Abstract
Background
Golimumab is a novel TNF-α inhibitor licensed to treat patients with active PsA. Although its clinical efficacy has been proven in clinical trials, its cost effectiveness is yet to be established.
Objectives
To estimate the cost effectiveness of golimumab among patients with active PsA from the UK NHS perspective.
Methods
A decision analytic model was used to simulate progression of a hypothetical cohort of active PsA patients on golimumab and other TNF-α inhibitors as well as palliative care. The clinical evidence was derived from clinical trials of TNF-α inhibitors and compared using mixed treatment models. The primary outcome measure was quality-adjusted life years (QALYs) estimated based on change in Health Assessment Questionnaire (HAQ) and Psoriasis Area Severity Index (PASI) from baseline. The annual acquisition cost of golimumab was assumed to be identical to annual cost of other subcutaneous TNF-α inhibitors. The resource use costs and outcomes were discounted at 3.5% over a period of 40 years. The uncertainty surrounding important variables was further explored using probabilistic sensitivity analyses (PSA).
Results
TNF-α inhibitors were significantly superior to palliative care but comparable to each other on Psoriatic Arthritis Response Criteria (PsARC), HAQ and PASI response. The incremental cost effectiveness ratio (ICERs) for golimumab compared to palliative care was £16,811 for PsA patients and £16,245 for a subgroup of PsA patients with significant psoriasis. At an acceptability threshold of £30,000 per QALY, the probability of golimumab being cost effective is 89%.
Conclusion
Once monthly, golimumab is a cost-effective treatment alternative for patients with active PsA. With its patient-focussed attributes, golimumab is likely to offer additional choice in PsA treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psoriatic arthritis (PsA) is a progressive, chronic inflammatory condition that can affect the joints and adjoining connective tissue in patients with psoriasis [1]. Signs and symptoms may range from mild synovitis to severe erosive arthropathy with joint stiffness, pain, swelling and tenderness and associated psoriasis of the skin and nails [1]. Surrounding tendons and ligaments also may be affected [1]. Psoriasis as such is seen in PsA patients even before the signs of joint disease have developed; the mean time to onset of joint disease being 10 years after development of first signs of psoriasis [2]. Dactylitis and synovitis affects 16–48% of PsA patients [2–4]. Because of the visibility of skin and nail involvement, patients with PsA may also suffer from poor psychosocial function and psychological consequences, such as embarrassment, self-consciousness and potentially from depression [5]. This further results in significant impairment of health-related quality of life (HRQoL). There can be substantial disability and morbidity associated with PsA with an increased rate of mortality [6]. PsA affects both the genders equally with age of onset being 30–55 years [6].
PsA has a significant economic burden with direct costs as high as $1.9 billion which include pharmacologic and non-pharmacologic treatment, hospitalization and physician visits [7]. Estimates from the National Database of the German Collaborative Arthritis Centres in 908 patients with PsA place the mean annual direct medical cost per patient at €3,162 and the mean indirect cost per patient at €11,075 in Germany in 2002 [8]. The major cost drivers of direct costs were hospitalizations and drug treatment [8]. A study by Mau and colleagues has shown a statistically significantly lower rate of employment for patients with PsA and an increased relative risk for unemployment with longer disease duration [9]. Based on the German PsA sample, only 63% of PsA patients were currently employed, even though the mean patient age was only 49 years [8]. Similar levels of work disability were observed in the Norwegian Disease Modifying Antirheumatic Drug (DMARD) study [10].
The goal of PsA therapy should be to improve upon the signs and symptoms of the disease and subsequently improve the quality of patient’s life [11, 12]. Traditionally, DMARDs were used to control the disease, but their use is largely derived from analogy of rheumatoid arthritis [1]. In addition, DMARDs usually have a slow onset of action, and close monitoring of the patient is required due to toxicities [13].
Newer developments in this area have focused on tumour necrosis factor-alpha inhibitors (TNF-α inhibitors) which have been shown to be efficacious in PsA patients [11]. These agents have shown marked improvements in skin and joint manifestations of PsA and have to a large extent improved patients’ QoL. Three TNF-α inhibitors agents are currently available for the treatment of PsA: etanercept, adalimumab and infliximab. Despite their efficacious results, TNF-α inhibitors are often perceived to be expensive treatments.
