Abstract
This cost-of-illness analysis based on information from 2973 patients with multiple sclerosis (MS) in Germany is part of a Europe-wide study on the costs of MS. The objective was to analyze the costs and quality of life (QOL) related to the level of disease severity. Patients from six centres (office- and hospital-based physicians) and patients enrolled in a database were asked to participate in the survey; 38% answered a mail questionnaire. In addition to details on the disease (type of disease, relapses, level of functional disability), the questionnaire asked for information on all resource consumption, medical, non-medical, work absence, informal care, as well as QOL (measured as utility). The mean age of the cohort was 45 years, and 18% of patients were 65 years of age or older. Forty-seven percent of patients had mild disease (Expanded Disability Status Scale [EDSS] score 0–3), 36% had moderate disease (EDSS score 4–6.5) and 12% had severe disease (EDSS score ≥7). The mean EDSS score in the sample was 3.8 (median 4.0), with a mean utility of 0.62. Costs and utility are highly correlated with disease severity. Workforce participation decreases from 73% in very early disease to less than 10% in the very late stages, leading to a tenfold rise in productivity losses in the late stages of disease. Hospitalisation and ambulatory visits rise by a factor of 5–6 between early and late disease; investments and services increase from basically no cost to € 2700; and informal care increases by a factor of 27 for patients with an EDSS score of 7 and by a factor of 50 for patients at the very severe end of the EDSS scale (8–9). Hence, total mean costs per patient are determined essentially by the distribution of the severity levels in the sample, increasing from approximately € 18 500 at an EDSS score of 0–1 to € 70 500 at an EDSS score of 8–9. The same is true for utility, which decreases from 0.86 to 0.10 as the disease becomes severe. However, the utility loss compared to the general population is high at all levels of the disease, leading to an estimated loss of 0.2 quality-adjusted life-years per patient. Relapses are associated with a cost of approximately € 3 000 and a utility loss of 0.1 during the quarter in which they occur. Compared with a similar study performed in 1999, resource consumption, with the exception of drugs, is somewhat lower. This is most likely due to a difference in the severity distribution of the two samples and to changes in health-care consumption overall in the country, such as the introduction of diagnosis-related groups (DRGs, Fallpauschalen).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
This study of costs and quality of life (QOL) of patients with multiple sclerosis (MS) in Germany is part of a multinational study in nine European countries on the burden multiple sclerosis (MS) [1], and was carried out during the spring of 2005. This paper presents details on data collection, cost calculations and results for Germany. (For a detailed discussion on the methods used for the study, see the general paper on methodology by Kobelt et al. in this supplement.)
As for all countries included in this European study, German results are presented from the societal perspective (all costs regardless of who pays, excluding transfer costs such as taxes). This eliminates one of the methodological differences that render comparisons between countries difficult. However, costs from the perspective of payers, i.e. health-care insurance companies, social services and pension funds, are also presented, as these are more relevant for individual organizations involved in paying for health-care.
The prevalence of MS in Germany varies somewhat between studies, in different regions and at different points in time, ranging from 43 to 145 per 100 000 inhabitants [2–5]. The number of patients suffering from MS in Germany is estimated at 100 000–120 000 [6]. However, few studies on the distribution of the course of MS and degrees of severity exist that would allow assessment of how representative a sample is.
An early study reported that patients with relapsing-remitting MS (RRMS) represented 63%, those with relapsing progressive MS (RPMS) or secondary progressive MS (SPMS) 25%, and those with primary progressive MS (PPMS) 12% [7]. This is not very different from a recent estimate for Europe by Pugliatti and colleagues, where RRMS was estimated at 45–65%, RPSM and SPMS at 20–40% and PPMS at 10–15% [4]. The distribution found in an earlier cost-of-illness study in Germany [6] was relatively close to these estimates, with 41% of patients with benign or relapsing disease, 35% with SPMS and 20% with PPMS (3% missing). Findings in our current study were very similar, with 40% of patients with RRMS and 47% with some form of progressive disease. However, 13% of patients were unsure regarding the course of their disease, indicating the general difficulty in defining precisely the time of conversion from relapsing to progressive disease at levels where both types are present. As a consequence, we have not used this information in our analysis.
