., Martinez-Martin, P., Forjaz, M.J., Spanish Research Group on Quality of Life and Aging: EQ-5D rated by proxy in institutionalized older adults with dementia: Psychometric pros and cons. Geriatr. Gerontol. Int. (2013) (Epub ahead of print)
Balboni, G., Coscarelli, A., Giunti, G., Schalock, R.L.: The assessment of the quality of life of adults with intellectual disability: the use of self-report and report of others assessment strategies. Res. Dev. Disabil. 34, 4248–4254 (2013)
Mahoney, F.I., Barthel, D.W.: Functional evaluation: the Barthel index. Md. State Med. J. 14, 61–65 (1965)
Shah, S., Vanclay, F., Cooper, B.: Improving the sensitivity of the Barthel index for stroke rehabilitation. J. Clin. Epidemiol. 42, 703–709 (1989)
Lewis, C., Shaw, K.: The (Original) Barthel Index of ADLs. Phys. Ther. Rehab Med. (2006)
Hébert, R., Bravo, G., Préville, M.: Reliability, validity and reference values of the Zarit Burden Interview for assessing informal caregivers of community-dwelling older persons with dementia. Can. J. Aging. 19, 494–507 (2000)
The World Bank: GDP per capita (current US$)|Data|Table, http://data.worldbank.org/indicator/NY.GDP.PCAP.CD
Reher, D.S.: Family ties in Western Europe: persistent Contrasts. Popul. Dev. Rev. 24, 203 (1998)
Vilaplana Prieto, C., Jiménez-Martín, S., García Gómez, P.: Trade-off between formal and informal care in Europe. Gac. Sanit. SESPAS 25(Suppl 2), 115–124 (2011)
PIB par habitant ($ US courants) | Données | Tableau, http://donnees.banquemondiale.org/indicateur/NY.GDP.PCAP.CD
Grobon, S.: Les ménages aisés envisageraient plus souvent de déléguer la prise en charge de leur proche parent dépendant. (2014)
Wildhagen, M.F., van Os, T.A., Polder, J.J., ten Kate, L.P., Habbema, J.D.: Explorative study of costs, effects and savings of screening for female fragile X premutation and full mutation carriers in the general population. Community Genet. 1, 36–47 (1998)
Prices and purchasing power parities (PPP)—Organisation for Economic Co-operation and Development. http://www.oecd.org/std/prices-ppp/eurostat-oecdmethodologicalmanualonpurchasingpowerparitiesppps.htm
Shemilt, I., Thomas, J., Morciano, M.: A web-based tool for adjusting costs to a specific target currency and price year. Evid. Policy J. Res. Debate Pract. 6, 51–59 (2010)
Life expectancy at birth, male (years)|Data|Table, http://data.worldbank.org/indicator/SP.DYN.LE00.MA.IN/countries
Life expectancy at birth, female (years)|Data|Table, http://data.worldbank.org/indicator/SP.DYN.LE00.FE.IN/countries
Musci, T.J., Caughey, A.B.: Cost-effectiveness analysis of prenatal population-based fragile X carrier screening. Am. J. Obstet. Gynecol. 192, 1905–1912 (2005)
Coppus, A.M.W.: People with intellectual disability: what do we know about adulthood and life expectancy? Dev. Disabil. Res. Rev. 18, 6–16 (2013)
Doran, C.M., Einfeld, S.L., Madden, R.H., Otim, M., Horstead, S.K., Ellis, L.A., Emerson, E.: How much does intellectual disability really cost? First estimates for Australia. J. Intellect. Dev. Disabil. 37, 42–49 (2012)
Chevalier, J., de Pouvourville, G.: Valuing EQ-5D using time trade-off in France. Eur. J. Health Econ. HEPAC Health Econ. Prev. Care. 14, 57–66 (2013)
Szende, A., Németh, R.: Health-related quality of life of the Hungarian population. Orv. Hetil. 144, 1667–1674 (2003)
Scalone, L., Cortesi, P.A., Ciampichini, R., Belisari, A., D’Angiolella, L.S., Cesana, G., Mantovani, L.G.: Italian population-based values of EQ-5D health States. Value Health. 16, 814–822 (2013)
Venturini, A., Giannini, B., Montefiori, M., Di Biagio, A., Mazzarello, G., Cenderello, G., Giacomini, M., Merlano, C., Orcamo, P., Setti, M., Viscoli, C., Cassola, G.: Quality of life of people living with HIV, preliminary results from IANUA (Investigation on Antiretroviral Therapy) study. J. Int. AIDS Soc. 17, 19581 (2014)
