Abstract
Objectives
To determine the costs and cost-effectiveness of a diagnostic strategy including computed tomography coronary angiography (CTCA) in comparison with invasive conventional coronary angiography (CA) for the detection of significant coronary artery disease from the point of view of the healthcare provider.
Methods
The average cost per CTCA was determined via a micro-costing method in four French hospitals, and the cost of CA was taken from the 2011 French National Cost Study that collects data at the patient level from a sample of 51 public or not-for-profit hospitals.
Results
The average cost of CTCA was estimated to be 180€ (95 % CI 162–206€) based on the use of a 64-slice CT scanner active for 10 h per day. The average cost of CA was estimated to be 1,378€ (95 % CI 1,126–1,670€). The incremental cost-effectiveness ratio of CA for all patients over a strategy including CTCA triage in the intermediate risk group, no imaging test in the low risk group, and CA in the high risk group, was estimated to be 6,380€ (95 % CI 4,714–8,965€) for each additional correctly classified patient. This strategy correctly classifies 95.3 % (95 % CI 94.4–96.2) of all patients in the population studied.
Conclusions
A strategy of CTCA triage in the intermediate-risk group, no imaging test in the low-risk group, and CA in the high-risk group, has good diagnostic accuracy and could significantly cut costs. Medium-term and long-term outcomes need to be evaluated in patients with coronary stenosis potentially misclassified by CTCA due to false negative examinations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The widespread diffusion of computed tomography coronary angiography (CTCA) has been driven by its ability to provide in a non-invasive fashion similar information to the conventional coronary angiography (CA). In 64-slice CT scanning, use of a contrast agent allows the visualisation of the coronary lumen and wall and results in a large 4-dimensional dataset of the heart obtained over the entire cardiac cycle. In addition to being non-invasive, since CTCA is usually done in outpatient care, it is also expected to be less costly than CA. Conventional CA has for many years been considered as the gold standard for coronary artery disease, with 100 % diagnostic accuracy. However, in the current context of healthcare cost containment, the question of adopting less expensive technology even at the cost of some loss in effectiveness may not be out of the question [1].
The average cost of CA in France in 2011, as estimated in the French Hospital Cost Database (Etude nationale des coûts, ENC), is 2,794€ (95 % CI 2,448–3,140€). This includes a national average hospital stay of 3.7 days. The cost of CTCA had not previously been estimated in France but, since it is generally carried out as an outpatient procedure, the cost was expected to be much lower than CA.
Given the different costs expected for the two diagnostic tests with similar aims to detect coronary stenosis, and the different outcomes expected in terms of diagnostic accuracy, cost-effectiveness analysis was the chosen economic tool in order to investigate both costs and consequences of diagnostic guidelines.
The objective was to ascertain the cost of CTCA in four hospitals participating in the EVASCAN study and to determine the cost-effectiveness of a diagnostic strategy including CTCA using 64-detector scanners in comparison with conventional CA in patients of a French multi-centre trial EVASCAN (Evaluation of CT Scanner), which included the largest number of intermediate- to high-risk stable and symptomatic patients with a clinical indication for coronary imaging reported to date.
Materials and methods
Study design and population
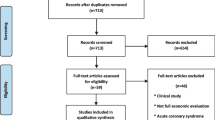
The methods and results of EVASCAN have been described elsewhere [2]. In short, a total of 757 adults referred from their primary care physicians for non-emergent invasive CA were enrolled in 40 French hospitals between June 2006 and May 2008. All patients had experienced chest pain and had suspected or stable coronary artery disease (CAD). The inclusion criteria stipulated the requirement for a CA to ensure that CTCA would be ethically compared to CA. Since the CA is considered to be the gold standard by which to measure CTCA, all patients underwent both procedures, even those who tested negative with CTCA, in order to be able to calculate the diagnostic accuracy of CTCA. A total of 11 patients were excluded due to the withdrawal of consent (n = 1), deviation from the protocol (n = 2) and either the CTCA and/or the CA not being completed (n = 8). Each of the remaining 746 patients underwent first CTCA followed by CA 1.7 ± 0.8 days later. The resulting images from each diagnostic technique were engraved on CD ROM for centralised blind analysis; diagnosis of significant coronary artery stenosis was based on the classic 50 % luminal narrowing threshold. The pretest probability of CAD was estimated using the Duke Clinical Score [3]. This score uses risk factors such as age and gender, co-morbidities such as diabetes and hypertension, a physical examination and other clinical factors to identify the level of risk of CAD. Patients were categorised as having low (1–30 %), intermediate (31–70 %) or high (71–99 %) estimated pretest probability of CAD. The economic analysis is based on the 705 patients for whom this risk level was calculable (Fig. 1). Each patient was asked to state a preference for either CTCA or CA. The effective radiation dose of medical imaging techniques was reported in milliSieverts (mSv). The time horizon was the diagnostic procedure only and costs were estimated from the viewpoint of the healthcare provider.