Similarly scarce is information on the proportions of patients at different levels of functional disability. The earlier cost-of-illness study found that approximately 49% of patients were at an Expanded Disability Status Scale (EDSS) score [8] below 4.0, approximately 33% at EDSS scores from 4.0–6.5 and 18% at EDSS scores ≥7.0. The distribution in our current study is similar, with 47% of patients below an EDSS score of 4.0, 36% at an EDSS score from 4.0–6.5 and 12% at an EDSS score ³ 7.0 (5% missing). However, Pugliatti and colleagues have estimated this distribution for Europe overall at approximately 45–55% for mild disease, 20–25% for moderate disease and 15–20% for severe disease. This would indicate that patients in the moderate and severe groups may be under-represented, and any extrapolation to the total cost of MS in Germany should therefore be based on an adjusted distribution.
Materials and methods
Study centres
Contrary to the majority of the countries in the study, German data were collected with the help of three clinical centres and three large private practices specialized in the treatment of patients with MS. In addition, patients in a database maintained by Business Solutions Medicine Online GmbH (BSMO) who had registered their interest in establishing contact with the MS Service Centre of Biogen Idec in Germany were included as an additional study centre. Although patients at all levels of disease severity are registered in this database, there is a strong bias towards early disease. The majority of patients have RRMS and roughly 70% are able to walk at least 500 m.
Data collection
Questionnaires were mailed directly from the clinics, and patients returned them fully anonymously (but including a formal consent for the utilization of the data) to the data-entry centre where they were entered into the Internet database on an ongoing basis.
The questionnaire collected data on MS-related inpatient and outpatient admissions, visits to physicians and other health-care professionals, and tests and procedures during the past 3 months. Details on medication, services such as home help or informal care (unpaid help) by family or friends were collected for the past month only, to ensure good-quality recall. Major investments such as transformations to the house or the car, or the purchase of a wheelchair, were collected for the past year. Productivity losses were captured through questions regarding employment status and normal working hours, early retirement (invalidity) and short- and long-term sick leave during the preceding 3 months.
In addition, the impact of the disease on patients’ QOL was assessed using the EQ-5D (EuroQol), a generic preference-based QOL instrument [9]. The instrument covers five domains of health-related QOL: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. The resulting combination of answers is translated into utilities (i.e. weights on a scale between 0 = death and 1 = full health) using a health status system developed with the general population. Such a system is available for Germany, but it is based on the EuroQol visual analogue scale rather than on assessment using the five QOL domains. For this reason, and also for comparability to the other countries in the study, the tariff based on decision-analytic tools developed originally together with the instrument for the UK population was used [10]. Finally, information on the patient’s disease course, the number of relapses and the level of functional ability was collected using detailed descriptions.
Cost calculations
In the societal perspective, unit costs should be assessed as opportunity cost (i.e. costs in the next best alternative). However, in Germany, often only tariffs or charges are available, and although these may or may not represent opportunity costs accurately, it is customary to use them for costing studies as the best approximation. When no tariff is available for a resource (e.g. for resources not included in the health-care services), market prices are used.
Productivity losses from the viewpoint of society are estimated with the human capital method, using the average gross national income plus employers’ cost. In addition, we present an estimate of “intangible costs,” i.e. costs of suffering, based on an approach developed earlier [11, 12]. General methodology for cost-of-illness studies recommends including intangible costs, but as QOL is not routinely collected in such studies, intangible costs are seldom calculated. Our estimates are based on the loss of quality-adjusted life-years (QALY) of MS patients compared to the general population, and are then combined with a threshold value of a QALY gained that payers appear to be willing to pay, implied from reimbursement decisions regarding new treatments.
For the payer perspective, tariffs are used for all resources, and all non-reimbursed items are excluded. Productivity losses in this case are based on daily reimbursements for short-term sick leave after 6 weeks and on invalidity pensions for early retirement. Table 1 presents details on the major sources for unit costs and summarizes the methods of cost calculations. All costs are for 2005 (adjusted using the consumer price index if necessary).
Analysis
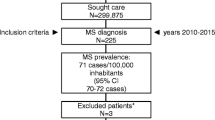
Resources used were multiplied with their unit cost and annualized. Only basic descriptive analysis is presented here, as the main objective of the study was to investigate the effect of disease severity on costs. As a consequence, it was more important to have sufficient numbers of patients at all levels of disease severity, as shown in Fig. 1, than a sample that perfectly matches prevalence estimates, and calculations presented here must be seen in the light of the sample specifics. Also, costs in health-care are generally highly skewed even at the same levels of disease severity, and in order, for instance, to estimate the long-term cost, estimates would have to be based on regression analysis and modelling.
