Abstract
Second-generation atypical antipsychotics such as clozapine, olanzapine, risperidone, quetiapine, ziprasidone, amisulpride and ariprazole offer the potential to reduce the significant health care resource demands in the treatment of schizophrenia through improved levels of initial clinical response and reduced levels of long-term acute relapse. However, the optimal sequencing of these drugs remains unclear. To consider this issue from a health economic viewpoint a decision model approach was used comparing healthcare costs and clinical outcomes when treating patients with alternative sequences of atypical antipsychotic treatment. Treated patients were assumed to be in a current acute episode with at least a 10-year history of disease and to be naive to previous atypical treatments. Treatment strategies were based on either first-line olanzapine or risperidone with switching to the alternative drug as second-line treatment following an inadequate clinical response to first-line drug therapy. Clinical response data were derived from a pivotal published comparative study of both olanzapine and risperidone. Published data on the long-term use of antipsychotic drugs where used wherever possible to populate the model for relapse rates during the maintenance phase. Health care resource data were defined for Germany based on expert clinical opinion. A treatment strategy of first-line olanzapine was shown to be cost saving over a 1-year period, with additional clinical benefits in the form of avoided relapses. The model suggests that over the first year of treatment a strategy of first-line olanzapine is associated with lower risk of additional relapse (0.33 fewer acute relapses per 100 patients per year) and with cost savings (€35,306 per 100 patients per year). There is a need for longer term direct in-trial comparisons of atypical antipsychotics to confirm these indicative results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Schizophrenia is a serious mental illness, with a generally accepted life-time prevalence of around 1% for the general adult population [1]. The majority of patients with schizophrenia are diagnosed in early adulthood, with their illness developing typically as a long-term chronic pattern of continuing acute relapses experienced over a period of many years. The chronic relapsing nature of schizophrenia can lead to significant resource demands on health care and social care systems. Patients often require specialist psychiatric hospital admission during periods of acute episode and frequently need significant longer term community-based support during the longer periods of symptom stability between acute episodes. The recent trend in most developed countries has been towards the expansion of community-based health care and support for patients with mental illness generally in the form of community mental health teams who develop close links and partnerships with community psychiatrists and out-patient care. When considered as a whole, the overall costs of acute treatment and longer term maintenance care for patients with schizophrenia represent a significant proportion of national health expenditure. In the United Kingdom, for example, schizophrenia has been reported to account for approx. 2.8% of all combined health service and community social care expenditures, and over 5% of all in-patient linked expenditure [2]. This consistent pattern of high levels of direct cost associated with schizophrenia is similar to that observed in other developed countries, including Germany [3, 4, 5, 6].
Antipsychotic drug-based therapy remains the mainstay of current treatment for the majority of patients who suffer from schizophrenia. The use of drug therapy both during an acute episode to control symptoms and continued over the longer term as a maintenance treatment to prevent episode relapse is strongly recommended in most treatment settings. Antipsychotic drugs as a whole can be considered in two general classes. The first group, conventional (or typical) antipsychotics, includes long-standing drugs such as haloperidol, which are recognised as being effective in treating the positive symptoms of schizophrenia (e.g. delusions, hallucinations and thought disorder). Their benefits against the negative symptoms of schizophrenia (e.g. social withdrawal, reduced motivation and blunting of emotions) are, however, not as clearly defined. These drugs are also strongly associated with increased levels of extrapyramidal symptoms (EPS) which can have severe impacts on patients’ quality of life and can lower treatment adherence [7]. The second group, newer (or atypical) antipsychotics, includes drugs such as clozapine, olanzapine, risperidone, quetiapine, ziprasidone, amisulpride and ariprazole. These drugs are generally thought to have greater efficacy against the negative symptoms of schizophrenia and causes fewer EPS symptoms, although variations do appear in EPS levels within the atypical drug class themselves [7].
