Abstract
Objective
In this study I aim to explore the statistical causes of country differences in mammography screening among women aged 50–69 years in 13 European countries. I focus on the relative importance of individual (e.g. age, education, etc.) and institutional (e.g. public screening programmes) factors in explaining these differences.
Data and methods
I use individual level data from the first three waves (2004–2006–2009) of the SHARE as well as regional and country level data on institutional factors. The analytical approach is based on multilevel statistical models, which allow me to analyse the contribution of individual and institutional factors in explaining the variation in breast cancer screening across European countries.
Results
I find that the standard deviation in screening rates across countries increases slightly from 19.5 to 20.8 per cent after controlling for individual factors. Observed individual factors such as age, education, health status, etc., do not significantly contribute to the explanation of cross-country differences. In contrast, after controlling for observed institutional factors such as the availability of an organised screening programme, the standard deviation drops from 20.86 to 12.92 per cent. These factors can statistically explain about 40 per cent of the between-country differences in screening rates. Moreover, I found that these institutional factors seem to prevent a woman from considering a mammogram “not necessary”.
Conclusion
This analysis provides important insights about patient’s attitudes and understanding of benefits of breast cancer prevention and highlights the importance of the availability of an organised screening programme for screening differences across European countries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common cause of cancer death in the member states of the European Union [1]. One in 9 women gets breast cancer at some point in her life and 1 in 30 dies from the disease [2]. Due to demographic trends a significantly higher proportion of women will be confronted with breast cancer in the future [3]. Moreover, breast cancer amounts to about 0.5–0.6 per cent of the total health-care expenditure of developed countries [2]. Mammogram screening is the best tool available for detecting breast cancer in the early stage and can reduce the rate of death from cancer. On the basis of several randomised clinical trials, the World Health Organisation concluded in 2002 that in areas with screening attendance of at least 70 per cent, a reduction in breast cancer mortality by about 25 per cent may be expected in women screened between ages of 50 and 69 years [5]. In light of this evidence, the International Agency for Research on Cancer expert working group, EU guidelines and national guidelines promote regular screening (i.e. every 2 years) for women of these ages [6].
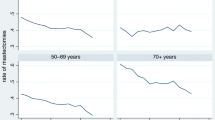
Despite these public health efforts to promote screening uptake, the use of screening mammography is not without controversy. Critics point to the problem that screening is associated with high rates of overdiagnosis and false-positive results [7–9], which might lead to overestimation of the effectiveness of screening.Footnote 1 As argued by Skinner [14] or Chandra et al. [15] the largest regional variations in treatment can be found in “supply-sensitive” care or “grey area medicine”, where the clinical value of treatment is debated and thus in which clinical judgment plays a key role. The discussion amongst the critics and advocates of breast cancer screening might be one reason why screening rates in most European countries remain far from 100 per cent and differ greatly, as shown by Fig. 1.
In this article I follow Bolin et al. [16] arguing that differences in healthcare utilisation might be explained (1) by differences in the demographic and socioeconomic composition of the population (individual factors) and (2) by differences in health system regulation, financing and provision (institutional factors). I do so by laying out some theoretical and empirical arguments for the association between these factors and mammogram uptake. A considerable amount of empirical and theoretical research exists in health economics on the predictors of screening and preventive behaviour. Theoretical economic models include those of Grossman [17], Cropper [18], Howard [19] and Fang [4]. Schueler et al. [20] provide a good review of the empirical literature on determinants of mammography screening uptake. While there is considerable evidence on factors associated with mammography screening, the reasons for country differences in screening remain unclear. This article advances the literature on the determinants of screening uptake by providing first time empirical evidence on the factors statistically explaining country differences. Awareness of the statistical causes behind these country differences might provide useful information for the design of appropriate public health policies for influencing mammography uptake within countries.
To analyse the individual and institutional causes of screening differences across 13 European countries, I take advantage of newly available internationally comparable individual-level data provided by the Survey of Health, Ageing and Retirement in Europe (SHARE). I combine these data with regional level data on institutional factors, which are taken from different sources. Plausibly, the most important institutional factor influencing screening uptake is whether a region has introduced an organised population-based screening programme. Within an organised screening programme eligible women (i.e. women aged 50–69 years) typically get regular (i.e. every 2 years) personal invitations to participate in free mammography screening at a location nearby imposing strong (economic) incentives fostering screening uptake. In Europe we see enormous regional differences, across and within countries, in the availability of such screening programmes and I exploit these differences to identify the impact of organised screening on screening uptake.
The analytical approach is based on multilevel statistical models. These models allow me to decompose the total variance in mammography screening rates into fractions that are due to differences in individual and regional factors within and between countries.
The remainder of the article is organised as follows: Sect. 2 illustrates the theoretical and empirical screening determinants. Section 3 presents some information on the data sets. Section 4 explains the empirical strategy and Sect. 5 presents the results. Section 6 discusses the results and adds some concluding remarks.
Mammography screening and its determinants
Individual factors: demand and supply side
Michael Grossman’s model of the demand for healthcare provides a useful framework for understanding the demand side, i.e. an individual’s demand for preventive healthcare services. In this model, the decision to undergo mammography screening is an investment decision. Such an investment is worthwhile if the expected present value of the reduction in disease and in the probability of death is larger than the opportunity costs of the intervention (compare [17, 18, 21] for a formalisation of these notions). Existing empirical studies—motivated by economic demand theory—found that individual factors like better education, better cognitive abilities, higher income, having a partner, younger age, a healthy lifestyle, and better health are associated with higher rates of receipt of screening (e.g. [6, 22]).
