Abstract
This cost-of-illness analysis is part of a Europe-wide study on the costs of multiple sclerosis (MS) and is based on information from patients in Austria. The objective was to estimate the costs and quality of life (QOL) related to the level of disease severity and progression. Questionnaires were sent to 2995 patients registered with a nationwide patient organization. Patients were asked to provide details regarding the type of disease, relapses, level of functional disability, resource consumption (medical and non-medical), work absence, sick leave and informal care, as well as QOL. Surveys from a total of 1.019 (34.0%) patients were used in the analysis, of which the mean (standard deviation [SD]) age was 50 (12.2) years; 70% of patients were female. Patients with mild disease (Expanded Disability Status Scale [EDSS] score 0–3) represented 41% of patients, 36% had moderate disease (EDSS score 4–6.5) and 22% had severe disease (EDSS score ≥7). The mean (SD) EDSS score in the sample was 4.4 (2.4), with a mean (SD) utility of 0.55 (0.32).
Costs are presented from the societal perspective as well as from the viewpoint of payers of care and invalidity. Mean total annual costs for an average patient in the sample were estimated at € 40.300 in the societal perspective, whereas payers’ costs were estimated at only half of this. Disease-modifying drugs represented a quarter of all costs in the payer perspective, but only 12% of societal costs. For society, the highest cost was the loss of productivity (36%), while payments for this loss (invalidity pensions and sick-leave compensation) accounted for only 21% of total costs to payers. Costs are highly correlated with disease progression, increasing four-fold from early disease to very severe disease (€ 16.000 to € 63.800). Mean annual costs per patient reported are thus determined by the distribution of disease severity in the sample. Workforce participation decreases from roughly 75% in early disease to less than 10% in the late stages, despite the fact that 70% of patients with an EDSS score of 8 or 9 are still below the official retirement age. Consequently, productivity losses increase over fivefold. In parallel, costs of informal care increase from € 325 per year at an EDSS score of 0–1 to over € 20.000 at an EDSS score of 8–9. Hospitalization is very infrequent in early disease, representing less than € 1.000 for patients with an EDSS score of 0–1, but increases steeply for patients with an EDSS score ≥5. QOL, measured as utility scores, decreases rapidly from almost 0.90 to 0.05 as disability becomes severe. However, the loss of utility is evident at all disease levels. Young patients with an EDSS score of approximately 2 have a utility that is 0.15 lower than matched individuals from the general population. This loss increases to approximately 0.4 for patients over 60 years of age with an average EDSS score of 6.0–6.5. Patients with a recent relapse had lower utility (–0.1) and higher costs (+ € 4.750).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The objective of this study was to analyze the costs and quality of life (QOL) related to disease severity and progression of patients with multiple sclerosis (MS) in Austria. The study is part of a multinational cross-sectional study in nine European countries and was performed during late spring 2005 [1]. The European study collected all resource consumption and productivity losses due to MS, and results are reported from the societal perspective. This eliminates one of the methodological issues when comparing across countries, represented by differences in coverage by social systems or insurances. Thus, all costs are accounted for regardless of who ultimately pays, and productivity losses are estimated using the human capital approach. This paper presents details on data collection, cost calculations and results for Austria. For a detailed discussion of the methods used for the study, see the general paper on methodology by Kobelt et al. in this supplement.
In order to analyze the effect of disease progression on costs and QOL, it is important to enroll a sufficient number of patients at all levels of disease severity to allow analysis. Selecting a sample that matches precisely the prevalence of the type of MS or the distribution across disease severity is of lesser importance. These can be adjusted using epidemiological data to estimate the total cost of MS in Austria. This was done in an earlier analysis in Austria, which followed a methodology similar to the current study [2]. The authors used a published epidemiological study that reported the prevalence of patients with mild, moderate and severe disease [3] and extrapolated costs to the total estimated population of around 8.000 MS patients in 1999. In the societal perspective, the annual cost per patient was estimated at € 47.000, of which public payers covered roughly one third [2]. Total cost for Austria was estimated at € 400 million.