Cost of coronary angiography
The EVASCAN study recorded the date of CA and the length of hospital stay (0–33 days). The ENC is constructed from activity-based costs collected at the patient level then aggregated to the DRG level. The total cost and standard deviation of outpatient CA were taken from the latest available version (2011) of the ENC and DRG schedule available on the website of the Technical Agency for Hospital Information (Agence technique de l’information sur l’hospitalisation, ATIH): http://www.atih.sante.fr. Using the average costs and standard deviation for CA carried out as an outpatient, the cost was modelled to a log normal density function to ascertain the 95 % confidence intervals and for re-sampling in the probabilistic sensitivity analysis.
Cost of CTCA
A micro-costing costing method was employed to estimate the average cost of CTCA. The volume of resources used was determined by direct observation of each stage of the diagnostic procedure. In addition, the case report forms (CRF) for each patient included the time required to analyse the images. Fixed and variable costs were included. The base-case hardware costs, including the cost of the CT scanner, were amortised over 5 years with a discount rate of 3 %. The price information was ascertained via the radiology service administrators and information from central purchasing service (Agence Générale des Equipements et Produits de Santé, AGEPS). Expert advice from the EVASCAN team and AGEPS was sought to ascertain the machine specification to be used in the costing given the variety of different CT scanners used in France and currently commercially available. In addition, three CT scanner manufacturers were interviewed: General Electric, Toshiba and Siemens. For all the hardware, the average maintenance for the base-case was estimated at 10 % per year of the original cost of the machine and the useful clinical life of the hardware was estimated at 5 years with no resale value. A 4-year lower limit and 8-year upper limit on the useful life of the hardware were employed in the probabilistic sensitivity analysis. The discount rate was varied between 0 and 5 %.
As far as personnel costs are concerned, the rate per minute for each agent involved was based on 212 working days per year for full time contracts and a 7.5-h contractual working day for a radiologist and medical secretary and a 10-h working day for the radiologist or executive administrator. No factors were included for lost time due to inefficiency or sickness. The patients’ travel time and work compensation payments were not accounted for in the micro-costing.
Both deterministic and probabilistic sensitivity analyses were carried out by varying a number of parameters such as salary costs, personnel time required for the test, hardware costs, consumable item costs, discount rate, length of useful life of the CT scanner, length of CT scanner time per test and the daily active hours of the CT scanner. The details of the micro-costing are contained in the online appendix. Using log normal functions for costs and the normal distribution for the other parameters, the probability density function for each parameter was simulated and the 95 % confidence intervals of CTCA cost were calculated by re-sampling 10,000 times the values of these parameters from their respective density functions. Since to the best of our knowledge, no correlations existed between the parameters, all parameters were assumed to be independent.
Cost-effectiveness analysis
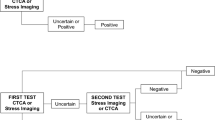
We assumed the following decision algorithm based on pretest probability of CAD from a prior risk assessment: high-risk patients would undergo CA without CTCA, intermediate-risk patients would undergo CTCA first, followed by CA if positive, and low-risk patients would not undergo any imaging test (Fig. 2). The measure of effectiveness was the diagnostic accuracy of the strategy including CTCA compared to the reference test CA in terms of correctly identified cases and non-cases (true negatives and true positives). A total of 10,000 estimations of the ICER were carried out by concurrently varying the cost and effectiveness using a non-parametric bootstrap method for the effectiveness and a probabilistic sensitivity analysis from the density functions already described for the costs.
Results
Patient-based analysis
Of the 746 patients studied for whom the CTCA was carried out on a 64-slice CT scanner, 65 % had suspected CAD and 35 % had previously known CAD. After risk level calculation using the Duke score, the population was split into three risk level categories: low, intermediate and high (Table 1). For 41 patients, the data required to calculate the Duke score was not available.