Alternatively, supply side factors have an important impact on screening behaviour. Agency theory [23] suggests that physician behaviour (i.e. whether a physician recommends screening) can be expected to influence the decision for screening, since asymmetric information is particularly widespread in healthcare markets, often forcing expert physicians to act on behalf of their less informed patients. Moreover, individual perception of risks is often biased [24]. Breast cancer is no exception in this regard and even women with a high risk of getting breast cancer may have false perceptions of the risks and the seriousness of breast cancer [25]. For this reason, physicians often act as agents for their less-informed patients, and they play an important role in determining mammography screening take-up.Footnote 2 Empirical evidence clearly indicates that women tend to follow their physician's advice for mammography screening [27, 28].
Institutional factors: demand and supply side
The Grossman model follows an individual-level approach wherein choices are made to maximise discounted lifetime utility subject to a number of monetary, time and other health system constraints [17]. In this model, changes in the constraints have influence on individual behaviour. Indeed, empirical studies reveal that public health interventions that influence financing, delivery, etc., and therefore change the constraints of an individual have a strong impact on screening rates [29]. Demand-side interventions that enhance access (e.g. mobile vans, transportation services), reduce perceived costs (e.g. coverage of mammography by insurance) or influence perceived benefits of screening (e.g. community education, mass media campaigns, introduction of guidelines) were very successful in increasing mammography use within a country [30]. Moreover supply side interventions like providing clear and non-conflicting guidelines for providers [31], or effort to change mammography prescribing behaviour of the physicians by reminder systems or bonus payments, significantly increased mammography use [32, 33].
An organised population-based screening programme is a comprehensive public health intervention that combines many of the interventions just mentioned. Eligible women get regular personal invitations to participate in free mammography screening.Footnote 3 Moreover the screening is offered at a location nearby, and women receive an information booklet that explains the pros and cons of mammography screening and that screening is accompanied by comprehensive quality assurance for equipment, radiographers (technicians) as well as radiologists.
From an economic point of view an organised screening programme should increase mammography uptake since it provides incentives in terms of reduced access costs, information costs, transaction costs as well as time costs and plausibly increases perceived benefits of mammography screening. The introduction of an organised screening programme plausibly “sends a message to providers” regarding the benefits of screening that might change physicians' priorisation, targets and incentives, making them more prone to recommend screening. Compared to the alternative of opportunistic screening,Footnote 4 organised screening puts a much greater emphasis on the quality of the screening process as measured by factors such as tumour characteristics, cancer detection rates and false-positive biopsy rates [34]. Thus, it provides better protection against the harms of screening—including overscreening, poor quality and complications of screening—and poor follow-up of those who test positively [34, 35]. Accordingly, Strumpf et al. [36] found that the implementation of an organised mammography screening programme in Quebec strongly increased adherence to cancer screening guidelines and screening uptake.
Data
I focus on women interviewed in the first three waves (2004–2006–2009) of the Survey of Health, Ageing and Retirement in Europe (SHARE) to explore variations in the uptake of breast cancer screening and associated factors influencing utilisation of mammography screening among women aged 50–69 years in 13 European countries. SHARE is a large representative micro data set providing detailed information on health, healthcare use and a variety of other socioeconomic characteristics of more than 30,000 individuals above the age of 50 years starting in 2004.Footnote 5 I restrict the sample to women aged 50–69 since for this group mammography screening is officially recommended at both the European level and the national level of the countries included. Therefore, the estimation sample consists of 11,409 observations in total. Table 1 provides descriptive statistics for the individual variables included in the empirical analysis for the sample as a whole as well as for each country separately. A more detailed description of the variables included and the exact specification can be found in Table 6 of the Appendix. The dependent variable measures whether a woman had a mammogram regularly (i.e. at least every 2 years) before 2007. To construct this variable I use retrospective information on mammography use collected in the third wave (2009) of the SHARE (SHARELIFE). SHARELIFE focusses on people’s life histories and contains detailed information on historical mammography screening use. Hence, I merge this information with the first and second wave but only use pooled data for all explanatory variables from the 2004 and 2006 waves.Footnote 6 Explanatory variables included individual factors like age (dummy variables for different age groups), health status (SAH and number of ADLs), a history of breast cancer, family structure (having a partner), income, education (as measured by ISCED-groups), cognitive abilities (as measured by verbal fluency), working, health behaviour and risk factors (taking medications). I included these variables because they have been debated in the theoretical and empirical scientific literature as important determinants of preventive behaviour (compare Sect. 2).Footnote 7 As Table 1 indicates, there are substantial differences in the means for almost each individual factor across the countries, which make it important to control for individual factors when analysing the impact of differences in institutions (see [16]).
Table 2 provides information on the institutional variables included in the empirical analysis. The data refer to the situation in the years 2004 as well as 2006 and are derived from different international sources (compare bottom of Table 2). Of special interest are the variables that refer to the availability and organisation of screening programmes in the different European countries. The most important variable includes whether an organised screening programme exists, since it imposes strong (economic) incentives fostering screening uptake (compare Sect. 2). In contrast to the other institutional variables, which are only available on the country level, I can take advantage of regional variation of the availability of an organised screening programme within countries [NUTS (Nomenclature of territorial units for statistics) 2 code level]. Some countries implemented an organised screening programme in all regions (e.g. Sweden, The Netherlands), whereas other countries offered it only in some regions (Italy, Denmark, Switzerland, Germany) and other countries (e.g. Poland, Greece) did not implement it at all.