Distribution of patients across severity levels. The sample contained enough patients at all levels of disease severity to analyze the effect of MS on costs: even the smallest group with an EDSS score of 6 contained 140 patients. However, it is evident that patients at very mild disease levels (i.e. low EDSS scores) are over-represented in the sample, and the effect of this on the mean cost per patient in the sample must be borne in mind
Results
Questionnaires were sent to 3625 patients in the six clinical centres, and to 3700 patients from the database. A total of 2840 questionnaires were returned, but 47 came back empty, with the indication that the patient did not want to participate (no interest, no time, did not have MS, <18 years of age). The overall response rate was 38% but slightly lower for patients in the database (35%).
Sample demographics
Table 2 presents details on the patient characteristics. A total of 2793 patients were included in the analysis. Almost half the patients (47%) came from the BSMO database, which biases the sample towards milder disease and younger age compared to expected prevalence. The mean age was 45 years (median 44 years, SD 11.1), 72% were female and 17.7% were 65 years or older. Forty-one percent of the patients were employed or self-employed. However, less than 40% of these patients worked full-time (42%), and most of those working shorter hours indicated that this was a consequence of their disease. In addition, 5.6% of those in employment (2.3% of the sample) were on long-term sick leave. Thirty-seven percent of patients had taken early retirement, the vast majority of these (90%) due to MS. On average, patients had to leave the workforce because of MS at the age of 40 years. Using patients with an EDSS score of 0 or 1 as the norm close to the population, the strong effect of the disease becomes evident (Fig. 2). While 73% of patients below 65 years of age were employed at an EDSS score of 0–1, this proportion drops to 10% for patients with an EDSS score of 7 and 4% for those with an EDSS score ³ 8. The majority of patients appear to be forced to stop working at an EDSS score of roughly 4–5; indeed, the proportion working drops from 65% with an EDSS score of 3 to 26% with an EDSS score of 5.
The effect of MS on work capacity. Work capacity decreases significantly with increasing disease severity. If workforce participation is assumed to be unaffected by the disease at the very early stages (i.e. with roughly 85% of patients between the ages of 20 and 65 years working), then employment is reduced fourfold (to roughly 20% at an EDSS score of 6.5), even taking age into account
Disease information
The average age at diagnosis was 35 years, and the time from first symptoms to diagnosis was approximately 3 years. Almost half of the patients indicated that they had progressive disease, while 13% stated that they did not know or omitted the answer, indicating the difficulty patients encountered with this question (Table 3). In contrast to this, patients appeared to have no hesitation in judging the level of functional ability, and 95% answered this question. The mean EDSS score was 3.8 (median 4), driven by the almost 40% of patients with an EDSS score £ 2. One quarter of patients stated that they had had a relapse (defined as lasting more than 48 h) during the past 3 months. However, as for the disease course, patients appeared to have difficulties in answering this question – 13% indicated that they were not sure and 3.3% did not answer the question.
QOL
The EQ-5D was completed by 91% of patients. Questionnaires with answers missing to any of the five questions were excluded, as no method for imputation of missing values exists. The mean utility score was 0.62, with no difference by gender, despite a higher mean EDSS score for men (4.3 compared to 3.6 in women). This is partly explained by the population EDSS scores, where women have somewhat lower scores than men of the same age.
Utility was significantly correlated with EDSS scores (p<0.001) and with having a relapse or not (Fig. 3). For patients with an EDSS score <5, thought to be primarily patients with RRMS, the difference was 0.09 (p<0.001), and for those at ≥5 it was 0.05 (p<0.05). Compared to the general population, patients in this sample had a utility reduced by 0.2, which over 1 year translates into a QALY loss of 0.2.
One of the major problems in daily activities that patients with MS encounter is fatigue. On a visual analogue scale ranging from 1 (best) to 10 (worst), 2705 patients in the sample (91%) scored 5.4 (SD 2.3) on average. Women had higher scores than men, and fatigue increased significantly by approximately 0.8 points during a relapse (p<0.001).
Costs
Health-care costs
Table 4 presents details on the use of medical resources during the respective recall period, including mean costs for users during the recall period calculated for each type of resource. Finally, mean annual costs for an average patient in the sample are calculated by assuming that the same proportion of patients would use the same quantity of resources during any time period identical to the recall period.