Lopez-Bastida, J., Oliva-Moreno, J., Perestelo-Perez, L., Serrano-Aguilar, P.: The economic costs and health-related quality of life of people with HIV/AIDS in the Canary Islands, Spain. BMC Health Serv. Res. 9, 55 (2009)
Burström, K., Johannesson, M., Diderichsen, F.: Swedish population health-related quality of life results using the EQ-5D. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 10, 621–635 (2001)
Khanna, R., Jariwala, K., Bentley, J.P.: Health utility assessment using EQ-5D among caregivers of children with autism. Value Health. 16, 778–788 (2013)
Canonici, A.P., de Andrade, L.P., Gobbi, S., Santos-Galduroz, R.F., Gobbi, L.T.B., Stella, F.: Functional dependence and caregiver burden in Alzheimer’s disease: a controlled trial on the benefits of motor intervention. Psychogeriatrics. 12, 186–192 (2012)
Yatsugi, S., Suzukamo, Y., Izumi, S.: Productive social activities in mothers of intellectually disabled children moderate the relationship between caregiver burden and self-rated health. Jpn. J. Public Health. 60, 387–395 (2013)
Bourgeois, J.A., Seritan, A.L., Casillas, E.M., Hessl, D., Schneider, A., Yang, Y., Kaur, I., Cogswell, J.B., Nguyen, D.V., Hagerman, R.J.: Lifetime prevalence of mood and anxiety disorders in fragile X premutation carriers. J. Clin. Psychiatry 72, 175–182 (2011)
Roberts, J.E., Bailey Jr, D.B., Mankowski, J., Ford, A., Sideris, J., Weisenfeld, L.A., Heath, T.M., Golden, R.N.: Mood and anxiety disorders in females with the FMR1 premutation. Am. J. Med. Genet. B Neuropsychiatr. Genet. 150B, 130–139 (2009)
Lubeck, D.P.: The costs of musculoskeletal disease: health needs assessment and health economics. Best Pract. Res. Clin. Rheumatol. 17, 529–539 (2003)
Landfeldt, E., Lindgren, P., Bell, C.F., Schmitt, C., Guglieri, M., Straub, V., Lochmüller, H., Bushby, K.: The burden of Duchenne muscular dystrophy: an international, cross-sectional study. Neurology. 83(6), 529–536 (2014)
Acknowledgments
The authors wish to thank the National Alliance of People with Rare Diseases (NAPRD), Bulgaria; Alliance Maladies Rares, France; ACHSE, Germany; Hungarian Federation of People with Rare and Congenital Diseases (RIROSZ), Hungary; Federazione Italiana Malattie Rare (UNIAMO), Italy; Associazione Italiana Sindrome X Fragile, Italy; the Consulta Nazionale delle Malattie Rare, Italy; Rare Diseases Sweden; Federación Española de Efermedades Raras (FEDER), Spain; Rare Disease UK; and Rare Diseases Europe (EURORDIS); Mosaiques, Association Le Goeland X-Fragile, France; Martin-Bell Baráti Közösség, Hungary; Associazione Italiana Sindrome X Fragile, Italy; Federación Española del Síndrome de X Frágil, Asociación para la integración de personas afectadas por X-Frágil u otro TGD en Andalucía, Asociación del Síndrome X-Frágil de Madrid, Extremadura y Cataluña, Spain.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
Supported by the Social/Economic Burden and Health-Related Quality of Life in Patients with Rare Diseases in Europe Project, which has received funding from the European Union within the framework of the Health Programme (Grant A101205). The Executive Agency of the European Union is not responsible for any use that may be made of the information contained herein.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Members of the BURQOL-RD Research Network listed in the Supplementary Annex 1.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chevreul, K., Gandré, C., Brigham, K.B. et al. Social/economic costs and health-related quality of life in patients with fragile X syndrome in Europe. Eur J Health Econ 17 (Suppl 1), 43–52 (2016). https://doi.org/10.1007/s10198-016-0784-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-016-0784-3