Further explanatory variables regarding the organisation of screening programmes (invitation rates, screening intervals, etc.) are presented in Table 2. Higher invitation rates, a shorter screening interval, a broader geographical spread, an extended age range as well as higher numbers of radiologists and mammography units are ceteris paribus all expected to lead to higher levels of utilisation, because they should lower information, transaction and access costs of screening.Footnote 8 Moreover, Table 2 provides other regulatory (e.g. physician payment, GP as gatekeeper) and demand side (i.e. incidence of breast cancer) differences than the ones mentioned above, which may affect mammography uptake.Footnote 9
The data indicate that there are remarkable differences in the availability and organisation of screening programmes across the countries.
Finally, I use data on the reasons why women did not regularly go for mammography screening. SHARELIFE explores the causes why women had never had or stopped having mammograms done regularly by focussing on perceived benefits, information issues, financial restrictions, time costs and availability of mammography services.Footnote 10 I defined “financial restrictions” when a women argued that mammogram screening was “too expensive” or “not covered by insurance” or when she stated having “no insurance”. Thus, this variable covers generally perceived co-payments to mammogram screening as reasons for not undergoing a mammogram.
There are enormous differences between countries in the self-stated reasons for not participating in screening (Fig. 2). Regarding “not considered to be necessary” 47 per cent of women in Denmark mention this factor as a cause for not getting a mammogram and only about 4 per cent of women in Sweden state this as a reason. As to “not usual” there are also wide variations. About 17 per cent of women in Denmark state this as a reason.
In contrast, in Sweden and The Netherlands this factor plays no significant role for not getting regular mammograms. “No information about this type of care” plays an important role in Greece (14.6 per cent) and Poland (4.6 per cent) for not undergoing mammograms. Financial restrictions play a very small role in not getting regular mammograms in most countries. Only in Greece (2.8 per cent), Germany (2.0 per cent) and Poland (2.4 per cent) some women state financial restrictions as reasons for not getting a mammogram. The minor relevance of “financial restrictions” reflects the fact that mammogram screening for women between 50 and 69 years is generally fully covered by insurance in all countries [43].
Estimation strategy
My basic regression models are linear multilevel models (MLM) [48]. MLM enables me to identify the individual and institutional determinants of mammography screening uptake. Furthermore MLM makes it possible to analyse whether the differences in individual and institutional factors across countries can explain country differences in screening rates.
I define the following two-level random-intercept model:
where y ij is whether a woman j in country i regularly gets (i.e. every 2 years) a mammography screening, x 0 is a constant, x ij is a vector of the explanatory variables, and β 0 and β are coefficient estimates. All variables are centred around zero by subtracting their grand mean from the individual values and thus the parameter β 0 can be interpreted as the mean intercept (i.e. screening ratio) across all individuals in all countries. The model has two error components: ν i is the random error for the ith country. This error term captures unobserved (country-specific factors like institutional differences in health system regulation, financing and the provision that cause differences in screening uptake across countries. ε ij is an individual-level random error for the jth woman within the ith country.Footnote 11 This error term captures unobserved individual factors like preferences for prevention, genetic factors, time preferences, exposure to screening when a friend is affected by breast cancer, etc., that might explain individual differences in screening uptake within a country.
I estimate seven variations of Eq. 1. The first model (Model 1) includes only an intercept mirroring average screening rates across countries. Model 2 adds individual and Model 3 institutional explanatory variables. Models 4–7 add the different reasons for not participating in regular mammography screening as explanatory variables. The rationale behind this strategy is to identify the individual (Model 2) and institutional (Model 3) determinants of mammography uptake within a country (“Analysis of determinants”) as well as the “channels” through which individual and institutional variables determine screening uptake (Models 4–7).
In a second step (“Analysis of variance”) this strategy allows me to analyse whether differences across countries in the individual and institutional screening determinants are related to screening differences across countries using the same sequential estimation strategy as above. To this aim I analyse the importance of the country level error v i term in Eq. 1 and whether the error term decreases after individual (Model 2), institutional (Model 3) and the other controls (Models 4–7) are included in the equation. The error term captures unobserved country-specific factors like institutional differences in health system regulation, financing and providing that cause differences in screening uptake across countries. The bigger the differences in screening rates across countries, the higher the value of the error term will be. The relative decrease in the error term as more individual and institutional controls are added to the model indicates the model’s ability to statistically explain cross-country differences. Finally, I calculate the intra-class correlation coefficients (ICC) as a relative indicator of country variation in screening rates:
The coefficient μ υ defines the proportion of variability in screening rates attributable to the level of the countries and is bounded between 0 and 1. The closer the ICC is to 0, the smaller the proportion of the total variance at the country level and the lower the relevance of country-specific factors (e.g. institutional factors) for differences in screening rates.
Results
Baseline results
Analysis of determinants
Table 3 presents the results of the analysis of screening determinants. The results of Model 1, used as the baseline model, indicate that the estimated mean screening rate across all countries is 63 per cent. Model 2 adds individual factors as explanatory variables. Almost all individual controls are significant.
More precisely, a younger age (reference category women aged 50–69 years), better health (as measured by self-assessed health and limitations in activities of daily livings), better education (i.e. higher ISCED, reference category ISCED 5–6) and better cognitive abilities (verbal fluency), having a partner, higher income, not working, a healthy behaviour (i.e. visit a dentist for prevention issues) and taking no medications (i.e. lower risk group) are consistently associated with higher rates of screening compliance (compare [6] for more details).