Amongst health-care costs, disease-modifying drugs (DMDs), which are used by half of the patients in the sample, represent, as expected, the largest proportion. Inpatient care and consultations each represent roughly one fifth of total health-care. One quarter of patients were hospitalized or admitted to a rehabilitation centre or nursing home in the preceding quarter (i.e. past 3 months), for an average length of stay of 21 days. The mean duration of hospitalization in the neurology ward was 14 days, while patients who were admitted to nursing homes (0.8%) stayed for an average of 45 days. Inpatient rehabilitation was used by 7.4% of patients for an average duration of 26 days. Medical consultations were frequent, with the majority of patients (78%) consulting a neurologist an average of 2.7 times during the 3-month period; 49% saw a general practitioner (3.4 times) and 53% had on average 19 physiotherapy sessions. In addition, 17% consulted with a urologist (twice on average) and 9% with an internist (2.5 times). Thus, despite a unit cost for consultations that is relatively low compared to countries with national health services such as Sweden or the United Kingdom, ambulatory care consultations represent half of all health-care costs aside from DMDs in Germany.
Mean total direct health-care costs amounted to € 17 165 per patient in the societal perspective, and 87% of these costs (€ 14 949) were paid for by the health-care system. Items that were not covered include some paramedical services and non-prescription drugs.
Direct non-medical costs
Contrary to health-care costs, the vast majority of the direct non-medical costs were borne by the patients themselves (Table 5). One quarter of patients had had to modify their living environment during the past year or required devices such as walking aids (6%) or wheelchairs (7%). Sixty percent of these costs, essentially modifications to the house or the car, had to be borne by patients themselves. Similarly, only 46% of services such as home help, childcare or transportation used by one quarter of patients were paid for by the health-care system. However, by far the largest cost was represented by informal care. Close to half the patients had received some form of help by family and friends, for an average of slightly less than 3 h per day. Annualized and calculated for the study sample, this represents approximately 500 h per MS patient per year and leads to an estimated cost of € 4400 per patient, borne by families and friends.
Productivity losses
Table 6 presents details of indirect costs. One in four of the patients in employment had had to take leave because of MS in the preceding 3 months, for an average of 19 days. This represents 5 days per quarter for patients in the group of employed patients. A further 64 patients in this group were on long-term leave, determined as being on sick leave, for more than 3 months. The largest impact on costs came, however, from the total loss of work capacity, with 34% of patients having retired early because of the disease. Total annual production losses to society were estimated at € 17 000 per average patient in the sample. Invalidity pensions for these patients were estimated to cover roughly 20% of total production losses.
Total costs
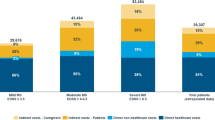
Mean total annual costs per patient in the sample, in both the societal and payer perspectives, are presented in Fig. 4. Production losses represent 42% of total costs in the societal perspective and 18% in the payer perspective. Of the total cost of € 40 000 per patient per year, slightly less than half (47.5%) is covered by health-care and social services.
Our calculations indicate that with a willingness to pay for a QALY of € 50 000, a level thought to be accepted by most reimbursement agencies, intangible costs would amount to roughly € 10 000 per patient in our sample.
Cost of a relapse
Using a patient questionnaire, it is difficult to define precisely what costs are related to a relapse, not least because it is difficult to define a relapse in itself. In order to approximate the cost caused by relapses, we estimated the difference in costs over 3 months between patients with and those without a relapse. As for utility, it is likely that exacerbations have a stronger impact on patients with limited disability and relapsing disease. Indeed, the cost for a relapse for patients with an EDSS score <5 was estimated at € 2955. The cost increase is essentially due to hospitalizations, visits, informal care and short-term sick leave.
Costs by level of disease severity
Costs are significantly correlated with disease severity and increase approximately fourfold from early to severe disease, and the mean cost per patient in the sample corresponds precisely to the costs for a patient with an EDSS score of 4 (the median in the sample) (Fig. 5). Informal care costs and production losses are concentrated in the high EDSS levels, while DMDs are used as per label much more in early disease. As a consequence, they represent as much as half of total costs for patients with an EDSS score £ 2, but only roughly 5% for patients with an EDSS score ≥7. Informal care costs increase from about € 600 per year for patients at low EDSS levels to over € 20 000 per year for patients at the highest EDSS levels.