Diagnostic accuracy: CTCA
In the patient-based analysis for the whole study population, the sensitivity and specificity of CTCA were 91 % (95 % CI 88–94) and 50 % (95 % CI 45–55) respectively (Table 2). Segments that could not be assessed by CTCA for a total of 13 patients out of the population of 746 patients (1.7 %) were counted as positive for stenosis and thus increasing the false positive rate. Six of these 13 patients had stenosis confirmed by CA, and 7 were found to be free of significant stenosis. If non-assessable segments were assumed to be negative for coronary stenosis, sensitivity and NPV would decrease, but specificity and PPV would increase [2]. Non-assessable segments were considered as positive test results for significant stenosis since this better reflects real world practice where an uncertain CTCA result would lead to referral for a conventional CA.
Diagnostic accuracy: CTCA triage in the intermediate-risk group
The clinical results from the EVASCAN study recommend individually evaluating each patient to determine pretest probability for significant CAD and using CTCA as the method of choice in the intermediate-risk group to exclude significant coronary stenosis. The resulting decision algorithm was used in this cost-effectiveness model and consisted of using conventional CA in the high-risk group, CTCA triage to rule out coronary stenosis in the intermediate-risk group, and no imaging tests in the low-risk group. This decision algorithm leads to 95 % of patients being correctly classified as with or without stenosis.
Cost of diagnostic procedures
The average cost of CTCA is 180€ (95 % CI 162–206€). Detailed results of the CTCA micro-costing are shown in the Appendix (online only). The total estimated cost of CTCA was shared almost evenly between personnel on the one hand, with equipment and supplies on the other. This estimate is based on the CT scanner having no down time; for example, in the base-case, the active daily operating time is 10 h per day. However, in real life, CT scanners may not be 100 % active and the average cost varies according to the annual volume (Fig. 3). The base-case results assume a length of a CTCA of 15 min which is comparable to the average length of any type of CT scanner examination for outpatients of 15.8 min for 80 % of the CT tests carried out in France [4]. Personnel costs included the post-scan image analysis mean time of 28 min by a doctor in radiology, patient preparation time and administrative time, such as making appointments or typing-up the CT scan results to be sent to the cardiologist.
Average cost of CTCA according to annual volume with deterministic sensitivity analysis. a Upper limit machine saturation cost estimate with CTCA machine time of 20 min and an 8-h machine day. b Base-case machine saturation estimate includes CTCA machine time of 15 min and a 10-h machine day. c Lower limit machine saturation cost estimate with CTCA machine time of 10 min and a 12-h machine day
According to 2011 ENC data, the average length of hospital stay in France for a patient undergoing CA was 3.7 days with 10 % of CA carried out as day-case procedures. The length of stay observed in the EVASCAN population ranged from 0 to 33 days with an average of 2.1 days. However, as we expect that more catheter-based procedures will be carried out as outpatient care in the future, the cost of CA was deduced from the average cost of outpatient CA reported in the ENC and the confidence intervals estimated from a log normal distribution. The average cost for CA in 2011 was estimated to be 1,378€ (95 % CI 1,126–1,670€).
Radiation exposure and patient preferences
The average radiation dose received during the CTCA in EVASCAN per patient was 17.2 ± 5.9 mSv. The patients showed an overall preference for CTCA with 58 % of patients declaring a preference for the non-invasive CTCA, and 12 % for conventional CA. For the remaining 30 % of patients, the data are either not available or the patient did not express a preference.
Cost of triage strategy
The triage strategy decision tree is shown in Fig. 2, and thus costs were estimated based on the clinical strategy of neither CTCA nor CA in the low-risk group (average age 59), CTCA triage in the intermediate group (average age 63) and CA in the high-risk group (average age 69). The base-case average cost of diagnosing stenosis in the EVASCAN population (n = 705 for whom risk information is available) was 1,085€ (Table 3).
Cost-effectiveness
An incremental cost-effectiveness ratio (ICER) was calculated to compare the costs and accuracies of CA alone versus the triage strategy of CTCA combined with CA. The accuracy or health effect in the ICER computation is the number of patients correctly diagnostically categorised as being free or not of significant stenosis. The CTCA triage strategy in the intermediate-risk group led to 95 % of the EVASCAN population being correctly classified versus 100 % with CA. The ICER is 6,380€ (95 % CI 4,714–8,965€) for each additional correct diagnosis that would be attained with a “CA for all” strategy compared to a triage strategy with CCTA that excludes negative CCTA from further testing.
