Abstract
Between just 1995 and 2003, the number of new chemical entities fell from 45 to 25, while the costs increased by two and a half times in the same period. Firms in the USA accounted for more than half of biotech drugs from 1982 to 2003. European firms are losing competitiveness. In this hostile environment for investment in pharmaceutical R&D, providing quick access to market for real innovations is the main challenge for regulatory agencies. More initiatives, more entrepreneurial spirit and easier work regulation are needed to facilitate the growth of firms in this field, especially in emerging economies like the Spanish. A new open source model proposes the use of pre-competitive public platforms formed by young and qualified human capital carrying out research in areas not sufficiently attractive for private initiatives, followed by the introduction of pharmaceutical companies to carry out the clinical research. The last step would be fast and effective approval by assessment agencies. Governments should, therefore, facilitate the regulation of socially effective innovations, bringing in manufacturers to take part in the post-clinical trial period after entering the market. The gathering of incentives between regulatory agencies and pharmaceutical industry must be approached through innovation and authorization stimulating systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
There is a growing feeling of crisis in the present innovation and development for new drugs system and its regulation [1, 2]. Providing quick access to market of real innovations is the main challenge of regulatory agencies. The scarcity of systematic information about new commercialized drugs, such as their relative efficacy or long term safety, to guide clinical practice, produces conflicts between the different interests in the decision making. Incomplete gathering of data, inadequate study designs, and failure in the effective communication of information to physicians and patients, are some of the important limitations in the regulation process that can put the public health in danger.
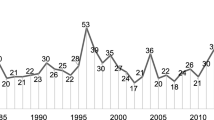
Assuming that the complete basic knowledge transfer process to its development and trading requires an average of 10–15 years, as well as an associated cost of $948 million US, the development of new drugs must improve the productivity of its value chain. Between just 1995 and 2003, the number of new chemical entities (NCEs) fell from 45 to 25, despite the costs increasing by two and a half times in the same period [3].
The pharmaceutical companies have 50% of the world expenditure on drugs concentrated in the USA, for example, in 2001 the USA spent 606$ per person on ambulatory drugs which compares with just 206$ spent in Spain. This is due to the rising number of formulations and the higher cost of the drugs. The pharmaceutical industry’s investment per person in R&D was nine times higher in the USA than in Spain [4].
The lead America has in research is, in great part, based on the close collaboration between the public and private sectors and the high investment in R&D made by the public sector, particularly the National Institutes of Health (NIH) and the public Universities, which amounted to $28,805 million dollars in 2005, representing almost 50% of the whole investment on research [4].
Pharmaceutical R&D requires a complex mix of physiology, pharmacology, target-oriented chemistry, genomics, molecular modelling, and structural biology in order to attempt to be successful. Only 1 out of 100 tested components gets to the human experiment phase, and from these only 1 in 5 ends up being an authorized drug. The pharmaceutical industry is obtaining fewer licences than before and it is really getting harder to develop a blockbuster [3, 4]. The next steps are a better understanding on the way we get ill, a major role of predictive medicine and a more individualized therapy. The benefits will be for the one who innovates by adapting needs to the final solving of demands.
According to a study led by Henry Grabowsky at Duke University [5], between 1993 and 2003, firms in the USA introduced 48% of first-in-class or novel drugs, 52% of biotech drugs, and 55% of orphan drugs, that treat rare diseases worldwide. The study notes that growth in the biotech sector has enhanced the USA’s world leadership in new drug introductions. USA firms accounted for more than half of biotech drugs from 1982 to 2003. Of all disease areas, cancer was the most common target of novel drugs, biotech drugs and orphan drugs. The USA has become the top country where new drugs are first launched and this has obvious benefits for USA patients.
From 1982 to 2003, 919 NCEs were introduced. From those, 42% were global, 13% were first-in-class, 10% were biotech and 8% were orphan. Since 1985–1988, there was a decline in overall drug introductions, but a slight increase in the number of global and first-in-class NCEs. Despite the pessimism about the decline of productivity, the industry is spending more and getting fewer drugs out, but the drugs are higher quality.