The calculations were based on an estimated prevalence of 98.5 per 100.000 inhabitants [3], which is similar to that of other countries in Europe [4]. The proportion of patients at different levels of disease severity was based on the Expanded Disability Status Scale (EDSS) [5] and estimated at 40% for patients with an EDSS score of <3, 32.6% for patients with an EDSS score of 3–6 and 27.4% for patients with an EDSS score of >6. The respective proportions estimated by Pugliatti and colleagues across Europe were 45 to 55% for mild disease (published range 33 to 80%), 20 to 25% for moderate disease (range 13 to 48%) and 15 to 20% for severe disease (range 5 to 39%). However, Pugliatti and colleagues used slightly different cutoffs for EDSS scores (<4, 4–6.5, >6.5), which appears to explain the higher prevalence of mild disease.
Compared to both the European estimates and the earlier Austrian study, it appears that patients with moderate disease, but possibly also those with severe disease, may be somewhat over-represented in our current study. This illustrates the possible impact of the method of patient enrollment and data collection in our study. Patients with very early disease may not yet be members of an MS patient society, and the anonymity of the survey does not permit verification of the self-assessed EDSS scores. The epidemiological study by Baumhackl and colleagues found a high correlation between physicians’ and patients’ assessment of disability [3], and the smaller proportion of patients with mild disease in our study must therefore be assumed to be a consequence of enrollment. However, the study enrolled sufficient patients at all levels of the disease to allow analysis, as shown in Fig. 1.
Contrary to disease severity, Ganzinger and colleagues reported difficulties with self-assessment of the MS course. The majority of patients rated their disease as secondary progressive MS (34%) or primary progressive MS (23%), while as many as 11% did not know. Compared to overall estimates for Europe, the proportion of patients with relapsing-remitting MS (RRMS) is thus underestimated (32% compared to 40 to 60%) [3, 4]. The authors, therefore, did not present cost estimates by disease type. We encountered the same difficulty in our current survey, and therefore do not exploit this information in our analysis.
Patients and methods
Methods
The study used a prevalence-based approach, and data were collected in a cross-sectional survey directly from patients, with the objective of including all costs due to MS. Patients were instructed to exclude consumption not related to MS in order to estimate costs of MS rather than costs of a patient with MS. As MS is well defined, and unrelated comorbidity is relatively low due to the young age of the patients, such a separation appears possible.
Patients
Questionnaires were sent out to all 2995 patient members of the Austrian MS Society, which is the only registered MS patient organization in Austria and represents an estimated 37% of MS patients in the country. There were no other ongoing surveys from this society at the time of this study. The questionnaire informed patients about the purpose of the study and how data would be used. Patients provided written consent to use the information they provided for research and publication.
Data collection
All data were collected with the questionnaires. Demographic background variables included age, gender, marital status, living situation and level of education. Disease information covered year of diagnosis, age at first symptoms, type of MS, relapses during the past 3 months and a self-assessment of the current EDSS level. Information was also collected on work capacity, including patients’ employment status, work changes due to the disease, short- and long-term sick leave and early retirement. Resource use collected concerned all MS-related health-care and non-medical consumption. To ensure high-quality data, the recall period was varied between different items, as major but infrequent events are easier to recall over longer time periods than, for example, drug use or home care. Health-care resources included inpatient and outpatient admissions, visits to physicians and other health-care professionals, tests and procedures and medication. With the exception of medication, consumption related to the preceding 3 months. Details on medication, services such as home help or informal care (unpaid help) by family or friends were collected for the past month only, while major investments such as transformations to the house or car or the purchase of a wheelchair were collected for the past year.
QOL data were collected in the form of utility, using a generic instrument (EQ-5D) [6]. Patients’ answers to the five questions addressing well-being are translated into utilities via a social tariff developed with the general population [7]. Utilities express preferences of the population for given health states, on a scale anchored between 0 (death) and 1 (full health). Negative values, i.e. health states considered worse than death, are possible on this scale, but for simplicity they were set to zero in this analysis. In order to ensure comparability across the countries participating in the study and due to the fact that no specific tariff for Austria is available, we used the tariff originally developed in the United Kingdom [7]. In addition, a visual analogue scale was used to measure patients’ level of fatigue during the last 48 h.