Model 3 adds institutional variables. The probability that a woman will get a mammogram increases by 38.7 percentage points if a region provides an organised population-based screening programme. Given the average screening rate of 63 per cent, this is a remarkable amount. Moreover, in countries where a screening programme is offered to women outside the age group of 50–69 years, the screening probability is on average 15 percentage points higher. After controlling for self-stated financial restrictions, other access problems (e.g. not near an available facility) (Model 4) and the reason “lack of information” (Model 5), the coefficients of the individual and institutional explanatory variables decrease slightly. For example, the coefficient of the organised screening programme drops from 0.387 to 0.370 (i.e. by about 4 per cent). This means statistically that the dummy for organised screening is negatively correlated with the variables included in Models 4 and 5 (i.e. financial restrictions, access and information problems). Thus, organised screening programmes seem to reduce perceived financial restrictions as well as access and information problems. However effects are very small, reflecting the fact that mammogram screening is generally fully covered by insurance in all countries [43]. However, including the variables “not usual” (Model 6) and especially “not necessary” (Model 7) as additional controls has a much bigger impact on the coefficients of the institutional explanatory variables. For example the coefficient of an organised screening programme drops statistically significantly by about 50 per cent from 0.37 (Model 5) to 0.19 (Model 7), indicating that the availability of an organised screening programme strongly reduces the probability that a woman believes a screening is “not necessary”. Moreover, the coefficients of many individual variables drop sharply towards zero (e.g. education, age, having a partner and income drop between 40 and 60 per cent, the decrease is statistically significant for the age categories, income and “ISCED 1 to 2”—category) and partly lose significance. This result suggests that being to elderly, having low income, being poorly educated and not having a partner are strongly related to believing that screening is not necessary.
In summary, individual and institutional factors can statistically explain screening uptake within a country. Quantitatively, the most important factor is the provision of an organised screening programme and a central mediating factor for most of the individual and institutional variables is whether a woman thinks that the screening is necessary or not.
Analysis of variance: what accounts for country differences?
A unique feature of multilevel regression analysis is the ability to partition the variance of the dependent variable at different levels (here country versus individual). Table 4 presents two absolute (standard deviation and variance) and one relative (ICC) indicator of variation in screening rates for the different models. For the sake of simplicity, I focus on the interpretation of the standard deviation and the ICC. Model 1 reveals a standard deviation in screening rates across countries of 19.5 per cent. After controlling for individual factors (Model 2), the standard deviation in screening rates between countries increases slightly by 1.3 percentage points to 20.8 per cent. Thus, country differences in individual factors (i.e. age, SAH, etc.) cannot explain country differences in screening rates but even lead to increased unexplained differences. By contrast, unexplained country differences in screening rates decrease sharply after controlling for institutional factors (e.g. organised screening programme). The standard deviation drops by 7.94 percentage points from 20.86 to 12.92 (i.e. in relative values by 38 per cent). The impact of the further inclusion of the self-stated reasons for not participating in screening to the regression model is analysed in Models 4–7. Stated financial restrictions and access problems (Model 4), “lack of information” (Model 5) and “not usual” (Model 6) cannot additionally explain differences in screening rates. In contrast, controlling for “not necessary” has an additional enormous impact on the unexplained standard deviation in screening rates among countries. Even after institutional and individual variables are controlled for, the standard deviation decreases by an additional 6.19 percentage points from 12.8 to 6.61 (i.e. in relative values by 52 per cent). Turning to the ICC results shows that in the model with no controls 21.3 per cent of the overall variance can be partitioned to the country level; this increases to 23.5 per cent after controlling for individual factors and drops sharply to 9.4 per cent when controlling for institutional factors. It drops further to 3.3 per cent when including all reasons for not participating.
Figure 3 visualises the additional explanatory power of each model and summarises the three striking results: First, observed individual factors cannot explain country differences. Second, observed institutional factors explain most of the country differences. For example, the higher screening rate in countries like Spain, The Netherlands, France or Sweden (compared to average screening rate across countries) is almost completely statistically explained by institutional factors (i.e. the availability of an organised screening programme and whether a screening programme is offered to women outside the age group of 50–69 years). Third, “not necessary” is the most important factor for explaining country differences even after individual and institutional factors are controlled for. For instance perceptions and attitudes to screening as measured by “not necessary" (Model 7) seem to be a big barrier in Denmark explaining much of the under-screening in this country (compared to the other countries).
Results of sensitivity analysis
Since the coefficients of the individual controls might differ across countries, I re-estimate the baseline models and additionally apply a random slope specification for the individual controls in each sequential model.Footnote 12 Thus, a random slope model adds a random error term to each coefficient in Eq. 1, accounting for possible differences in the relationship between explanatory and dependent variable across countries. Results are presented at the bottom of Table 4 and show slightly smaller standard deviations and variances. Thus, adjusting for random slopes explains country differences slightly better than not allowing for random slopes. However, in general, results remain robust if we allow for random slopes.