Golimumab, the first once-monthly subcutaneous TNF-α inhibitor, has demonstrated efficacy in active and progressive PsA patients in treating both rheumatic as well as psoriatic components [13]. However, similar to other TNF-α inhibitors, it is likely to be perceived as an expensive treatment adding further pressure on the healthcare budgets. The present economic analyses therefore were aimed to evaluate the long-term cost effectiveness of golimumab 50 mg once monthly compared to palliative care. This was assessed in conjunction with other subcutaneous TNF-α inhibitors to assess its relative position compared to palliative care. The NICE guidance at the time of this analysis stipulated the use of etanercept or adalimumab as the choice of TNF-α inhibitor in PsA [6]. As a result, infliximab was hardly used in clinical practice in the UK. We therefore restricted our comparators to subcutaneous TNF-α inhibitors such as etanercept and adalimumab. A separate analysis was also performed for the subgroup of PsA patients with a significant psoriatic component at baseline (BSA of ≥3%).
Methods
Patients and interventions
Our economic analyses focused on active PsA patients defined by the presence of at least 3 swollen and 3 tender joints, negative rheumatoid factor, at least 1 subset of PsA, and the presence of plaque psoriasis with a qualifying lesion at least 2 cm in diameter [13]. Of these patients, 73% had significant psoriasis at baseline [13]. For these patients only, the treatment benefits on psoriasis were estimated using the Psoriasis Area Severity Index (PASI). The treatment benefit on the rheumatic component was modelled for all patients including those with no significant psoriasis. The effect of TNF-α inhibitor on rheumatic component was estimated using the Health Assessment Questionnaire (HAQ). The baseline scores for HAQ and PASI were derived from golimumab clinical trial in PsA and were assumed to be 1.02 and 9.9, respectively [13]. Patients entered the model at the age of 47 and 60% were men [13].
Model overview
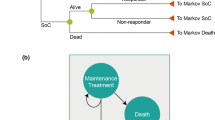
The model structure in terms of cohort flow is displayed below in Fig. 1 [14]. This model has been adopted from a recent study by Cummins and colleagues to include golimumab as an additional treatment alternative and thus relates very closely with that model [14]. The model can briefly be summarised as having a 1st cycle of 0–12 weeks, a 2nd cycle of 13–24 weeks, and thereafter annual cycles [14]. A study by Rogers and colleagues has also used a similar model [15]. The primary difference between our model and the Rogers model is the inclusion of a second 12 weeks cycle stretching from week 13 to week 24 in our analysis. This second cycle was included based on golimumab trial analysis which suggested continued improvement in the psoriatic component of the disease beyond initial 12 weeks [13]. Patients enter the model in an active PsA state. Those responding to treatment as estimated by Psoriatic Arthritis Response Criteria (PsARC) response at 12 weeks continue with treatment whilst non-responders are withdrawn from treatment and moved on to palliative care [14]. Patients continuing on TNF-α inhibitors were assumed to have an annual withdrawal rate due to loss of response or side effects, and this withdrawal probability was estimated to be 16.5% annually [15].
Our analysis compared the licensed dose of golimumab (50 mg) with palliative care comprising non-biologic DMARDs. In addition, we also investigated the relative cost effectiveness of other subcutaneous TNF-α inhibitors compared palliative care.