Cost
In the main analysis, costs are estimated from the societal perspective, including all costs at full, regardless of who ultimately pays, with indirect costs valued according to the human capital method. However, costs incurred by social insurance systems are also estimated, using tariffs for services covered by the statutory insurances and invalidity pensions and sick leave compensations to estimate indirect costs.
Unit costs were collected during the first quarter of 2005 and are generally based on 2004/2005 prices. If a source was from an earlier year, prices were inflated to 2004/2005 levels using the consumer price index (CPI). Table 1 presents details on the major sources for unit costs and summarizes the methods of cost calculations. All costs are in 2005 € (adjusted using the CPI if necessary).
When no standard unit cost was available (e.g. for transformations to the house or car or for home help), patients’ indications were used. However, costs quoted were systematically verified, and outliers (defined as more than one standard deviation from the sample mean) were corrected and taken to the standard deviation. For devices (e.g. wheelchairs), costs indicated were verified against an average available cost. For the perspective of the payer, only those costs identified by patients as having been covered by insurance were included.
Informal care was considered a direct cost. Valuation was based on the disposable income, i.e. the net income after social contributions (18%) and income tax (∼33%) after taxes. There are other methods to estimate the cost of informal care, in particular using the cost of a professional care-giver (replacement cost) or productivity losses by employed relatives and friends. Using the disposable income generally produces estimates that are between these two methods, with the use of replacement cost usually producing the highest cost.
In the societal perspective, production losses were valued using the cost of labour, i.e. average gender-specific gross salary including employers’ contribution (25%). The cost of sick leave was adjusted for patients’ working h, while early retirement due to MS for patients <65 years of age was based on average national working h. In the payer perspective, invalidity pension payments were used for patients on early retirement. For short-term sick leave no cost was included, as no compensation is paid from the insurances for the first 12 weeks, which corresponds to the maximum length of short-term sick leave defined in our study. For long-term sick leave, per diem compensation was included.
Analysis
An online database was created to facilitate data entry and analysis. The database was designed so that data entry errors were minimized, e.g. requiring key cells to be filled in, applying numeric checks regarding the number of days/h/etc possible during a certain time period, as well as logical checks concerning combinations of certain answers, such as employment status and early retirement, combinations of disease-modifying drugs (DMDs), etc. Subsequently, each resource was multiplied by the relevant unit cost and scaled up to 1 year. In the results section, the cost per patient is reported both for the entire sample during 1 year and for those patients who used the resource during the given time period. Where relevant, the mean quantities are also reported in the same way. For the analysis of costs and utilities by EDSS level, the mean values were calculated.
The cost of a relapse was estimated from the difference in costs incurred by patients with a relapse during the 3 preceding months compared to patients without a relapse. For this calculation, we used information from patients at an EDSS score <5, as relapses diminish over time. Patients at severe EDSS levels may therefore not only incur fewer relapses, but these levels also may not have the same impact in patients who are already physically dependent.
Results
Patient demographics and background information
The questionnaires were returned by 1047 patients, establishing the response rate at 35%. Twenty-eight patients returned the questionnaire empty, leaving a total of 1.019 patients (34%) who were included in the analysis. Of the sample, 70.4% were female and the mean age was 50 years (Table 2). Fifty-five percent of patients were between 40 and 59 years of age, and the proportion of patients over 65 was 13%.
In our sample, 30.4% of patients were employed or self-employed, two thirds of them full-time. Six patients were on long-term sick leave, while 47.1% of patients were early retired, the vast majority of these (94.5%) because of their MS (Table 2). This is almost identical to the findings in the study by Ganzinger and colleagues [2], where 28% of patients were employed and 41% were on early retirement, despite a somewhat younger population (mean age of 47 years compared to 50 in our study). About one in four employed had taken short-term sick leave during the past 3 months due to MS.