Another restrictive assumption within the multilevel model framework is that the country-specific effects (error terms) are uncorrelated with the individual and institutional explanatory variables. This assumption can be relaxed by a country fixed effects regression approach allowing for possible correlation of the country-specific effects with the explanatory variables. Thus, I re-estimate Models 1–7 by including a dummy variable for each country in Eq. 1 instead of inserting a country-specific random error term. However, a problem of the country fixed effects regression approach is that one cannot control for institutional variables that vary across countries, but display no within-country variability (i.e. extended age range). Thus, Table 5 presents the results of fixed effects regression without controlling for country-level institutional variables, i.e. for whether a country provides screening for an age range beyond 50–69 years. The results can be summarised as follows: First, the coefficients of the individual and institutional explanatory variables are quite similar to those in the multilevel models. Second, the differences between the country dummies greatly decrease after controlling for (1) an organised screening programme and (2) “not necessary” as the reason for not participating in screening. This is shown by the variances and standard deviations of country dummies presented at the bottom of Table 5. I calculate the variance (Var) of country dummies as follows:
where 13 is the number of countries included, di is the dummy of country i, and d is the average of the 13 country dummies as calculated by
The variance for Model 1 is a measure of the total differences in screenings rates between countries, while the varianced for Models 2–7 are measures of the remaining unexplained differences when taking account of an increasing number of individual and institutional characteristics as well as the different reasons for not participating in regular mammography screening. The results indicate the convergence of the variance of country dummies as one moves from Model 1 with enormous differences across countries (variance 355 per cent, standard deviation 18.85 per cent) to Model 3 with much lower differences (variance 202 per cent, standard deviation 14.24 per cent) to Model 7 with only minor differences (variance 53 per cent, standard deviation 7.34 per cent) in screening rates across countries. Thus, the results found are also robust to these changes in specification.
Finally, I tried alternative institutional variables (see Table 2 for these variables) for explaining country differences in screening rates. Since these variables are only available on the country level it is not possible to put them into the models simultaneously. The results are presented in Table 7 of the Appendix. At the top of the table, results are presented without controlling for whether an organised screening programme exists. At the bottom of Table 1 a control for the availability of an organised screening programme is presented. I find a strong statistically significant positive relation between screening uptake and “Age Rate Extended” as well as “Personally Invited”. Countries that offer screening to women beyond the age range of 50–69 years had a 27 percentage point higher screening uptake rate compared to countries that do not. Moreover, countries that have a 10 percentage point higher invitation rate have a 6.2 percentage point higher screening uptake rate. The results change strongly if I control for an organised screening programme indicating a strong correlation between the availability of an organised screening programme and both variables. The association between “Age Rate Extended” and screening uptake drops by 50 per cent but still remains significant and the relation between invitation rate and screening uptake vanishes. The other institutional variables (mammogram units, gatekeeper, fee for service, etc.) show, with and without controlling for an organised screening programme, no statistically significant relation to screening uptake and hardly influence the coefficient of organised screening demonstrating the dominant role of organised screening in explaining mammography decisions. Finally, the relation between screening uptake and breast cancer incidence as well as breast cancer mortality is analysed. Without controlling for organised screening I find a positive correlation between screening and incidence and a negative correlation between screening and mortality. However, the correlation between incidence of breast cancer and screening uptake turns out to be negative if I control for organised screening, indicating a positive association between breast cancer incidence and the availability of an organised screening programme.
Discussion and conclusion
The purpose of this article was to explore the statistical causes of country differences in screening rates of woman aged 50–69 using data from the SHARE. The results indicate that observed individual factors (i.e. age, education, etc.) can statistically explain within-country variation in screening rates but cannot explain between-country variations. Further, observed institutional factors (i.e. the availability of an organised screening programme and whether screening is provided beyond the age group of 50–69 years) can statistically explain about 40 per cent of the between-country differences in screening rates. The impact of these institutional factors seems to be mediated to a major extent (by about 50 per cent) by avoiding that a woman believes a screening is “not necessary”. This result might indicate that the availability of a screening programme increases perceived benefits of screening reflecting that national screening programmes have reduced mortality over time [7]. It also might resolve concerns about false-positive treatments, the treatment of potentially benign tumours or identifications of mainly low-risk cancers, since there is some evidence that organised screening protects better against harms compared to traditional opportunistic screening [34].Footnote 13 In contrast, the availability of an organised screening programme is merely associated with solving financial restrictions and access problems. This is hardly surprising since screening is generally fully covered by insurance in all countries independently of the kind of screening programme (organised vs. opportunistic) available [43]. Third, and additionally to the indirect association of “not necessary” with individual and institutional factors, differences across countries in whether a woman believes that screening is not necessary can statistically explain an additional 50 per cent of the between-country differences.
Policy implication
The availability of an organised screening programme (i.e. differences in the institutional framework) is strongly correlated with current screening differences across European countries. Since it is not clear to what extent people have an understanding of harms (e.g. overtreatment, anxiety associated with false-positive results) associated with screening when weighing up the costs and benefits of screening, this analysis can provide some insights about patient attitudes and understanding of benefits of breast cancer prevention and it highlights the possible importance of the availability of an organised screening programme for screening differences across European countries. If policy further intends to increase screening rates, changes in the institutional setting should address the perceived necessity of screening. Since patients often follow physician recommendation, public health interventions might address physicians’ recommendations on screening. In the context of an increasing debate on benefits, harms and side effects of screening, a balanced communication of these effects by public health authorities and physicians might help to ensure informed choice and consent of women.
The study has a number of limitations that need to be taken into account when interpreting the results: First, since I used survey data the results might be affected by non-response bias. The overall response rate was around 62 per cent and differs across countries (compare footnote 5). However, as shown in an experiment by Keeter et al. [49], even large differences in response rates may not lead to significant differences in key explanatory variables. Second, self-reported mammography use might be affected by reporting and recall bias. Caplan et al. [50] found in a US study that overall agreement between self-reported mammogram screening rates and automated records of mammographic examinations was high (82.7 per cent), but self-reported rates exceeded record rates by 8.2 per cent. Thus, using self-reported mammography in this study may have resulted in a slight overestimation of mammography use.