Given these clinical advantages, the newer second-generation atypical antipsychotics have extended the available treatment options for schizophrenia and offer the potential to reduce both the need and duration of hospital admissions through improved levels of initial clinical response and reduced levels of long-term acute relapse during maintenance therapy. The recent National Institute for Clinical Excellence (NICE) technology appraisal of antipsychotics into the treatment of schizophrenia clearly recommends that second-generation oral atypical antipsychotics should be considered as a first-line treatment option for newly diagnosed patients for and patients in relapse who have unsatisfactory management or unacceptable adverse events from conventional therapy [8]. Although grouped together, the atypical drugs do have their own unique efficacy and adverse event profiles which allows them to be differentiated on clinical and economic terms [9, 10].
Study design
Aims and objectives
This study developed a decision model to compare healthcare costs and clinical outcomes in the form of the number of acute relapses and days spent in acute episodes. In line with the recommended use of antipsychotic therapy the model includes both the acute and maintenance phases of therapy. The model structure was used to considered alternative sequences of second-generation atypical antipsychotic treatments in patients with an established history of schizophrenia. The model was informed by previous modelling studies which have compared conventional and atypical antipsychotics [11, 12, 13]. This contribution presents the model structure, parameter estimates and baseline results from the perspective of the German healthcare system.
Population
The model is designed to consider the costs and outcomes for a simulated cohort of patients who are currently suffering from an acute episode of schizophrenia, and who are being considered for first-line treatment with a second-generation atypical antipsychotic. Patients are assumed to have a long-term history of relapsing schizophrenia and to have no other concurrent psychotic diagnoses or other significant health issues. In addition, patients are assumed to have not received any form of previous treatment with atypical antipsychotics. Data for the model is drawn from pivotal clinical trials of atypical antipsychotic, which are generally based in cohorts of adult patients with schizophrenia, having Brief Psychiatric Rating Scale (BPRS) scores of at least 24.
Model structure
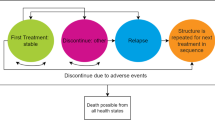
The model structure (Fig. 1) was split into two distinct treatment phases. The first, the acute phase, reflects an initial 3-month period of acute treatment in which the treatment intent is aimed primarily at reducing the current acute symptoms and stabilising the patient. The model is used to describe the level of hospitalisation and length of stay required and to treat patients with atypical antipsychotics. The second, the maintenance phase, is a phase of prolonged longer term preventative treatment aimed at preventing acute relapses. Acute relapses are defined in the model as new episodes of acute symptoms that require clinical treatment. The pattern of repeated acute relapses of symptoms is typically seen in the majority of patients with diagnosed schizophrenia. The second element of the model applies a risk of acute relapse and, again, associates these episodes with a likelihood of hospitalised treatment and cost. A summary of key model terminology and assumptions is provided in Table 1.
Acute treatment phase
For the first phase of the model a simple decision-tree approach was used to track the resource use, costs and clinical outcome experience of patients from the point at which atypical antipsychotics are prescribed until the acute clinical symptoms are brought under control (defined in the model as an adequate clinical response-in line with trial outcome definitions). The model was designed to consider and compare two alternative treatment approaches, strategies A and B. These treatment strategies were based on alternative first-line options using olanzapine and risperidone, respectively. Patients failing to achieve an adequate first-line clinical response were switched in the model to the alternative atypical drug as a second-line treatment option. Failure to achieve a clinical response after two separate atypical antipsychotic treatments was considered in the model as patients having treatment-resistant disease. In this case patients were then assumed to have treatment based on the antipsychotic drug clozapine, in line with standard clinical practice, for the remainder of the treatment period.
Maintenance phase
The second phase of the model was based on a health transition (or Markov) model, tracking the longer term treatment experience of patients and the associated health care resource use and costs. Patients were assumed to remain on the atypical antipsychotic drug to which they responded during their acute treatment. Patients remained in a stable condition with controlled symptoms unless they experienced an acute relapse of symptoms (Fig. 1). The ongoing risk of an acute relapse was considered in the model using 3-monthly risks based on outcome data from published longer term clinical studies of second-generation atypical antipsychotics [14, 15]. On having an acute relapse patients in the model were moved into one of two possible health states depending on the setting of their treatment for the new acute episode: hospitalised-based or community-based care. The risk of suicide attempt, and completion, and associated health care costs were included in the model for each predicted acute relapse. Each acute relapse in the model was given an expected duration of 3 months. Therefore the primary economic analysis, which was based on 1 year of treatment, consisted of the initial acute treatment period (3-months) followed by three cycles of maintenance treatment (9 months).