Discussion
This study aimed to provide extensive and detailed current data on resource consumption, workforce participation and QOL for patients with MS at all levels of disease severity. Results confirm that costs and QOL are highly correlated with disease severity, as shown in previous studies. Thus, it is important to consider all average results per patient reported here in the light of the distribution of severity in the sample. Indeed, the sample may not be fully representative of the overall patient population. As a consequence of the method of patient inclusion, patients in late disease may be under-represented. The BSMO database, which provided slightly less than half of the patients in the sample, contains a majority of early-disease patients with RRMS (mean EDSS score of 3.2), and despite a lower response rate amongst these patients compared to patients recruited in the clinical centres, the effect is substantial. Also, two of the six clinical centres provided patients with early disease, with a mean EDSS score of 2.7 and 3.2, respectively.
As a consequence, almost half of the patients in the sample are at an EDSS level <4, which clearly affects the study results. Over half of the patients are treated with DMDs, indicated in early disease, while current penetration of these drugs in Germany is estimated at around 35%. This is, however, not exclusively due to the inclusion of patients with early disease; it is also likely that patients on treatment are more motivated to participate in a mail survey.
Another effect of the sample composition is that when estimating the mean cost per patient in the sample, some of the costs that are very high in late disease, such as large investments in transformation of the house, the use of home care (Pflegedienst) as well as informal care, may be under-represented. This becomes evident when comparing the results to our earlier study in Germany [6], where the mean cost per patient for investments was between two and three times higher. While this is partially due to patients with more severe disease in the sample, an additional and most likely stronger reason is the combination of a less rigorous exclusion of outliers in these self-reported costs combined with a much smaller sample size. Similarly, services such as home care and home help were substantially higher in the earlier study, essentially for the same reasons.
However, other cost differences between the two studies are not explained by the sample composition. In the earlier study, a number of resources – amongst them home care and visits to the neurologist – were valued using true opportunity costs (i.e. recalculating the cost of the time used), while in the current study, tariffs (i.e. lower unit costs) were used even in the societal perspective. According to accepted methodology, costs should be estimated from the societal perspective and resources should hence be valued in using opportunity costs. In well-functioning free markets, market prices represent opportunity costs, but in health-care, costs are generally controlled. In most health-care systems, tariffs are used to set incentives, in addition to paying for a service. For instance, in a fee-for-service system such as that in Germany, if service appears to be used too frequently, payment for it is reduced to discourage usage. Alternatively, to induce more frequent usage of a given service, payment would be increased. These incentives often work less than perfectly, as they fall within an entire framework of different incentives or disincentives and controls, making it difficult to judge how well they represent true costs. In this situation, it is habitual in Germany to perform costing studies using the tariffs also in the societal perspective, but to include all copayments and non-reimbursed items, as well as productivity losses. For the payer perspective, only those items reimbursed, at a tariff net of any copayment or rebate, are included.
Health-care costs excluding DMDs are slightly lower in the current study than in the earlier study, when adjusted for the time difference, particularly as far as outpatient visits are concerned. Again, this is partly due to the sample selection. However, another reason may be that in the early years of the use of DMDs, patients were more closely monitored for safety reasons. Also, most patients in the earlier study were recruited in university clinics, where patient management tends to be more intensive. This latter fact also explained the high proportion of patients using DMDs (43%) at a time when overall penetration was estimated at 10–15%. The study hence reported adjusted drug usage. If a similar adjustment were made in the current results (∼35% DMD usage instead of 50%), costs would decrease by approximately € 3150.
A large number of different studies have shown that costs are highly correlated with EDSS score, and these findings are therefore expected. Our current study confirms again that costs, as well as QOL, need to be considered in the light of the level of functional disability rather than for a population as a whole. Workforce participation in patients between 18 and 65 years of age decreases from roughly 70–75% in early disease to less than 10% in the late stages. Hospitalization and ambulatory visits increase by a factor of 5–6 from between early and late disease; investments and services increase from basically no cost to € 2700; productivity losses increase tenfold; and informal care increases by a factor of 27 for patients with an EDSS score of 7, and by a factor of 50 for patients at the very severe end of the EDSS scale (scores of 8–9). Hence, total mean costs per patient are driven essentially by the distribution of the severity levels in the sample, increasing from approximately €18 500 at an EDSS score of 0–1 to € 70 500 at an EDSS score of 8–9.