Third, results found in this study can only be interpreted as statistical causes (or statistical associations). No causal link between mammography use and the explanatory variables can be established. For example, information on possible driving factors behind the strong association between screening rates and the availability of an organised screening programme is missing. Plausibly, an organised screening programme imposes strong (economic) incentives fostering screening uptake as argued in this study. However, I cannot rule out that other unobservable factors like region-specific public debates and scientific disagreement on the effectiveness of screening might be correlated with the availability of organised screening programmes and might drive the strong effects found in this study.Footnote 14 Longitudinal data might be used to trace the effects of changes in the screening behaviour on corresponding changes in the implementation of screening programmes. Such analysis might be possible in the future as additional waves of SHARE data will be collected. Establishing further evidence on this link may be an important subject for future research and may also be of relevance for health policy.
Notes
Moreover, critics argue that problems associated with overdiagnosis and false positives might not be communicated adequately by public health communicators and physicians. For example screening invitations are not balanced regarding harms and benefits (compare Gøtzsche and Nielsen [10] or Gummersbach et al. [11]) and physicians often do not adequately communicate possible harms of screening (compare Wegwarth and Gigerenzer [12]). Furthermore, awareness of women regarding potential overdetection of indolent breast cancer is minimal [13]. Compare for the definition of overdiagnosis and false positives [8].
Note that physicians might depart from perfect agency especially if the financial incentives they face deviate from the interests of the patient (see McGuire [19] for a critical discussion). Moreover, with regard to risk communication in clinical practice in the context of cancer, physicians might have limited expertise to help patients make informed medical decisions as discussed by Schwartz et al. [26] or Wegwarth and Gigerenzer [12].
In the EU-member states the characteristics of organised screening programmes are widely generalisable [37].
Opportunistic screening happens when someone asks their doctor or health professional for a mammogram.
The SHARE used computer-assisted personal interviewing methods among participants drawn from probability samples in all contributing countries [38]. The sampling procedure followed a complex probabilistic multistage design to produce estimates representative of the non-institutionalised population above the age of 50 years in each country [38]. The SHARE overall response rate in 2004 was 61.8 per cent, being lowest in Switzerland (38 per cent) and highest in France (74 per cent). Compare Börsch-Supan and Jürges [39] for a comprehensive description of the methodology used.
I assumed that a woman had a screening regularly in 2004 (wave 1) and in 2006 (wave 2) if she stated in the SHARELIFE (wave 3 of the SHARE) that she always had a mammogram regularly at least every 2 years before 2007. I decided not to use the third wave of the SHARE, because many explanatory variables that are included in wave 1 and wave 2 are not included in wave 3. To account for repeated observations I calculated robust standard errors that considered clustering at the individual level. As a robustness test, I calculated all regressions using the last wave of SHARE data. The results were substantially unchanged and are available upon request.
For example better education may increase the use of screening services, implying more efficiency in producing health (e.g. [17]). A better educated woman may be more likely to understand the benefits of mammography screening. In addition, these women may be more prone to recognise the early warning signs of breast cancer and be more apt to visit a physician when symptoms first occur. Wübker [6] provides detailed reasoning, based on economic theory (i.e. human capital models), for including the above-mentioned variables. Moreover he tests them empirically using the SHARE database.
Autier et al. [45], p. 1188, argued that “the total radiologists registered in a country could represent a reasonable approximation to those specialising in mammography”.
For example a fee for service payment system might be associated with higher supply of screening, since doctors have financial incentives to increase the volume of screening and theory and empirical evidence show that physicians respond to incentives [23]. In contrast gatekeeping is expected to lead to less use of mammogram screening since women might see a general physician before going to the specialist (who is often provider of mammograms). A higher demand for breast cancer screening as measured by incidence rates should be associated with more screening activity.
SHARELIFE is wave 3 of the SHARE and focusses on people's life histories and contains detailed information on historical mammography screening use. The question in SHARELIFE is “What are the reasons you [have never had/stopped having] mammograms regularly?” It provides the following possible answers: (1) not affordable, (2) not covered by health insurance, (3) did not have health insurance, (4) time constraints, (5) not enough information about this type of care, (6) not usual to get this type of care, (7) no place to receive this type of care close to home and (8) not considered to be necessary. Compare Table A1 in the “Appendix” for the detailed definition of variables.
By assumption both error terms have zero mean and constant variances (σ 2, σ 2 ε ).
So far I assumed that the coefficients of the individual variables are identical for each country. However the impact of these variables might depend on the institutional framework within a country. For example, whether income has an impact on the individual screening decision depends on whether a woman has to pay for screening.
However, it might also reflect that invitation leaflets are not balanced with regards to benefits and harms of screening (compare [10, 11]). Gummersbach et al. [11] found that in different European counties (Germany, France, Spain and Italy) the information on side effects and risks provided by the brochures was generally of poor quality and none of them referred to the problem of overdiagnosis.
Considerable differences might exist in the scientific and public debate about screening benefits and harms across countries. For example, in Sweden leading professionals are positive about screening and two major RCTs were undertaken in there [5]. In contrast, influential Cochrane collaborators in Denmark [10] have heavily criticised the randomised trials, not least one of the Swedish trials. These country differences in the public debates and scientific disagreement on the harms and benefits of screening might influence both the differences in the availability of screening programmes as well as the differences in screening rates between Sweden and Denmark. However, I control for these unobservable impact factors within my sensitivity analysis using country-fixed effects. Moreover, I exploit regional differences in the availability of organised screening programmes (i.e. Copenhagen versus other Danish regions) to identify the impact of organised screening on screening uptake. Thus, only if public debates and scientific disagreement on the effectiveness of screening differ also within countries and are correlated with regional availability of organised screening programmes might the results be driven by these differences.