The reference diagnostic test CA is on average 293€ (95 % CI 249–337€) more per patient. The results of the bootstrap and sensitivity analysis are shown as a scatter plot of 10,000 ICERs presented on the cost-effectiveness plane (Fig. 4). The horizontal axis shows the difference in diagnostic accuracy between the strategies of CTCA triage in the intermediate group and the comparator of CA for all. The vertical axis represents the cost difference between the triage strategy and the comparator. The scatter plot is contained in the south-west quadrant. In other words, CTCA is a cost-reducing/quality-reducing innovation, decrementally cost-effective compared to CA with an ICER of 6,380€ spared on average per correct diagnosis lost.
Scatter plot on the cost-effectiveness plane showing the difference in costs and diagnostic accuracy from EVASCAN data using 10,000 bootstrap replicates with the reference test of CA (for all risk levels) on average 293€ more expensive per patient. The lines circling the scatter plot represent the 50 and 95 % confidence intervals
Discussion
The EVASCAN population is the largest used for a diagnostic accuracy study to date in the population of patients with stable or suspected CAD. With such a large sample size of 705 patients from 40 centres, quality controlled data and CTCA and CA being analysed in a blinded fashion at central core laboratories, the EVASCAN results are robust. The diagnostic accuracy results of EVASCAN (NPV 84 %) support findings of other authors that conclude that CTCA is good for ruling out disease in the intermediate-risk group [5, 6], particularly for patients who cannot undergo CA or who have uncertain results from diagnostic work-up procedures such as stress tests [7]. All the EVASCAN patients, of whom 14 % were at low risk of CAD, had been referred to the hospital for catheter CA between June 2006 and June 2008 despite the fact that guidelines do not recommend invasive CA for low-risk patients [8]. This implies that some cardiologists may be prepared to catheterise patients at very low risk of having CAD based on the clinical information available to them.
Since more and more catheter-based procedures are carried out in ambulatory care [9], it seemed appropriate to compare CTCA carried out in ambulatory care with outpatient CA, despite the fact that the average length of stay in the EVASCAN population for CA was 2.2 days. A limit of the study is the use of different methods for evaluating the cost of the two techniques. The CTCA cost estimate is estimated from a micro-costing analysis which is considered to be the most precise costing method [10], and the CA cost estimate was carried out using a quality-controlled activity-based method employed in a sample of 51 public hospitals. The most modest cost estimate for the CA has been employed in the analysis (outpatient vs. inpatient) and the results show no overlap between the confidence intervals of the cost of the two diagnostic techniques.
Offering a reduction in cost per patient of 293€, the CTCA triage strategy compared to CA was decrementally cost effective and spared 6,380€ per diagnosis missed. The triage strategy whereby CTCA positive results are validated by CA would result in 95.3 % of patients (n = 672) being correctly classified and 4.7 % (n = 33) incorrectly classified. Of these 33 incorrectly classified patients, 21 are low-risk patients who have risk of adverse cardiac events very similar to that of the general population [11, 12]. The remaining 12, a group representing 1.7 % of the EVASCAN population, are intermediate-risk patients with a false negative result from CTCA triage who suffered a relatively high dose of radiation and are at risk for adverse cardiac events such as myocardial infarction. The average cost of care in a French public hospital, as estimated in the 2011 ENC for a myocardial infarction, is 4,463€. After a myocardial infarction, life expectancy can be expected to reduce by between 3 and 13 years [13]. Therefore, in the unlikely case scenario where all of the 1.7 % patients with false negative results proceed to myocardial infarction, the average loss in life expectancy for the EVASCAN population may be between 0.6 and 2.7 months at an average cost of care of 78€. However, a negative CTCA result does not imply that no treatment is given. These patients would continue to be monitored by their cardiologist for suspected or stable angina, in particular with medication. No high-risk patients would be incorrectly classified since they would proceed directly to CA. For the 7 % of the EVASCAN population (50 patients) who had a false positive result from the CTCA, the costs of the subsequent CA carried out to refute the positive diagnosis are included in the total cost analysis (Table 3) and represent 9 % of the average cost per patient in the triage strategy. It appears that only the patients who need urgent bypass or angioplasty would be failed by this strategy of triage, which is unlikely to occur in a patient population of stable or suspected CAD with intermediate risk. The advantage of CA is that should stenosis be found interventions such as coronary artery bypass surgery can be carried out immediately. However, many studies agree that there is evidence that the extent and severity of CAD defined at CTCA predicts all-cause mortality, whereas patients with a normal CTCA have an excellent prognosis [14]. Advocating a decrementally cost-effective strategy is unusual in the medical literature. As noted by Nelson et al., physicians forced to implement such strategies may choose not to advertise it because of the inherent ethical dilemma. The potential loss in diagnostic accuracy for patients at intermediate risk with normal CTCA results does not affect the prognosis of patients [14] and may be compensated by reduced procedural risk.