Efficacy estimates and transitions
Since no head-to-head efficacy data between TNF-α inhibitors were available, evidence synthesis techniques were used to estimate their relative efficacy compared to palliative care. We used the evidence synthesis model developed by Cummins and colleagues and adopted it to include golimumab as a treatment alternative [14]. Briefly, the patient outcomes of interest were PsARC response to treatment, the effect on HAQ score and, in the subgroup of patients with significant psoriasis at baseline, the effect on PASI score. The latest available endpoints were used in this evidence synthesis. Thus, for PsARC, the data used were response data at week 12 or 14, depending on the study. For HAQ, the data used included week 12 and 24 for adalimumab, week 14 and 16 for infliximab, week 12 for etanercept and week 14 for golimumab. Similarly, for PASI, the data used included week 24 for adalimumab, week 14 and 16 for infliximab, week 24 for etanercept and week 14 for golimumab. Efficacy estimates were derived using incremental treatment effect for TNF-α inhibitors. In particular, the probability of PsARC response with treatment was modelled as comprising of the probability of PsARC response with placebo and a treatment-related increment, this being on the log-odds scale. Similarly, the HAQ change given PsARC response with treatment was also modelled as comprising of the HAQ change given PsARC response with placebo plus a treatment-related increment. The HAQ and PASI change among non-responders was estimated using similar technique. The PASI was modelled as an aggregate across patients with or without a PsARC response. The trial analysis suggested that the PASI change and HAQ change were not correlated, and therefore, no such correlation was assumed in the group of patients with significant psoriasis. The HAQ change from baseline to the last randomised data point of up to week 24 is the main outcome of interest and is the main determinant of the outcomes of the economic model. The HAQ change is assumed to be identical for the subgroups with or without significant psoriasis at baseline based on a separate analysis of golimumab trial data [13]. The evidence synthesis model by Cummins and others included infliximab as a comparator. The authors had access to patient-level data from infliximab and golimumab RCTs, and their model included estimates based on patient-level information on etanercept. Therefore, we preferred to adopt their model even though our analysis excluded infliximab as a comparator [13]. The data sources used in the evidence synthesis model are displayed in Fig. 2.
For those coming off treatment, rebound was modelled under two scenarios: rebound equal to gain with natural history disease progression thereafter, and rebound equal to the natural history disease progression as would have occurred from baseline with palliative care alone. These scenarios are further depicted in Fig. 3. The natural history disease progression for HAQ was assumed to be as per the evidence synthesis from Woolacott study and was estimated at 0.0719 annually and 0.016 for 12 weeks [16].
While PASI scores do vary through time for a patient, no further progression was assumed for patients receiving TNF-α inhibitor therapy [15]. Patients coming off TNF-α inhibitors were assumed to rebound back to their baseline PASI values and remain at that value for rest of the analysis period. The mortality multipliers for psoriatic arthritis were derived from a study by Wong and others and were estimated to be 1.60 for females and 1.66 for males [17].
Costs
Perspective
UK NHS perspective was adopted on costs. The base year for prices was 2009 and the drug prices were taken from BNF58. The full-year drug acquisition cost of golimumab used in the analysis was £9,295; equivalent to adalimumab and etanercept. All other costs except for drug costs have been inflated using PSSRU HCHS index. Indirect costs in terms of productivity loss have been excluded.
Drug administration and monitoring costs
The administration and monitoring costs for golimumab were assumed to follow the same schedule as other subcutaneous TNF-α inhibitors and were derived from the study by Cummins and colleagues [14]. The detailed costs have been outlined in Table 1. In summary, the administration costs for subcutaneous TNF-α inhibitors included two outpatient visits to rheumatology department and 4 h of staff nurse time during first 24 weeks of treatment. No further administration cost was assumed. The resultant estimated total costs of administration were £330.71 for first 12 weeks, £91.50 for 12–24 weeks and, £0 annually thereafter for all the three subcutaneous TNF-α inhibitors including golimumab. The ongoing monitoring costs for subcutaneous therapies included one outpatient visit to rheumatology department in first 12 weeks followed by two outpatient visits annually. The resultant estimated total costs of monitoring were £182.28 for first 12 weeks, £72.30 for 12–24 weeks and, £313.30 annually thereafter for all the three subcutaneous TNF-α inhibitors including golimumab.
Costs associated with PsA
The ongoing cost of PsA was derived from the previous appraisal of TNF-α inhibitors in PsA by Bravo Vergel and colleagues, who estimated a resource use cost of £357.57 (SE: £231.3) per point increase in HAQ per year with a constant of £1,182.09 (SE: £416.1) [18]. Patients remaining on treatment were assumed to incur 85% of these costs while those withdrawing from treatment and moving on to palliative care were assumed to incur 100% of these costs. Similarly, for patients with significant psoriasis, ongoing costs as a function of PASI were derived from a survey of 20 dermatologists. This data collection focussed on inpatient, consultant-led outpatient, nurse-led outpatient and phototherapy costs associated with different PASI scores. For psoriatic patients with significant psoriasis, an additional cost of £167 per PASI point increase was applied based on the results of this survey [14].