References
von Karsa, L.: Cancer screening in the European Union: Report on the implementation of the Council recommendations on cancer screening. European Commission, Luxembourg (2008)
OECD: Health at a Glance 2009: OECD Indicators, 5th edn. OECD (2009)
Ferlay, J., Autier, P., Boniol, M., Heanue, M., Colombet, M., Boyle, P.: Estimates of the cancer incidence and mortality in Europe in 2006. Ann. Oncol. 18(3), 581–592 (2007)
Fang, H.: Estimating dynamic discrete choice models with hyperbolic discounting, with an application to mammography decisions. NBER working paper series, vol. 16438, Cambridge (2010)
Vainio, H., Bianchini, F.: IARC handbooks of cancer prevention: Volume 7: breast cancer screening. IARC Press, Lyon (2002)
Wuebker, A.: Who gets a mammogram amongst European women aged 50–69 years? Health Econ. Rev. 2(6), 1–13 (2012)
Kalager, M., Zelen, M., Langmark, F., Adami, H.-O.: Effect of screening mammography on breast-cancer mortality in Norway. N. Engl. J. Med. 363(13), 1203–1210 (2010)
Raftery, J., Chorozoglou, M.: Possible net harms of breast cancer screening: updated modelling of Forrest report. Brit. Med. J. 343, d7627 (2011)
Bleyer, A., Welch, H.G.: Effect of three decades of screening mammography on breast-cancer incidence. N. Engl. J. Med. 367(21), 1998–2005 (2012)
Gøtzsche, P.C., Nielsen, M.: Screening for breast cancer with mammography. Cochrane Database of Systematic Reviews, Issue 1. Art. No.: CD001877 (2011). doi:10.1002/14651858.CD001877.pub4
Gummersbach, E., Piccoliori, G., Zerbe, C.O., Altiner, A., Othman, C., Rose, C., Abholz, H.: Are women getting relevant information about mammography screening for an informed consent: a critical appraisal of information brochures used for screening invitation in Germany, Italy, Spain and France. Eur. J. Public Health 20(4), 409–414 (2011)
Wegwarth, O., Gigerenzer, G.: There is nothing to worry about’’: gynecologists’ counseling on mammography. Patient Educ. Couns. 84, 251–256 (2011)
Hersch, J., Jansen, J., Irwig, L., Barratt, A., Thornton, H., Howard, K., McCaffery, K.: How do we achieve informed choice for women considering breast screening? Prev. Med. 53, 144–146 (2011)
Skinner, J.: Causes and consequences of regional variations in health care. In: Pauly, M.V., McGuire, T.G., Barros, P.P. (eds.) Handbook of Health Economics, vol. 2, pp. 46–93. North-Holland, Amsterdam (2012)
Chandra, A., Cutler, D., Song, Z.: Who ordered that? The economics of treatment choices in medical care. In: Pauly, M.V., McGuire, T.G., Barros, P.P. (eds.) Handbook of Health Economics, vol. 2, pp. 398–425. North-Holland, Amsterdam (2012)
Bolin, K., Lindgren, A., Lindgren, B., Lundborg, P.: Utilisation of physician services in the 50+ population: the relative importance of individual versus institutional factors in 10 European countries. Int. J. Health Care Finance Econ. 9(1), 83–112 (2009)
Grossman, M.: On the concept of health capital and the demand for health. J. Polit. Econ. 80(2), 223–255 (1972)
Cropper, M.L.: Health, investment in health, and occupational choice. J. Polit. Econ. 85(6), 1273–1294 (1977)
Howard, D.H.: Life expectancy and the value of early detection. J. Health Econ. 24(5), 891–906 (2005)
Schueler, K.M., Chu, P.W., Smith-Bindman, R.: Factors associated with mammography utilization: a systematic quantitative review of the literature. J. Womens Health (Larchmt) 17(9), 1477–1498 (2008)
Dardanoni, V., Wagstaff, A.: Uncertainty and the demand for medical care. J. Health Econ. 9(1), 23–38 (1990)
Lairson, D.R., Chan, W., Newmark, G.R.: Determinants of the demand for breast cancer screening among women veterans in the United States. Soc. Sci. Med. 61(7), 1608–1617 (2005)
McGuire, T.: Physician agency. In: Culyer, A., Newhouse, J. (eds.) Handbook of Health Economics, vol. 1, pp. 461–536. North-Holland, Amsterdam (2000)
Viscusi, W.K.: Do smokers underestimate risk? J. Polit. Econ. 98(6), 1253–1269 (1990)
Gigerenzer, G., Mata, J., Frank, R.: Public knowledge of benefits of breast and prostate cancer screening in Europe. J. Natl Cancer Inst. 101, 1216–1220 (2009)
Schwartz, L.M., Woloshin, S., Welch, H.G.: Risk communication in clinical practice: putting cancer in context. J. Natl. Cancer Inst. Monogr. 25, 124–133 (1999)
May, D.S., Kiefe, C.I., Funkhouser, E., Fouad, M.N.: Compliance with mammography guidelines: physician recommendation and patient adherence. Prev. Med. 28, 386–394 (1999)
Meissner, H.I., Breen, N., Taubman, M.L., Vernon, S.W., Graubard, B.I.: Which women aren’t getting mammograms and why? Cancer Causes Control 18(1), 61–70 (2007)
Peek, M.E., Han, J.H.: Disparities in screening mammography. Current status, interventions and implications. J. Gen. Intern. Med. 19(2), 184–194 (2004)
Legler, J., Meissner, H.I., Coyne, C., Breen, N., Chollette, V., Rimer, B.K.: The effectiveness of interventions to promote mammography among women with historically lower rates of screening. Cancer Epidemiol Biomarkers Prev. 11(1), 59–71 (2002)