The EVASCAN study did not collect follow-up data on clinical outcomes for the patients and thus could not measure the medium- to long-term outcomes for these patients. A study comparing three different imaging methods to angiography in the investigation of patients with stable chest pain found that, in terms of QALYs gained after 3 years of follow-up, there was little difference between any of the techniques [15], and that there was no significant difference in cost-effectiveness between systematic CA and the non-invasive test groups, although this may be due to a deviation from the protocol whereby negative functional tests still lead to referral to angiography, perhaps due to doctor prejudice leading to a preference for interventional versus non-invasive testing. Other cost-effectiveness analysis (CEA) studies of similar populations that consider longer-term clinical outcomes are usually based on modelling data. One such study concluded that CTCA is a cost-saving technique which avoids some unnecessary CA but with a slight detriment in patient outcomes [16]. Another found that health outcomes between different strategies with or without CTCA were very similar. The study also found that costs calculated from tariffs could actually be higher with CTCA partly due to incidental findings such as pulmonary nodules [17]. However, in another modelling analysis from a societal point of view, CTCA demonstrated similar gains in QALY over a 10-year period to CA—but at a lower cost [18]. This wide variety of results is partly due to the fact that modelling studies rely on many assumptions in order to be able to estimate the cost per life gained, yet for diagnostic tests, which only indirectly save lives, this measure may not be pertinent. Thus, for imaging studies, more disease-specific measures such as cost per case identified, or cost per correct diagnosis, as we have used here, are often employed [19, 20]. Our cost analysis covers the cost of the diagnostic techniques only and does not incorporate any treatment costs or other follow-up events linked to incidental findings.
Correct risk classification is one of the main keys to the feasibility of a CTCA-based triage. Many different methods are available to classify the risk level of patients. The Duke Clinical Score used in EVASCAN is based on an American population and so might not be as pertinent to the EVASCAN population as the ESC HeartScore. In addition, different studies use different algorithms to test risk and these may not reflect the actual practice of cardiologists, who may use more simple techniques such as definition by age, gender and symptoms [21], or simply their clinical judgement. Another study using the score of Morise et al. concluded that the most important step for physicians in choosing between the two diagnostic techniques is made on the basis of a clinical estimation of disease likelihood [22, 23]. Not employing an accurate method to stratify the patients into risk groups may result in an incorrect therapy being prescribed. In the high- and intermediate-risk group, the average Duke score overestimates the actual risk of CAD as demonstrated by a lower actual prevalence as measured by the CA results.
The overall clinical benefit to the patient must outweigh any untoward risk such as radiation exposure or adverse events, as well as bearing in mind patient preferences. Both CA and CTCA incur radiation exposure for the patient. The average radiation dose per patient received from CTCA was 17.2 ± 5.9 mSv. Other studies report ranges of effective doses for CTCA of between 5 and 30 mSv with a median value of 12 mSv [24]. For conventional CA, where X-ray technology is also used, effective doses of between 2.0 and 15.8 with an average effective dose of 7 mSv are reported [25]. At these low doses of radiation, there is still considerable uncertainty about the overall effects and increased cancer risk. This is due to the fact that there is no easy way to differentiate between the effects of medical device radiation, other “man-made” sources and those from naturally occurring radiation, nor any way to distinguish cancers that occur due to radiation exposure rather than other causes. One study based on 34 patients undergoing CTCA in 2009 reported that, for a median CT effective dose of 22 mSv, one radiation-induced cancer would develop for every 790 CTCA carried out on 60-year-old men [26]. In the strategy of CTCA triage in the intermediate-risk group, the strategy confirmed by EVASCAN, all high-risk patients would be correctly identified, at an average radiation dose for all risk levels of over 10 mSv. For comparison, annual background radiation is typically 1–3 mSv depending on the location. In the French hospital network, older model CT scanners with less than 64 slices are considered to have insufficient diagnostic accuracy for CTCA. However, new generation models have been shown to be cost-effective for difficult-to-image patients with known CAD and with scanning protocols that incur lower radiation doses. Given the current high cost of these scanners, it is unlikely that they will be universally available to all patients and 64-slice CT scanners remain very much the mainstay in the French public setting [27].