This is reinforced when analyzing differences between the seven study centres in our sample. The distribution of disease severity, and hence the mean EDSS score, drives resource usage (most strongly, the use of DMDs and informal care) and production losses due to loss of work capacity due to the disease (Table 7, Fig. 6).
The same is true for utility, which decreases from 0.86 to 0.10 in the overall sample as the disease becomes more severe, and is correlated with the mean EDSS score of the centre samples. However, the utility loss compared to normative values in the population is high at all levels of the disease. In the general population, utility scores fall in relation to age, from around 0.94 at an age of 20–30 years to 0.75 at 70–80 years. This compares to a mean utility of around 0.75 for patients in the age group between 18 and 29 years (with an EDSS score of approximately 2) and a utility of 0.75 in our study. At older ages (70–79 years), the mean EDSS score was approximately 6.3 and utility 0.36. The total loss of QOL in the sample was estimated at 0.2 QALYs, from which an intangible cost of approximately € 10 000 was derived.
Compared to the other countries included in this European study, German patients included in this sample are at the high end of the range in terms of inpatient days, outpatient consultations and physiotherapy sessions, despite the fact that they have the lowest mean EDSS score in the series. This is explained by medical tradition and payment mechanisms (fee-for-service), and is well known. However, tariffs for these services are comparatively low to discourage overprovision, and hence total costs controlled for EDSS are not fundamentally different in Germany than from the rest of the countries. Differences between countries are concentrated for the most part in very late disease, where the support provided to patients is highly variable.
This study provides information at a very detailed level concerning consumption of resources by patients with MS in Germany. When taking the necessary precautions, results within the study can be compared to results in other countries, and differences can be explored [1]. Overall, differences in the German results compared to other countries in the study are partly explained by differences in the sample, but more so by medical traditions and the unique way in which the health-care system is organized.
References
Kobelt, G, Berg J, Lindgren P, Jönsson B (2006) Costs and quality of life of multiple sclerosis in Europe. J Neurol Neurosurg Psychiatry. Online publication, May 11 (doi: 10.1136/jnnp.2006.090365)
Rosati G (2001) The prevalence of multiple sclerosis in the world: an update. Neurol Sci 22: 117–139
Pugliatti, M, Sotgiu S, Rosati G (2002) The worldwide prevalence of multiple sclerosis. Clin Neurol Neurosurg 202: 82–191
Pugliatti, M, Rosati R, Carlton H et al. (2006) The prevalence and incidence of multiple sclerosis in Europe. Eur J Neurol 13: 1–23
Hein T, Hopfenmuller W (2000) Hochrechnung der Zahl an Multiple Sklerose erkrankten Patienten in Deutschland. Nervenarzt 71: 288–294
Kobelt, G, Lindgren P, Smala A, Jönsson B, Group Gms (2001) Costs and quality of life in multiple sclerosis. A cross-sectional observational study in Germany. Eur J Health Econ 2: 60–68
Poser S (1994) The epidemiology of multiple sclerosis in southern Lower Saxony. In: Firnhaber W, Lauer K (eds) Multiple sclerosis in Europe: an epidemiological update. Darmstadt, Germany: Leuchtturm-Verlag/LTV Press
Kurtzke J (1983) Rating neurological impairment in multiple sclerosis and expanded disability status scale (EDSS). Neurology 33: 1444–1452
The EuroQol Group (1990) EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 16: 199–208
Dolan P (1995) A social tariff for EuroQol: results from a UK general population survey. Centre for Health Economics, University of York: York
Kobelt G, Lindgren P, Parkin D, Jönsson B (2000) Costs and quality of life in multiple sclerosis. A cross-sectional observational study in the United Kingdom. SEE/EFI Working Paper Series in Economics and Finance No. 399. Stockholm School of Economics; Stockholm, Sweden
Eichler H, Kong SX, William C, Gerth, PM, Jönsson B (2004) Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value in Health 7: 518–528
Acknowledgement
K. Berger (Medical Economics Research Group, Munich); O. Ström (Stockhkolm Health Economics, Sweden)
Conflict of interest
No information supplied.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kobelt, G., Berg, J., Lindgren , P. et al. Costs and quality of life of multiple sclerosis in Germany. Eur J Health Econ 7 (Suppl 2), 34–44 (2006). https://doi.org/10.1007/s10198-006-0384-8
Issue Date:
DOI: https://doi.org/10.1007/s10198-006-0384-8