Of those currently in employment, one fifth (21.9%) had to reduce their working h and 18.4% had to change the type of work because of their disease. This corresponds to about 12% of the sample, and again compares well to the 10% identified by Ganzinger and colleagues [2]. For almost 70% of these patients, the change in work meant a reduction in income of roughly one third.
Figure 2 shows the proportion of patients <65 years of age employed at different levels of EDSS. While early in the disease three quarters of patients are working, this proportion shrinks to 9% for patients with an EDSS score of 7 and to 1.5% above an EDSS score of 7, despite the fact that 78 and 70% of patients, respectively, are still below the normal retirement age. The sharpest reduction in the ability to work, or the time at which patients can obtain early retirement, occurs at approximately an EDSS score of 3, where work capacity is reduced from 75 to 43%, and then again at an EDSS score of 5, where it is reduced to less than 20%.
Disease information and QOL
Disease information
On average, patients were 35 years old at diagnosis, with first symptoms occurring approximately 3 years earlier (Table 3). Similarly to earlier studies, patients appeared to have difficulties with defining the type of their MS. Thirty-six percent of patients declared having RRMS, 55% a progressive form of the disease, while approximately 10% did not know or did not answer. The proportion of patients with relapsing MS in our sample is thus slightly lower than the European estimates by Pugliatti and colleagues [4] and the findings in Austria [3].
Patients found it apparently easier to assess the level of disability, and only 1.5% omitted this answer. The mean (SD) EDSS score in the sample was 4.4 (2.4), with 49.8% of patients at an EDSS of 0–4 (Table 3). Forty-one percent of patients had mild disease (defined in our study as an EDSS score <4), 36% had moderate disease (EDSS score 4–6.5) and 22% were severely disabled (EDSS score ≥7).
Seventeen percent of patients had experienced an exacerbation during the preceding 3 months, defined in the questionnaire as symptoms lasting more than 48 h. However, 10% of patients were unsure and 3% did not answer the question, indicating the difficulty in defining a relapse, particularly in the later stages of the disease with more permanent disability.
QOL
Almost all patients completed the EQ-5D (91%), and the mean utility in the sample was estimated at 0.55. This is lower by 0.27 than the score estimated for an age- and gender-matched sample of the general population.
Utility decreases significantly with worsening disease (Fig. 3). This is the case even when accounting for the fact that utility decreases with age in the general population (from approximately 0.94 for the age group 18–29 years to approximately 0.70 for those ≥80 years of age), and age and advanced disease are interrelated. Young patients with an average EDSS score of slightly below 2 had a utility reduction of 0.15, while patients over 60 years, with an average EDSS score of approximately 6, lost on average roughly 0.4 in utility.
The utility loss due to a relapse was calculated as the difference in the mean utility scores of patients with and without a relapse. Patients with a relapse had on average a utility loss of 0.1 (p<0.05).
Costs
Direct health-care costs
Table 4 presents details on the use of medical resources during the respective recall periods. In our sample, 25.8% of patients had been hospitalized during the past 3 months, most often in neurology wards or rehabilitation centre, for an average of 27.0 days. The average length of stay is determined by half of those patients admitted for rehabilitation for an average duration of 22 days, and a small proportion of patients admitted to nursing homes for an average of 58 days. Stays in neurology wards, however, lasted an average of 16 days. Of the total annual cost per patient in the sample, over 40% were due to rehabilitation.
Seventy-six percent of patients had had hospital outpatient or specialist visits during the past 3 months, most often to neurologists (49%), general practitioners (46%) and physiotherapists (22%). Thirty-five percent of patients had had day admissions at hospitals, rehabilitation centres or nursing homes, and these represented 73% of total visit costs.
The majority of patients (84.5%) were using some medication. DMDs were used by 40% of patients and represented two thirds of total drug costs. They were also the largest contributor to total mean annual cost per patient in the sample. Fifty-eight percent received other prescription products, with antispasticity drugs being the most common (30.4%), followed by anti-inflammatory drugs (23.4%) and antidepressants (22.1%).
Direct non-medical costs
Twenty-six percent of patients had made some sort of investment because of their MS during the past year, most often to help mobility (Table 5). Modifications to the house were made by 12.5% of patients, including stair lifts and elevators, which were the most expensive item. Walking aids, including wheelchairs and scooters, were purchased by 12.7% of patients, while 3.1% made transformations to the car.