Outcomes
The primary outcome measure was quality-adjusted life years (QALYs). This was based on a regression recently published in an assessment report for a technology appraisal [15]. The assessment report also included three separate algorithms submitted by individual manufacturers during the appraisal. We selected the algorithm developed by Rogers and colleagues as an independent source used by the National Institute of Health and Clinical Excellence (NICE) during the appraisal [15]. The regression estimated the utility based on both HAQ and PASI scores and is displayed below.
Cost effectiveness analyses
In the base case, cost effectiveness was estimated for all PsA patients. A separate analysis was conducted for patients with significant psoriasis (BSA ≥ 3%). An annual discount rate of 3.5% was applied for costs and benefits. Multiple one-way sensitivity analyses were conducted varying the parameters such as baseline HAQ score, baseline PASI score, analysis time horizon, HAQ rebound assumptions, HAQ reduction beyond the first two cycles, withdrawal rates and natural history HAQ progression. The uncertainty surrounding important variables was further explored using probabilistic sensitivity analyses (PSA) with 10,000 simulations.
Results
Cost effectiveness analyses
The results of the indirect comparison between TNF-α inhibitors and palliative care are displayed in Table 2. Results showed that all TNF-α inhibitors were significantly superior to palliative care in PsARC response in all patients and change in PASI score from baseline for patients with significant psoriasis. Among the three TNF-α inhibitors, the incremental treatment effect compared to palliative care estimated as PsARC response, HAQ reduction and PASI reduction was similar with no significant differences between the TNF-α inhibitors.
In the cost effectiveness analysis for PsA patients, etanercept generated 7.69 mean QALYs for a mean total cost of £94,578 over 40 years. This was followed by golimumab with mean QALYs of 7.34 at a mean total cost of £94,151. Due to its lower PsARC response and fewer patients continuing on treatment, adalimumab generated lower mean QALYs (6.97) for a lower mean total cost (£86,410) compared to the other TNF-α inhibitors. A similar trend was also observed among subset of PsA patients with significant psoriasis and no psoriasis as displayed in Table 2.
The results of the cost effectiveness analyses demonstrate that for a patient with active PsA, golimumab was a cost-effective treatment alternative with the Incremental Cost Effectiveness Ratio (ICER) of £16,811 per QALY compared to palliative care. The ICERs for other TNF-α inhibitors were similar with all the three subcutaneous TNF-α inhibitors well within the acceptable threshold of £30,000 per QALY as displayed in Table 3. Among patients with significant psoriasis at baseline, the ICER for golimumab compared to palliative care was £16,245 per QALY. In this psoriatic subgroup, other TNF-α inhibitors also were cost effective with ICERs similar to golimumab.
The results of one-way sensitivity analysis suggested that changing the rebound assumption to ‘rebound to natural history’ had significant impact on resultant ICERs with all the three TNF-α inhibitor ICERs increasing above £30,000 per QALY threshold. The analysis was less sensitive to baseline HAQ or PASI score changes as well as change in treatment withdrawal rates. All the parameters explored in the one-way sensitivity analyses and their impact on the golimumab ICERs have been displayed in Table 4. The PSA indicated that the probability of golimumab being cost effective is 50 and 89% at £20,000 and £30,000 per QALY thresholds respectively as displayed in Fig. 4.
Discussion
Golimumab is the first and only once-monthly subcutaneous TNF-α inhibitor agent offering convenient dosing regimen and reduced injection site reactions [13]. Apart from demonstrating the treatment benefit on conventional outcomes such as PsARC, PASI and ACR, golimumab has also demonstrated significant benefits on nail psoriasis and inhibition of structural damage its clinical trials [13]. The aim of this analysis was to assess the cost effectiveness of golimumab at the licensed dose of 50 mg. Although the cost effectiveness of golimumab has not been assessed in any of the previous studies, the results of other TNF-α inhibitors in this analysis were similar to those observed in literature. Two previous published analyses have used a similar methodology and model structure to this analysis [14, 18]. The study by Cummins and colleagues reported ICERs for both etanercept and adalimumab to be around £20,000 per QALY threshold. The results from analysis by Rogers and others were also similar with comparable ICERs at £17,000 per QALY.