Clinical effectiveness
Clinical response rate
Data on clinical response were taken from the international pivotal olanzapine-risperidone comparative study [15]. The study was based on adult patients with a formal diagnosis of DSM-IV schizophrenia, schizophreniform disorder or schizoaffective disorder and BPRS scores (derived from Positive and Negative Symptoms Scale, PANSS, score) of at least 42, indicating moderate–severe disease symptoms. Clinical response was primarily defined in the trial as patients showing at least a 40% improvement in their PANSS score from baseline values. These data showed a significant advantage in favour of olanzapine (Table 2) and were the definition of clinical response used in the model to represent patients continuing treatment on their initial drug. In addition, the published comparative study also reported clinical response data using a range of different levels of a minimally required improvement in PANSS score (based on at least a 30% and at least a 50% improvement in PANSS). At all levels of defined clinical response, olanzapine had a clear and significant advantage over risperidone [15].
The HGAJ study [16] was by far the largest of the short-term studies of olanzapine. This trial reported that 52% of patients on olanzapine achieved the defined level of clinical response, compared to 34% of patients on haloperidol, where clinical response was based on at least a 40% improvement in BPRS scores from baseline. This suggests that the data from the comparative study by Tran et al. [15] are likely to be conservative in terms of the absolute response rates for olanzapine. A recently published trial by Gureje and colleagues [17] has also considered olanzapine and risperidone in the management of schizophrenia. The study found that based on a PANSS score change of at least 20% from baseline olanzapine resulted in a clinical response in 75% of patients, compared to 47% of patients treated on risperidone. Using a definition of response of at least a 40% improvement, the corresponding response rates were 22% and 13%, respectively.
Acute relapse risk
Where available, published data were used in the model to calculate corresponding 3-monthly risks of patients experiencing an acute relapse for each antipsychotic treatment included in the model (Table 3). However, at the point of developing the model there existed only limited published data on the longer term use of atypical antipsychotics and their associated acute relapse rates. The majority of this published long-term data related to olanzapine.
Due to this limitation the maintenance phase was separated into two distinct time periods (year 1 and year 2+) in line with available published data. For the first year of treatment acute relapse data for olanzapine were taken from a pooled analysis of the three main randomised controlled trial extensions comparing olanzapine to the typical antipsychotic drug haloperidol [14]. The largest of these was the HGAJ international study which dominates the pooled data. In the overall pooled 1-year risk data 19.7% of patients on olanzapine (dose range 2.5–20 mg, n =628) experienced an acute relapse of diagnosed schizophrenia, compared to 28.0% of patients treated with the standard conventional antipsychotic drug haloperidol (dose range 5–20 mg, n =180). The comparative study of olanzapine and risperiodone [15] reported 28-week data equating to relapse rates of 12.1% for olanzapine and 32.3% for risperidone (a relative risk for risperidone of around 2.6). This suggests a likely advantage towards olanzapine over risperidone in longer term relapse rates. However, no comparable data were available for risperidone for the full 1-year time period.
Therefore for this analysis we used assumed relapse rates for risperidone based on data taken from a recent economic model of schizophrenia which also included risperidone as a treatment alternative [11]. This study used an annual relapse rate for risperidone of 23.4% for year 1, based on an average over the olanzapine and haloperidol trial extension data. Again, no published trial data were found for either treatment covering acute relapse periods beyond 1 year of follow-up. A conservative assumption was therefore used, setting both treatments to equivalent relapse rates based on an annual risk of 9.4% [11].
Suicide risk
Default data on suicide risk at acute episode (13.1%) were taken a recently published schizophrenia model from the United States [13] and a study of suicide rates in patients suffering from acute schizophrenia [18]. Suicide completion rates were set to 23% [11].