Over the past month, 24.5% of patients had used some type of community service, for an average duration of 79 h. Home help accounted for the majority of resource use and costs, with an average of 86 h per month for the 11.7% of patients using the resource.
For 57.9% of patients, family and friends had provided informal care during the past month, for an average of 147 h per month. This corresponds almost to a full-time work schedule and is determined by patients at the severe disease level who need support for far longer than a normal working day. Annualized and calculated for the entire sample, patients use informal care for an average of more than 1000 h.
Productivity losses
Table 6 summarizes work capacity and costs due to production losses. One in four employed patients had taken sick leave for a mean of 17.3 days, or 113 h when taking part-time work into account. However, these costs represented only 3% of indirect costs, which were entirely determined by early retirement due to MS by 44.5% of patients. In the societal perspective, production losses represent 36% of total annual costs for an average patient in the sample.
Total costs
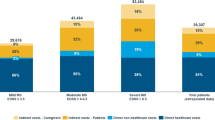
The total societal costs per MS patient in our sample average € 40.300 per year. The majority of these costs (63.6%) are direct costs, with drugs constituting the largest single direct-cost item (Fig. 4).
Total mean annual costs per patient (2005 €). As in all disabling diseases, particularly when they strike people early in life, production losses and informal care are very high, representing together roughly half of all costs in the societal perspective. In the payer perspective, drugs represent the largest single cost. Overall, approximately half of total costs are covered by social insurance, leaving a large part of the burden to patients and their families
The total cost per MS patient and year carried by the health-care and social systems amounts to € 20.012, i.e. roughly half of the total societal cost. In this perspective, drugs amounted to 34%, with DMDs accounting for close to 25%.
Cost of a relapse
Patients with a relapse had significantly higher costs during the quarter in which the relapse occurred than patients without. For patients with an EDSS score <5, costs increased by approximately € 4.700 (societal perspective). The increase is predominantly due to hospitalization (∼€ 1.100); ambulatory care visits and day admissions (∼ € 1.670); and short-term sick leave (∼ € 500). This is different from findings in the majority of the other countries, where the largest cost increase is most often due to informal care.
Intangible cost
The utility loss of our sample compared to the general population was used to calculate intangible costs by multiplying a life-year with utility to obtain quality-adjusted life years (QALYs). In total, 278 QALYs were lost in the entire sample, which corresponds to 0.273 QALY lost per MS patient. To these we applied a benchmark value of € 50.000 per QALY, implied from reimbursement decisions in Europe as an acceptable level to pay for a QALY gained with a treatment [8]. This translates into intangible costs of € 13.650 per patient and year, an additional cost not included in our presentation of total costs.
Effect of functional status on cost
Figure 5 shows costs in relation to functional status. Both direct and indirect costs increase with rising EDSS levels, from a mean cost of € 16.000 for an EDSS score of 0–1 to € 54.500 for an EDSS score of 7 and € 63.800 for very severe patients. However, the distribution of costs changes as the disease progresses. Costs for services, inpatient care and informal care increase with more advanced disease. Neurologist visits, tests and drugs increase in the earlier stages of disability but decrease thereafter. Also, short-term sick leave increases at first but decreases with higher EDSS levels, due to both the higher proportion of patients on early retirement and the higher age of patients, leading to normal retirement. The largest cost increase at very severe disease levels is, however, due to informal care.