In our study, more adverse events were recorded for the CA examination (30 events) than CTCA (8 events); however, the detail of the adverse events was not recorded. With respect to patient preference, given the non-invasive nature of CTCA, the reduction of patient discomfort and the fact that it does not require hospitalisation, it is not surprising that 58 % of patients declared a preference for CTCA over CA. Another study reporting patient preferences also found that CT scanning was the most preferred technique compared to other imaging techniques or digital subtraction angiography before carotid endarterectomy. [28].
Notwithstanding guidelines or patient preferences, the choice of diagnostic test will be influenced by the available techniques in the local health setting. In 2002, a government directive was issued to ensure coverage of 10 CT scanners per million inhabitants for the whole of France but with varying regional targets. The presence or absence of a CT scanner in the local hospital could determine whether or not the patient is referred for CTCA. Nevertheless, more health care services do not necessarily lead to improved outcomes [29]. In the USA, there has been a shift from carrying out CT scans in hospitals in inpatient and outpatient settings to physicians’ offices. As a result, there has been rising concern at the reduction in quality alongside an increase in the provision of these services, leading to overuse in some areas [29]. In addition, physician preferences rather than the patient health-state may influence decision making. This may lead to inappropriate use of either technique [15]. For example, if guidelines are not followed, and CTCA used indiscriminately regardless of patient risk level, the CTCA false positive results could lead to unnecessary further tests such as CA and over-treatment in the low-risk groups. In the absence of a triage strategy, if the CTCA technology is used indiscriminately without consideration of pretest risk, it is possible that overall diagnostic spending would increase [19] and, depending on the health-care setting and payment systems, financial incentives can also lead to inappropriate use [29]. However, with careful evaluation of pretest probability of CAD, CTCA triage for intermediate-risk patients is a cost-saving strategy in comparison with the strategy of “CA for all”: on average, the per patient saving is 293€. The trade-off of these potential monetary savings is a slight reduction in accuracy and a small increase in the average radiation dose.
Abbreviations
- AGEPS:
-
Paris University Hospital Central Purchasing Service (Agence Générale des Equipements et Produits de Santé)
- ATIH:
-
Technical Agency for Hospital Information (Agence technique de l’information sur l’hospitalisation)
- CA:
-
Coronary angiography
- CAD:
-
Coronary artery disease
- CEA:
-
Cost-effectiveness analysis
- CI:
-
Confidence interval
- CRF:
-
Case report forms
- CTCA:
-
Computed tomography coronary angiography
- DRG:
-
Diagnosis-related group
- ENC:
-
French National Cost Database (Etude nationale des coûts)
- FN:
-
False negative
- FP:
-
False positive
- ICER:
-
Incremental cost-effectiveness ratio
- mSv:
-
MilliSieverts
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- TN:
-
True negative
- TP:
-
True positive
References
Nelson, A.L., Cohen, J.T., Greenberg, D., Kent, D.M.: Much cheaper, almost as good: decrementally cost-effective medical innovation. Ann. Intern. Med. 151, 662–667 (2009)
Gueret, P., Deux, J.F., Bonello, L., Sarran, A., Tron, C., Christiaens, L., Dacher, J.N., Bertrand, D., Leborgne, L., Renard, C., Caussin, C., Cluzel, P., Helft, G., Crochet, D., Vernhet-Kovacsik, H., Chabbert, V., Ferrari, E., Gilard, M., Willoteaux, S., Furber, A., Barone-Rochette, G., Jankowski, A., Douek, P., Mousseaux, E., Sirol, M., Niarra, R., Chatellier, G., Laissy, JP.: Diagnostic performance of computed tomography coronary angiography—results from the Prospective National Multicenter Multivendor EVASCAN study. Am J Cardiol 111, 471–478 (2013)