EPS and anticholinergic medication
The model included an estimate of the expected cost for anticholinergic drug treatment used to prevent the development of EPS. Data on the proportion of patients on each atypical drug requiring anticholinergic drug treatment were taken from the comparative Tran et al. [15] study (olanzapine 19.8%, risperidone 32.9%). The cost of medication was based on a maximum 6 mg/day dose of biperiden. Montes and colleagues [19] reported similar differences in incidence rates for EPS, at 18% for olanzapine and 46% for risperidone.
Health care resource data
A clinical focus group was used to estimate the expected levels of healthcare resource use and cost in terms of hospitalisation rates and levels of community care in both the acute and maintenance phases of the model. This group consisted of four experts selected to provide clinical and health economic experience of treating schizophrenia in Germany. These experts were all based in Germany and consisted of a leading published health economist in the mental health sector and three leading clinical psychiatrists/psychotherapists who are involved in leading regional and national professional groups in the area of mental health (including Association of German Nervenärzte, Deutsche Gesellschaft für Psychiatrie, Psychotherapie und Nervenheilkunde, Göttingen Research Association for Schizophrenia and German-Language Society for Psychotraumatologie).
Hospitalised care
Default data used in the model for acute hospitalisation admission rates, average admission length of stay and costs per hospital day are shown in Table 4. The model applied an extended hospitalisation stay for patients who required a second-line treatment of their acute symptoms to account for the time spent on initial first-line treatment.
Community-based care
Community-based care was represented in the model in three ways. Patients in an acute episode could be expected to have community-based care for the complete duration of their acute episode (termed in the model as non-hospital acute episodes). Alternatively, patients could move into the community for the remainder of their acute episode following a discharge from an initial period of hospitalisation (post-hospital acute episodes). Finally, patients with stabilised symptoms were assumed to remain community-based during the maintenance phase (stable maintenance) unless they moved on to experience further acute relapses. In the model community care was split into two key elements (Table 5): clinical management costs (i.e. the cost of providing direct clinical management through psychiatrists, general practitioner or specialist out-patient services) and residential-based costs (i.e. the costs per day of any form of sheltered housing or home support).
Drug costs
The daily cost assumed for each of the atypical antipsychotic drugs is detailed in Table 6. Drug doses were based on recommended levels for Germany and an analysis of typical prescribing in Germany using Mediplus IMS data for 2004. Olanzapine and risperidone were costed assuming per day doses of 10 and 4 mg, respectively. Unit costs for drugs were taken from published German drug cost data [20].
Suicide-related costs
A default cost of suicide attempt was based on the diagnosis-related group (DRG) code 449: poisoning and toxic effects of drugs for both analyses. However, no DRG-based cost existed for Germany at the time of this analysis, nor did we identify any other specific estimated of cost related to suicide. We therefore adopted an estimate taken from an Italian cost estimate of €1,996 per episode. For comparison a cost of US $1,860 per suicide attempt (1995 values) was originally used in the Palmer et al. [11, 21] model. We assumed a relative cost ratio of 30% for the cost of suicide completion (estimated at €599) compared to suicide attempt, as observed in the Palmer et al. [11] model.
Quality of life adjustment
The model includes clinical outcomes based on quality-adjusted life years (QALYs). Few data were available on utility weights for health states specific to the area of schizophrenia. Revicki et al. [22] have previously published the most widely quoted data on utility weights related to schizophrenia. This study used a standard gamble approach with clinician assessment and suggested the following utility weights: acute symptoms (positive) as in-patient, 0.56 (used in the model for acute in-patient care); acute symptoms (negative) as out-patient, 0.60 (used in the model for acute community-based care); excellent function as out-patient, 0.83 (used in the model for stable maintenance). Alternative utility data were also considered from a Canadian study of risperidone and clozapine [23]. The Revicki et al. study data was, however, felt to provide the most comprehensive data available in the published literature.
Results
Primary analysis
The primary analysis focused on treatment costs and benefits experienced during the first year of treatment as no existing data were identified for relapse rates beyond a 1-year follow-up period. Strategy A resulted in costs of €3,226,028 per 100 patients compared to €3,261,334 per 100 patients for strategy B, representing a saving of €35,306. Over the 1-year period the model predicted that strategy A resulted in 0.33 fewer acute relapse per 100 patients, equating to a QALY gain of 0.05 per 100 patients. First-line olanzapine was therefore shown to be cost saving over the 1-year period, with additional clinical benefit, in the form of avoided relapses.