Mean annual costs per patient by disease severity (2005 €). Most resources increase steadily as EDSS score increases, with the exception of DMDs that are indicated for early relapsing disease, and sick leave, as patients at the severe levels are rarely working. The largest cost increase comes from informal care and services such as home care and home help
Discussion
The aim of this study was to investigate current total costs of MS in Austria. The study is part of a European analysis in nine countries, which has both advantages and inconveniences. The standardized methodology allows for performing studies in a relatively short timeframe, and results can to some extent be compared. This is rarely the case when comparing two or several studies performed using different approaches to sampling, data collection and inclusion, unit costing and analysis [9]. More often than not, comparisons are not possible. Even within this European study, comparisons must be made with care as, on the one hand, the severity distribution in the samples differs slightly between countries and, on the other hand, unit prices for health-care resources and social services are quite different across Europe. We have therefore focused on total costs for different levels of disease severity, expressed as functional disability (EDSS), and the proportion of costs represented by different resource types. In our opinion, this provides more useful data than a calculation of the total cost in a given country, which would need a perfect prevalence sample. Also, the findings can be combined with data on the natural history of MS to estimate lifetime costs for a patient with MS.
The Austrian study is to some extent an exception as not only is a recent detailed epidemiological study available [3] but, in addition, a resource survey using the methodology developed in our earlier studies [10, 11, 12] was performed [2]. Thus, results of our current study can be compared to these earlier data (Fig. 6). It is striking to see the similarities and relatively easy to explain the differences.
Comparison to a similar study in 1999 [2]. The study in 1999 was performed using a similar methodology and questionnaire. The sample distribution was slightly different, with somewhat more patients with mild disease than in our current study, as patients were recruited via clinical centres rather than a patient association. Costs for 1999 were adjusted to 2005 costs using the consumer price index for comparison. Inpatient care was almost twice the cost in 1999, most likely due to a difference in unit costs (per diem cost in 1999, DRG [Fallpaupschalen in 2005]) but also due to a general shift towards outpatient care. Approximately the same proportions of patients used DMDs in the two studies. This is most likely a consequence of patients being recruited from university and specialized centres, where new treatments are used earlier and more intensively than in general practice
Direct costs excluding informal care are almost identical, when adjusted for the difference in timing (€ 20.500 in the earlier study versus € 19.600 in the current analysis). However, within these costs there is an important difference. Hospitalization costs in the earlier study are almost exactly twice those found in 2005, while exactly the opposite is the case for ambulatory care costs. This denotes the general trend towards more outpatient care, reinforced by payment mechanisms such as diagnostic research group (DRG) payments (Fallpauschalen). Ganzinger and colleagues reported an average length of stay in hospital wards of 18 days and in rehabilitation clinics of 27 days. This compares to 10–15 days and 22 days, respectively, in our study. However, this difference in length of stay does not explain the large difference in costs, which is due to the unit price used. Most likely, Ganzinger and colleagues at the time evaluated hospitalization using the per diem cost, while we used DRGs, which will avoid outliers and generally lead – at least on the introduction of DRGs – to lower costs.
Indirect costs are considerably lower in our study, the reason being mostly differences in the mode of calculations. In contrast to Ganzinger and colleagues, we did not include the cost of changes in the work situation in our calculations. However, the proportion of patients who had to retire early due to MS was almost identical (44.5 compared to 41.3% in the earlier study), and the small difference is explained by a slightly higher mean age and longer disease duration in our sample.
Informal care costs were also lower in our study, and the reason for this difference is less obvious. Part of it may be due to more patients living alone in our sample (28 vs. 18%), with therefore less continuous and easy access to informal care. Another reason is a difference in the unit cost for an hour of informal care: we calculated the disposable income as gross salary less 18% social contributions and 33% average taxes, while the earlier study deducted only the tax rate.
DMD usage in the two studies was similar (40 compared to 38% in the earlier survey), despite the difference in timing. One would have expected usage to be lower in the earlier study, as these drugs were relatively new at that time. One explanation may be that a proportion of the patients were recruited in specialized clinics where these treatments would be used earlier and more often. It may, however, also be the case that new drugs are rapidly adopted in Austria and provided to patients suitable for treatment.
Compared with the other countries in our European study, costs in Austria are in the mid-range. However, inpatient costs and ambulatory care consultations are at the high end of the range. Length of stay was the longest, and the number of consultations among the highest. Similarly, DMDs were intensively used, while the cost of services was low compared to other countries.