Pryor, D.B., Shaw, L., McCants, C.B., Lee, K.L., Mark, D.B., Harrell, F.E., Muhlbaier, L.H., Califf, R.M.: Value of the history and physical in identifying patients at increased risk for coronary artery disease. Ann. Intern. Med. 118, 81–90 (1993)
Agence National d’Appui à la Performance. Imagerie scanner-IRM rapport de benchmark. http://www.anap.fr/uploads/tx_sabasedocu/rapport_BenchImagerie_dec2010.pdf (2010). Accessed 10 July 2013
Budoff, M.J., Dowe, D., Jollis, J.G., Gitter, M., Sutherland, J., Hamamert, E., Scherer, M., Bellinger, R., Martin, A., Benton, R., Delago, A., Min, J.K.: Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J. Am. Coll. Cardiol. 52, 1724–1732 (2008)
Mowatt, G., Cummins, E., Waugh, N., Walker, S., Cook, J., Jia, X., Hillis, G.S., Fraser, C.: Systematic review of the clinical effectiveness and cost-effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of coronary artery disease. Health Technol Assess. 12(17):iii-iv, ix-143 (2008)
Marano, R., De Cobelli, F., Floriani, I., Becker, C., Herzog, C., Centonze, M., Morana, G., Gualdi, G.F., Ligabue, G., Pontone, G., Catalano, C., Chiappino, D., Midiri, M., Somonetti, G., Marchisio, F., Olivetti, L., Fattori, R., Bonomo, L., Del Maschio, A.: NIMISCAD Study Group. Italian multicenter, prospective study to evaluate the negative predictive value of 16- and 64-slice MDCT imaging in patients scheduled for coronary angiography (NIMISCAD-Non Invasive Multicenter Italian Study for Coronary Artery Disease). Eur. Radiol. 19, 1114–1123 (2009)
ACC/AHA guidelines for coronary angiography. Circulation (1999). doi:10.1161/01.cir.99.17.2345
Le Corvoisier, P., Gellen, B., Lesault, P.F., Cohen, R., Champagne, S., Duval, A.M., Montalescot, G., Elhadad, S., Montagne, O., Durand-Zaleski, I., Dubois-Randé, J.L., Teiger, E.: Ambulatory transradial percutaneous coronary intervention: a safe, effective and cost-saving strategy. Catheter. Cardiovasc. Interv. 81, 15–23 (2013)
Drummond, M., Sculpher, M., Torrance, G., O’Brien, B., Stoddart, G.: Methods for the Economic Evaluation of Health Care Programmes, pp 72–78. Oxford University Press, Oxford (2005)
Hulten, E.A., Carbonaro, S., Petrillo, S.P., Mitchell, J.D., Villines, T.C.: Prognostic value of cardiac computed tomography angiography: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 57(10), 1237–1247 (2011)
Fazel, P., Peterman, M.A., Schussler, J.M.: Three-year outcomes and cost analysis in patients receiving 64-slice computed tomographic coronary angiography for chest pain. Am. J. Cardiol. 104, 498–500 (2009)
Boersma, H., van der Vlugt, M.J., Arnold, A.E.R., Deckers, J.W., Simoons, M.L.: Estimated gain inlife expectancy—a simple tool to select optimal reperfusion treatment in individual patients with evolving myocardial infarction. Eur. Heart J. 17, 64–75 (1996)
Bastarrika, G., Schoepf, U.J.: Coming of age: coronary computed tomography angiography. J. Thorac. Imaging 25(3), 221–230 (2010)
Thom, H., West, N.E.J., Hughes, V., Dyer, M., Buxton, M., Sharples, L.D., Jackson, C.H., Crean, A.M.: Cost-effectiveness of initial stress cardiovascular MR, stress SPECT or stress echocardiography as a gate-keeper test, compared with upfront invasive coronary angiography in the investigation and management of patients with stable chest pain: mid-term outcomes from the CECaT randomised controlled trial. BMJ Open 4, (2013). doi:10.1136/bmjopen-2013-003419
Genders, T., Meijboom, W.B., Meijs, M.L., Schuijf, J.D., Mollet, N.R., Weustink, A.C., Pugliese, F., Bax, J.J., Cramer, M.J., Krestin, G.P., de Feyter, P.J., Hunink, M.G.: CT coronary angiography in patients suspected of having coronary artery disease: decision making from various perspectives in the face of uncertainty. Radiology 253(3), 734–744 (2009)
Ladapo, J.A., Jaffer, F.A., Hoffmann, U., Thomson, C.C., Bamberg, F., Dec, W., Cutler, D.M., Weinstein, M.C., Gazelle, G.S.: Clinical outcomes and cost-effectiveness of coronary computed tomography angiography in the evaluation of patients with chest pain. JACC 54(25), 2409–2422 (2009)