Sensitivity analysis
Acute relapse rate
As no data were available to populate the model for experience of acute relapse for either treatment beyond year 1, a set of sensitivity analysis were run to consider potential difference in acute relapse rates, continued over a longer term period of up to 3 years (Table 7). The table shows the baseline case when the model was run over a 3-year period with the relapse rates of both treatments set to equal baseline levels (i.e. 0% absolute difference in rates). Under these conditions we see that the model produces a cost saving of €21,096 per 100 patients. We then considered a range of absolute differences in the relapse rate set in favour of olanzapine (as observed in the comparative study) for years 2 and 3 in the model, leaving all the other baseline parameters fixed (Table 6). The overall additional cost of strategy A decreased as the difference in relapse rate increased, reflecting savings from avoided relapses.
Hospitalisation rate
As part of the sensitivity analyses we also considered a range of alternative hospital admission rates (50–100%) for patients during an acute episode of schizophrenia. Strategy A remained cost saving during year 1 unless hospital admission rates dropped to just below 20% in which case the olanzapine first-line strategy was associated with additional costs. The sensitivity of the cost per QALY and per avoided relapse to the acute hospitalisation rate is shown in Fig. 2. This shows that the level of hospitalisation expected for acute episodes of schizophrenia is a very strong driver of the cost saving and cost-effectiveness for antipsychotic drug treatment choices.
Discussion
Analysis summary
Using a decision modelling approach, our analysis showed that over a 1-year time period first-line treatment with olanzapine provided significant overall treatment cost savings of €35,306 for a simulated cohort of 100 patients. These were attributable mainly to the reduced duration of hospitalisation during the acute episode as more patients had an adequate clinical response to olanzapine. The model also predicted that using first-line olanzapine avoided approximately one acute relapses for every 300 patient treated. It is difficult to place this level of benefit into a framework of clinical meaning as decision makers really need to consider this level of benefit against the expenditure required to achieve it. In this case the relative value in avoiding acute relapses is easy to consider as we also predict a cost a saving alongside the avoided relapse. Certainly the rate of relapse avoidance is low (at one avoided relapse per 300 treated patients), but nevertheless each acute relapse can have a significant impact on a patient. What is clear is that a patient’s quality of life, and that of his or her carers, is certainly much reduced during an acute episode of schizophrenia. Outcomes were, again, driven by the differences observed between treatments in terms of initial clinical response rates and the expected level of acute relapse.
It was clear from the analysis that the clinical and economic benefit of treatment came from two specific areas. Firstly, the shortened hospital duration for patients who achieved an adequate clinical response during their first-line acute treatment, with a 30-day expected additional length of stay indicated for patients who required second-line treatment. This led to higher levels of cost savings associated with the increased proportion of patients who achieved good first-line response (approx. 10% difference between strategies). Secondly, benefits were driven by the avoidance of long-term relapse, and its associated hospitalisations.
Sensitivity analyses confirmed the most influential model parameter as the expected level of acute relapse over the longer term. Expanding the analyses out to 3 years across a range of theoretical differences between olanzapine and risperidone in terms of relapse rates resulted in significant increase in avoided acute relapses (Table 6). Also the level of hospitalisation rate expected for an acute episode is a key model parameter, with rates below 20% suggesting additional costs for olanzapine first-line strategies.
Model validity and limitations
A number of limitations need to be highlighted when considering the validity of the model, its parameter values and the results generated. The level of avoided acute relapse in the model (at around 3–4 per 1,000 patients) between the two treatment strategies may seem initially low given the clear differences in the clinical data used in the model for each of the drugs. Clinical response rates were 36.8% and 26.7%, respectively, for olanzapine and risperidone. However, this is explained by the fact that patients switch treatments on having a poor response to first-line therapy. The overall effect was a 10% difference in the number of patients who became maintained on a long-term olanzapine treatment and hence benefited from the improvement in acute relapse rate (a 3.7% lower risk for olanzapine). Therefore the 10 patients combined with a 3.7% risk reduction combined with other model parameters resulted in 0.33 avoided relapses per 100 patients.