Yasmeen, S., Romano, P.S., Tancredi, D.J., Saito, N.H., Rainwater, J., Kravitz, R.L.: Screening mammography beliefs and recommendations: a web-based survey of primary care physicians. BMC Health Serv. Res. 12, 32 (2012)
Mandelblatt, J.S., Yabroff, K.R.: Effectiveness of interventions designed to increase mammography use: a meta-analysis of provider-targeted strategies. Cancer Epidemiol Biomarkers Prev. 8(9), 759–767 (1999)
Li, J., Hurley, J., DeCicca, P., Buckley, G.: Physician response to pay-for-performance: Evidence from a natural experiment. NBER working paper series, vol. 16909, Cambridge (2011)
Miles, A., Cockburn, J., Smith, R.A., Wardle, J.: A perspective from countries using organised screening programmes. Cancer 101(5 Suppl), 1201–1213 (2004)
Levin, T.R., Jamieson, L., Burley, D.A., Reyes, J., Oehrli, M., Caldwell, C.: Organized colorectal cancer screening in integrated health care systems. Epidemiol. Rev. 33(1), 101–110 (2011)
Strumpf, E.C., Chai, Z., Kadiyala, S.: Adherence to cancer screening guidelines across Canadian provinces: an observational study. BMC Cancer 10, 304 (2010)
Commission of the European Communities—COM. Report from the commission to the council. The European Parliament, The European Economic and Social Committee and the Committee of the Regions. (2008). http://ec.europa.eu/health/ph_determinants/genetics/documents/com_2008_882.en.pdf, download: 20.08.2012
Andreyeva, T., Michaud, P.C., van Soest, A.: Obesity and health in Europeans aged 50 years and older. Public Health 121, 497–509 (2007)
Börsch-Supan, A., Jürges, H.: The survey of health, aging and retirement in Europe—methodology. Technical Report, Mannheim Research Institute for the Economics of Aging, Mannheim (2005)
Kooperationsgemeinschaft Mammographie: Evaluationsbericht 2008–2009: Ergebnisse des Mammographie-Screening-Programms in Deutschland. Berlin, (2012)
Giorgi, D., Giordano, L., Ventura, L., Frigerio, A., Paci, E., Zappa, M.: Mammography screening in Italy: 2004 survey and 2005 preliminary data. Epidemiol. Prev. 31(2–3 Suppl 2), 7–20 (2007)
Jørgensen, K.J., Zahl, P.-H., Gøtzsche, P.C.: Breast cancer mortality in organised mammography screening in Denmark: comparative study. Brit. Med. J. 340, c1241 (2010)
Schopper, D., de Wolf, C: Breast cancer screening by mammography: International evidence and the situation in Switzerland, Krebsliga Schweiz. (2007). http://assets.krebsliga.ch/downloads/short_mammo_report_final_el_.pdf, download: 20.07.2012
National Cancer Institute; International Cancer Screening Network: Organization of breast cancer screening programs in 27 ICSN countries, 2007–2008. 2012. http://appliedresearch.cancer.gov/icsn
Autier, P., Ouakrim, D.A.: Determinants of the number of mammography units in 31 countries with significant mammography screening. Br. J. Cancer 99(7), 1185–1190 (2008)
European Observatory on Health Systems and Policies. Health Systems in Transition (HiT) series (2012). Diverse country reports available from: http://www.euro.who.int/en/who-we-are/partners/observatory/health-system-reviews-hits/full-list-of-hits. Download: 20.09.2012
Biesheuvel, C., Weigel, S., Heindel, W.: Mammography screening: evidence, history and current practice in Germany and other European countries. Breast Care 6(2), 104–109 (2011)
Rice, N., Jones, A.: Multilevel models and health economics. Health Econ. 6, 561–575 (1997)
Keeter, S., Miller, C., Kohut, A., Groves, R.M., Presser, S.: Consequences of reducing nonresponse in a national telephone survey. Public Opinion Q 64, 125–148 (2000)
Caplan, L.S., Mandelson, M.T., Anderson, L.A.: Validity of self-reported mammography: examining recall and covariates among older women in a health maintenance organization. Am. J. Epidemiol. 157, 267–272 (2003)
Acknowledgment
The author thanks Marc Dusheiko, Jens Harbecke, Jan Kleibrink, Jürgen Maurer, Maren M. Michaelsen and all participants of the PhD seminar on health economics and policy in Grindelwald for valuable comments. All the remaining errors are mine. This paper uses data from SHARELIFE release 1, as of November 24th 2010 or SHARE release 2.3.1, as of July 29th 2010. The SHARE data collection has been primarily funded by the European Commission through the 5th framework programme (project QLK6-CT-2001- 00360 in the thematic programme Quality of Life), through the 6th framework programme (projects SHARE-I3, RII-CT- 2006-062193, COMPARE, CIT5-CT-2005-028857, and SHARELIFE, CIT4-CT-2006-028812) and through the 7th framework programme (SHARE-PREP, 211909 and SHARE-LEAP, 227822). Additional funding from the U.S. National Institute on Aging (U01 AG09740-13S2, P01 AG005842, P01 AG08291, P30 AG12815, Y1- AG-4553-01 and OGHA 04-064, IAG BSR06-11, R21 AG025169) as well as from various national sources is gratefully acknowledged (see http://www.share-project.org/t3/share/index.php for a full list of funding institutions).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wübker, A. Explaining variations in breast cancer screening across European countries. Eur J Health Econ 15, 497–514 (2014). https://doi.org/10.1007/s10198-013-0490-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-013-0490-3