Amemiya, S., Takao, H.: Computed tomographic coronary angiography for diagnosing stable coronary artery disease a cost-utility and cost-effectiveness analysis. Circulation 73, 1263–1270 (2009)
Shaw, L.: Cost-effectiveness and future implications for cardiovascular imaging. Can. J. Cardiol. 29, 350–357 (2013)
Heller, D.: Adapting cost-effectiveness analysis to radiology: from the boardroom to the bedside. Eur. Radiol. 10(suppl 3), S344–S346 (2000)
Diamond, G.A., Forrester, J.S.: Analysis of probability as an aid in the clinical diagnosis of coronary artery disease. N. Engl. J. Med. 300, 1350–1358 (2009)
Morise, A.P., Haddad, W.J., Beckner, D.: Development and validation of a clinical score to estimate the probability of coronary artery disease in men and women presenting with suspected coronary disease. Am. J. Med. 102, 350e6 (1997)
Dorenkamp, M., Bonaventura, K., Sohns, C., Becker, C.R., Leber, A.W.: Direct costs and cost-effectiveness of dual-source computed tomography and invasive coronary angiography in patients with an intermediate pretest likelihood for coronary artery disease. Heart 98, 460–467 (2012)
Hausleiter, J., Meyer, T., Hermann, F., Hadamitzky, M., Krebs, M., Gerber, T.C., McCollough, C., Martinoff, S., Kastrati, A., Schömig, A., Achenbach, S.: Estimated radiation dose associated with cardiac CT angiography. JAMA 301, 500–507 (2009)
Mettler, F.A., Huda, W., Yoshizumi, T.T., Mahesh, M.: Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 248, 254–263 (2008)
Smith-Bindman, R., Lipson, J., Marcus, R., Kim, K.P., Mahesh, M., Gould, R., Berrington de Gonza´lez, A., Miglioretti, D.L.: Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch. Intern. Med. 169(22), 2078–2086 (2009)
Westman, M., Al, M., Burgers, L., Redekop, K., Lhachimi, S., Armstrong, N., Raatz, H., Misso, K., Severens, J., Kleijnen, J.: A systematic review and economic evaluation of new-generation computed tomography scanners for imaging in coronary artery disease and congenital heart disease: Somatom Definition Flash, Aquilion ONE, Brilliance iCT and Discovery HD. Health Technol. Assess. (2013). doi:10.3310/hta17090
Patel, S.G., Collie, D.A., Wardlaw, J.M., Lewis, S.C., Wright, A.R., Gibson, R.J., Sellar, R.J.: Outcome, observer reliability, and patient preferences if CTA, MRA, or Doppler ultrasound were used, individually or together, instead of digital subtraction angiography before carotid endarterectomy. J. Neurol. Neurosurg. Psychiatry 73, 21–28 (2002)
GAO Report to Congressional Requseters Medicare Part B Imaging services Rapid Spending Growth and Shift to Physician Offices Indicate Need for CMS to Consider Additional Management Practices. http://www.gao.gov/new.items/d08452.pdf?bcsi_scan_628cd39dca2568d2=Afz8M7ObHpLJ87IonfGt+8zAzkEBAAAARMdqAw==&bcsi_scan_filename=d08452.pdf (2008). Accessed 4 aril 2014
Acknowledgments
Many thanks to the medical corps, statisticians, administrators, and purchasers who helped with the micro-costing. In particular, thanks to (in alphabetic order): Elisabeth Aoun (AGEPS), Dr. Michel Cymbalista (Montfermeil Hospital), Catherine Deneux (Centre Chirurgical Marie Lannelongue), Dr. Jean-François Deux (Henri Mondor Hospital), Dr. Franck Digne (Max Fourestier Hospital), Jean-Eric Lefèvre (AGEPS), Dr. Jean-François Paul (Centre Chirurgical Marie Lannelongue), Professor Alain Rahmouni (Henri Mondor Hospital), Rémy Raymond (Henri Mondor Hospital). This study was fully supported by a grant from the French Ministry of Health (Grant STIC IC 050126).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Darlington, M., Gueret, P., Laissy, JP. et al. Cost-effectiveness of computed tomography coronary angiography versus conventional invasive coronary angiography. Eur J Health Econ 16, 647–655 (2015). https://doi.org/10.1007/s10198-014-0616-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-014-0616-2