The most comprehensive of the clinical data on atypical antipsychotics covered olanzapine and risperidone, which formed the basis to the treatment strategy comparisons. However, direct head-to-head treatment comparisons of olanzapine and risperidone conducted within the context of randomised clinical trials were limited and restricted to shorter-termed acute care studies. When looking at longer term maintenance therapy, only olanzapine had published estimates of expected relapse rate that covered periods of up to 1 year of treatment (from the pooled short-term trial extension data). The comparison trial [15] made some comparison to risperidone, but this was limited to 28-weeks treatment. It did, however, suggest that differences existed between the treatments, in terms of acute relapse rates. Additional clinical trial data on the longer term use of atypical antipsychotics would strengthen the models ability to consider the possible benefits gained from avoiding acute relapses. This aspect of care needs further clinical research over a longer time period to clarify these potential differences between treatments. Ideally, these data should come from direct head-to-head studies or have inclusion criteria that maximise the ability to make cross study comparisons of outcome data.
The model considers direct treatment costs only and therefore ignores any cost implications of acute relapse on indirect costs such as impacts on carers and lost productivity in patients who are currently employed. The model is also based firmly on outcome data derived from clinical trials in patients with schizophrenia. As such the patient groups are very clearly and tightly defined around patients with no other concurrent diagnosis or complications which therefore limits the generalisability of the model to a wider target patient groups that may be appropriate for treatment with second-generation atypical drugs in clinical practice. Finally, in the absence of any published clinical data comparing the effects of these drug treatments in alternative sequences we assumed that treatment response data could be applied equally in both the first-line and second-line setting. Without clinical data specifically looking at the effects of these drugs given after failure on previous atypical drugs this is a difficult assumption to confirm.
Conclusion
The primary focus of this modelled analysis was directed at olanzapine and risperidone. This was partly in recognition that these are the two main atypical drugs used in clinical practice in Germany. It was also driven by the fact that the majority of head-to-head and longer term published data on atypical antipsychotics were based on these two drugs. The analysis suggests that first-line use of olanzapine has potential cost and clinical benefit advantages over first-line risperidone in atypical naive patients with a history of relapsing schizophrenia. However, there is nothing drug specific about the ‘core’ model framework, limited it to these treatments and it would be possible to use the model, together with suitable clinical data and/or assumptions, to compare a wider set of treatment strategies involving other atypical antisychotics such as quetiapine, ziprasidone, amisulpride and ariprazole.
References
Goldner EM, Hsu L, Waraich P, Somers JM (2002) Prevalence and incidence studies of schizophrenic disorders: a systematic review of the literature. Can J Psychiatry 47:833–843
Knapp M (1997) Costs of schizophrenia. Br J Psychiatry 171:509–518
Rice DP (1999) The economic impact of schizophrenia. J Clin Psychiatry 60 [Suppl 1]:4–6
Garattini L, Rossi C, Tediosi F, Cornaggia C, Covelli G, Barbui C, Parazzini F (2001) Direct costs of schizophrenia in Italian community psychiatric services. Pharmacoeconomics 19:1217–1225
Salize HJ (2001) Costs of schizophrenia—what we know (not)? Psychiatr Prax 28 [Suppl 1]:21–28
Schulenburg JM Graf von der, Uber A, Höffler J, Trenckmann U, Kissling W, Seemann U, Müller P, Rüther E (1998) Untersuchungen zu den direkten und indirekten Kosten der Schizophrenie: eine empirische Analyse. Gesundh Okon Qual Manage 381–87