This analysis attempted to combine the most recent data and robust assumptions from both these analysis, and therefore, the results obtained are consistent with the previously published work. Since both the evidence synthesis model and the economic analysis model used in this analysis were adopted from Cummins and others, a more detailed description of the approach is available elsewhere [14]. However, it is important discuss the fundamental assumptions that significantly impact the results. We assumed rebound equal to gain in our base case analysis and explored the impact of changing the rebound assumption to natural history in the sensitivity analysis. In the true clinical practice, we expect the rebound effect to be in between these two extreme scenarios. However, in the absence of any conclusive evidence of rebound effect in the literature, it is difficult to ascertain the true impact of this assumption on the resultant ICERs. Similarly, in the absence of any data in literature on PASI natural progression and PASI rebound following loss of response, we assumed PASI to remain constant as long as the patient was responding and revert back to baseline following loss of response. This is in accordance with a recent NICE appraisal [19]. Due to lack of any available data, it is impossible to determine whether this assumption is optimistic or conservative and how much PASI should be varied over time to estimate the underlying uncertainty. We also excluded the adverse events (AEs) from our analysis. The clinical trials have demonstrated that the AEs associated with TNF-α inhibitors to be infrequent, minor and not significantly different from palliative care. We therefore do not anticipate AEs to have a significant impact on the costs or QALYs and thus on the final results. On the contrary, the utility estimation method significantly affects the ICERs. We selected EQ-5D in the base case as it is recommended by NICE and has been used in previous analyses. We believe that EQ-5D is a more appropriate scale in PsA due to its domains (mobility, self care, usual activities and pain) that capture highly relevant information on parameters affecting a PsA patient. Utilities derived using other elicitation methods, however, may significantly impact the ICERs.
The evidence synthesis used in the analysis resulted in non-significant differences between golimumab and other TNF-α inhibitors which is consistent with the previous published work. Due to the non-significant point estimates and the surrounding uncertainty, we decided not to compare golimumab with other TNF-α inhibitors and derive comparative ICERs. With comparable results across a range of efficacy and cost parameters, we believe that TNF-α inhibitors should be viewed as a class and golimumab as a novel addition to the treatment armamentarium.
Conclusion
In this analysis, we compared golimumab and other subcutaneous TNF-α inhibitors with palliative care. All the TNF-α inhibitors were significantly superior to palliative care on all outcome measures and were cost effective within the acceptability thresholds. Golimumab thus exerts no significant additional burden on the healthcare system above and beyond the existing TNF-α inhibitors. With no significant differences between the efficacy, safety and cost effectiveness of TNF-α inhibitors, the choice of TNF-α inhibitor is likely to be driven by patients and the healthcare professionals.
References
Gladman, D.D., Antoni, C., Mease, P., Clegg, D.O., Nash, P.: Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann. Rheum. Dis. 64(II), ii14–ii17 (2006)
Krueger, G.G.: Clinical features of psoriatic arthritis. Am. J. Manag. Care 8, S160–S170 (2002)
Gottleib, A., Korman, N.J., Gordon, K.B., et al.: Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 2. J. Am. Acad. Dermatol. 58, 851–864 (2008)
Helliwell, P.S.: Therapies for dactylitis in psoriatic arthritis. A systematic review. J. Rheumatol. 33, 1439–1441 (2006)
Mease, P.J.: Assessing the impact of psoriatic arthritis on patient function and quality of life: lessons learned from other rheumatologic conditions. Semin. Arthritis Rheum. 38, 320–335 (2009)
TAG 104. Etanercept and infliximab for the treatment of adults with psoriatic arthritis. NICE technology appraisal guidance 104. July 2006:18
Williams, J.P., Meyers, J.A.: Immune-mediated inflammatory disorders (I.M.I.D.s): the economic and clinical costs. Am. J. Manag. Care 8, S664–S681 (2002)