Overall, however, the study confirms the results of the earlier Austrian analysis and the general findings in most previous analyses in the field. Specifically, the costs of MS increase up to fourfold with rising EDSS levels, while utilities (QOL) are substantially lower than those of the general population at all levels of disease severity, and decrease dramatically with progressing disease. Relapses are associated with a high short-term increase in costs and decrease in utility.
The distribution of patients in our study is possibly slightly biased towards patients with severe disease, due to our method of patient recruitment. However, if we were to adjust the distribution using the epidemiological study by Baumhackl and colleagues [3], total current costs in Austria, based on 8.100 patients, could be estimated at € 350–400 million per year, which is similar to the results of the earlier study.
These estimates depend on whether patients have assessed their level of disability accurately. Although it seems that patients had little hesitation regarding this question, and there was a good correlation in a small test of the questionnaire between patients’ answers and physician-assessed scores, the results nevertheless indicate that it is difficult to distinguish between an EDSS score of 3 and 4 and between a score of 6 and 6.5. One would have to say, however, that all scales are subject to inter-rater variability, and hence some of these uncertainties would also be present when scores are assessed by physicians.
This study provides an in-depth analysis of the total societal costs in Austria for MS. The results show the close link between costs, utilities and disease severity. This is to our knowledge the first Europe-wide study that has evaluated the cost structure of the MS burden since the introduction of DMDs. A number of studies were performed shortly after their introduction, one of them in Austria, and our results are very similar due in part to the use of the same methodology. Although cost-of-illness studies cannot be used to assess the cost-effectiveness of different treatment alternatives, the results of this study provide up-to-date information for cost-effectiveness evaluations of new therapeutic options in MS.
References
Kobelt G, Berg J, Lindgren P, Fredrikson S, Jönsson B (2006) Costs and quality of life of multiple sclerosis in Europe. J Neurol Neurosurg Psychiatry. Published online 11 May 2006
Ganzinger U, Badelt C, Vass K et al. (2004) Krankheitskosten der multiplen Sklerose in Österreich. Querschnittstudie unter Brücksichtigung der Lebensqualität, Der Nervenarzt
Baumhackl U, Eibl G, Ganzinger U et al. (2002) Prevalence of multiple sclerosis in Austria. Results of a nationwide survey. Neuroepidemiology 21: 226–234
Pugliatti M, Rosati R, Carton H et al. (2006) The prevalence and incidence of multiple sclerosis in Europe. Eur J Neurol 13: 1–23
Kurtzke J (1983) Rating neurological impairment in multiple sclerosis and expanded disability status scale (EDSS). Neurology 33: 1444–1452
The EuroQol Group (1990) EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 16: 199–208
Dolan P (1995) A social tariff for EuroQol: results from a UK general population survey. Centre for Health Economics, University of York: York
Eichler H, Kong S, Gerth W, Al E (2004) Use of cost-effectiveness analysis in health care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Val Health 7: 518–528
Kobelt G (2004) Economic evidence in multiple sclerosis: a review. Eur J Health Econ 5: 54–62
Kobelt G, Lindgren P, Parkin D, Jönsson B (2000) Costs and quality of life in multiple sclerosis. A cross-sectional observational study in the United Kingdom. SEE/EFI Working Paper Series in Economics and Finance No. 399. Stockholm School of Economics; Stockholm, Sweden
Henriksson F, Fredrikson S, Masterman T, Jönsson B (2001) Costs, quality of life and disease severity in multiple sclerosis. A cross-sectional study in Sweden. Eur J Neurol 8: 27–35
Kobelt G, Lindgren P, Smala A, Jönsson B, Group GMS (2001) Costs and quality of life in multiple sclerosis. A cross-sectional observational study in Germany. Eur J Health Econ 2: 60–68
Acknowledgement
Ulrika Lilja (Stockholm Health Economics), Steve Hass (Elan Corporation)
Conflict of interest
No information supplied.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kobelt, G., Berg, J., Lindgren , P. et al. Costs and quality of life of multiple sclerosis in Austria. Eur J Health Econ 7 (Suppl 2), 14–23 (2006). https://doi.org/10.1007/s10198-006-0382-x
Issue Date:
DOI: https://doi.org/10.1007/s10198-006-0382-x