Gerlach J (1999) The continuing problem of extrapyramidal symptoms: strategies for avoidance and effective treatment. J Clin Psychiatry 60 [Suppl 23]:20–24
National Institute of Clinical Excellence NICE (2002) Guidance on the use of newer (atypical) antipsychotic drugs for the treatment of schizophrenia. Technology appraisal guidance no 43, sect 1.2. NICE: London
Tandon R (2002) Safety and tolerability: how do newer generation “atypical” antipsychotics compare? Psychiatr Q 73:297–311
Remington G, Kapur S (2000) Atypical antipsychotics: are some more atypical than others? Psychopharmacology (Berl) 148:3–15
Palmer CS, Revicki DA, Genduso LA, Hamilton SH, Brown RE (1998) A cost-effectiveness clinical decision analysis model for schizophrenia. Am J Manage Care 4:345–355
Almond S, O’Donnell O (2000) Cost analysis of the treatment of schizophrenia in the UK. A simulation model comparing olanzapine, risperidone and haloperidol. Pharmacoeconomics 17:383–389
Alexeyeva I, Mauskopf J, Earnshaw S, Stauffer VL, Gibson JP, Ascher-Svanum H, Ramsey J (2001) Comparing olanzapine and ziprasidone in the treatment of schizophrenia: a case study in modelling. J Med Econ 4:179–192
Tran PV, Dellva MA, Tollefson GD, Wentley AL, Beasley CM Jr (1998) Oral olanzapine versus oral haloperidol in the maintenance treatment of schizophrenia and related psychoses. Br J Psychiatry 172:499–505
Tran PV, Hamilton SH, Kuntz AJ, Potvin JH, Andersen SW, Beasley C Jr, Tollefson GD (1997) Double-blind comparison of olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorders. J Clin Psychopharmacol 17:407–418
Tollefson GD, Beasley CM Jr, Tran PV, Street JS, Krueger JA, Tamura RN, Graffeo KA, Thieme ME (1997) Olanzapine versus haloperidol in the treatment of schizophrenia and schizoaffective and schizophreniform disorders: results of an international collaborative trial. Am J Psychiatry 154:466–474
Gureje O, Miles W, Keks N, Grainger D, Lambert T, McGrath J, Tran P, Catts S, Fraser A, Hustig H, Andersen S, Crawford AM (2003) Olanzapine vs risperidone in the management of schizophrenia: a randomized double-blind trial in Australia and New Zealand. Schizophr Res 61–:303–314
Meltzer HY, Okayli G (1995) Reduction of suicidality during clozapine treatment of neuroleptic-resistant schizophrenia: impact on risk-benefit assessment. Am J Psychiatry 152:183–190
Montes JM, Ciudad A, Gascon J, Gomez JC EFESO Study Group (2003) Safety, effectiveness, and quality of life of olanzapine in first-episode schizophrenia: a naturalistic study. Prog Neuropsychopharmacol Biol Psychiatry 27:667–674
Anonymous (2002) Rote Liste 2002 (Online). http://www.rote-liste.de, accessed November
Palmer CS, Revicki DA, Halpern MT, Hatziandreu EJ (1995) The cost of suicide and suicide attempts in the United States. Clinical Neuropharmacology 18 [Suppl 3]: S25–S33
Revicki DA, Shakespeare A, Kind P (1996) Preferences for schizophrenia-related health states: a comparison of patients, caregivers and psychiatrists. Int Clin Psychopharmacol 11:101–108
Glennie, Judith L (1997) Pharmacoeconomic evaluations of clozapine in treatment-resistant schizophrenia and risperidone in chronic schizophrenia—summary. Canadian Coordinating Office for Health Technology Assessment: Ottawa
Deckert C, Höffler J, Kortmann J, Linden M, Roth GD, Struck M, Clouth J, Czekalla J (2001) Cost-analysis of schizophrenia treatment in Germany. a comparison of olanzapine, risperidone and haloperidol using a clinical decision model. Gesundh Okon Qual Manage 6:161–166
Conflict of interest
No information supplied.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beard, S.M., Maciver, F., Clouth, J. et al. A decision model to compare health care costs of olanzapine and risperidone treatment for schizophrenia in Germany. Eur J Health Econ 7, 165–172 (2006). https://doi.org/10.1007/s10198-006-0347-0
Issue Date:
DOI: https://doi.org/10.1007/s10198-006-0347-0