Huscher, D., Merkesdal, S., Thiele, K., Ziedler, H., Schneider, M., Zink, A.: Cost of illness in rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis and systemic lupus erythematous in Germany. Ann. Rheum. Dis. 65, 1175–1183 (2006)
Mau, W., Listing, J., Huscher, D., Zeidler, H., Zink, A.: Employment across chronic inflammatory rheumatic diseases and comparison with the general population. J. Rheumatol. 32, 721–728 (2005)
Wallenius, M., Skomsvoll, J.F., Koldingsnes, W., et al.: Work disability and health-related quality of life in males and females with psoriatic arthritis. Ann. Rheum. Dis. 68, 685–689 (2009)
Kyle, S., Chandler, D., Griffiths, C.E.M., et al.: Guideline for anti-TNF-a therapy in psoriatic arthritis. Rheumatology 44, 390–397 (2005)
Kavanaugh, A.F., Ritchlin CT, GRAPPA Treatment Guideline Committee.: Systematic review of treatments for psoriatic arthritis: an evidence based approach and basis for treatment guidelines. J. Rheumatol. 33, 1417–1421 (2006)
Kavanaugh, A., McInnes, I., Mease, P., et al.: Golimumab, a new human tumor necrosis factor-α antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis. Arthritis Rheum. 60, 976–986 (2009)
Cummins, E., Asseburg, C., Punekar, Y. et al.: Cost-effectiveness of infliximab for the treatment of active and progressive arthritis. Value in Health (2010)
Rogers, M., Epstein, D., Bojke, L., et al.: Etanercept, Infliximab and Adalimumab for the treatment of Psoriatic Arthritis: a systematic review and economic evaluation. Final Report 4th December 2009; Available at http://www.guidance.nice.org.uk/TA/WaveR/36
Woolacott, N., Bravo Vergel, Y., Hawkins, N., et al.: Etanercept and infliximab for the treatment of psoriatic arthritis: a systematic review and economic evaluation. Health Technol. Assess. 10, 1–265 (2006)
Wong, K., Gladman, D.D., Husted, J., et al.: Mortality studies in psoriatic arthritis: results from a single outpatient clinic. I. Causes and risk of death. Arthritis Rheum. 40, 1868–1872 (1997)
Bravo Vergel, Y., Hawkins, N.S., Claxton, K., et al.: The cost-effectiveness of etanercept and infliximab for the treatment of patients with psoriatic arthritis. Rheumatology 46, 1729–1735 (2007)
NICE TAG 199. Etanercept, infliximab and adalimumab for the treatment of psoriatic arthritis (review of technology appraisal guidance 104 and 125). NICE 2010. Available at http://www.nice.org.uk/nicemedia/live/13110/50422/50422.pdf accessed on 18th April 2011
Antoni, C., Krueger, G., de Vlam, K., et al.: Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann. Rheum. Dis. 64, 1150–1157 (2005)
Antoni, C.E., Kavanaugh, A., Kirkham, B.: Sustained benefits of infliximab therapy for dermatological and articular manifestations of psoriatic arthritis: results from the Infliximab Multinational Psoriatic Arthritis Controlled Trial (IMPACT). Arthritis Rheum. 4, 1227–1236 (2005)
Mease, P.J., Goffe, B.S., Metz, J., et al.: Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet 356, 385–390 (2000)
Mease, P.J., Kivitz, A.J., Burch, F.X., et al.: Etanercept treatment of psoriatic arthritis safety, efficacy, and effect on disease progression. Arthritis Rheum. 50(7), 2264–2272 (2004)
Mease, P.J., Gladman, D.D., Ritchlin, C.T., et al.: Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a randomised placebo controlled trial. Arthritis Rheum. 52(10), 3279–3289 (2005)
Genovese, M.C., Mease, P.J., Thomson, G.T., et al.: Safety and efficacy of adalimumab in treatment of patients with psoriatic arthritis who had failed disease modifying antirheumatic drug therapy. J. Rheumatol. 34(6), 1439 (2007)
Acknowledgments
This study was jointly funded in full by Schering-Plough Ltd and Centocor Ortho Biotech Inc, and the writing of this paper was funded in full by Schering-Plough Ltd.
Conflict of interest
Ewen Cummins and Christian Asseburg have no conflicts of interest. Christian Asseburg was employed by Swedish Institute of Health Economics when this study was conducted. Yogesh Punekar and Manishi Prasad are employed by Schering-Plough Ltd. Jacqueline Buchanan was employed by Centocor Ortho Biotech Inc when this study was conducted.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cummins, E., Asseburg, C., Prasad, M. et al. Cost effectiveness of golimumab for the treatment of active psoriatic arthritis. Eur J Health Econ 13, 801–809 (2012). https://doi.org/10.1007/s10198-011-0335-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-011-0335-x