Current drug safety concerns could also cause a slowdown in the rate of innovative drug introductions and novel oncology drugs, for example, addressing life-threatening diseases with greater efficacy, but also subject to unknown safety risks at the time of approval. These are difficult trade-offs for the regulatory agencies.
In Spain, the research at public centres usually works without adequate incentives to cover the most urgent health necessities. Since the incentives for researchers are more directed to publishing, in terms of impact factor, less attention is paid to the development of patents and there is little effort put into identifying the researchers’ enterprise potential. There have been some improvements in recent years, but almost everything has still to be done in development. There is little R and less D. The causes of our delay, in a great part stemming from cultural and educational roots, make the priority of biomedical innovation in economic policy in a sensible and explicit way much to be recommended.
The growth of risk capital detected in Spain in recent years will help to improve the investment in this field, as long as it can find solvent initiatives that allow us, because of their quality and quantity, to move up from the lowly positions in competitiveness in every global ranking we have looked at. The information about attractive and potential market opportunities is crucial for the university students who are going to face a hostile labour market if we want to orient some of them towards an entrepreneurial initiative. The implanting of subjects that give an impulse to the enterprising spirit from childhood onwards is crucial so that the next generations see enterprise as something natural as well as a professional expectation that is solid and has a future. Biotechnological enterprise is one of the most attractive alternatives in terms of the creation of new markets and the development of our innovative potential. The affordability of new technologies promotes this development, so it is important to find financing formulas that are able to make a profit from ideas that have a good chance of success in the biotechnological field. Finally, education, advising, and greater capabilities of people to make good plans for new companies and for solid and viable projects will be the elements that will facilitate the growth of these firms in Spain. To sum up: what are needed are more initiatives, more entrepreneurial spirit and a less ridiculous work regulation [6, 7].
In this situation, there must be a better incentive model than the NCEs’ present protection and price system, which is starting to show signs of exhaustion and to not fully convince any of the parts in the game. The roles of the regulators, manufactures, etc., start being seriously questioned [8, 9]. All the agents must try to get the best balance between technological progress and the sustainability of the public health system as a whole.
A new open source model proposes to change present incentives in their location. It claims the opening of technological development’s first phase (public use of the molecules basic knowledge) and the improvements of innovation with the use of pre-competitive public platforms formed by young and qualified human capital making research in insufficiently attractive areas for private initiatives.
In other technological sectors, like the development of new software, the implementation of the model is rapid. The open source approach means that the code-source, from which a number of researches can be made, is accessible in free on the Internet for everyone who wants to use it and modify it, with the condition that the changes and improvements introduced are also in the public domain. The benefit, therefore, is performed by the one who works with the final client and has to adjust the software to the necessities of the client. This advantage, that anyone can contribute, leaves projects open to abuse, either by well-meaning dilettantes or intentional disrupters. Constant self-policing is required to ensure its quality.
In a way, that is how they worked on the development of the human genome project which counted on a sufficient dose of altruistic enthusiasm, public–private collaboration and a critical mass, all working on the same direction.
Under the assumption “You share–I share” and the help of bioinformatics, biotechnological platforms are being built. Many areas can be the pioneers in the use of these platforms, for example, research on new indications for non-patentable drugs or components, or drugs with expired patents, such as statins for HIV/AIDS and aspirin for cancer, or research on orphan drugs for diseases with low incidence or with poor response, such as drug addictions and eating disorders in developed countries, or for diseases that only affect developing countries, e.g., malaria.
In the case of the open source model, the tackling of open and voluntary collaboration would be in the young enterprises, held on the Internet, located in university centres and in public or non-profit private research centres. The natural process would be focused on business projects with shared risk; i.e., with public and private capital input when the developing costs start to be larger than the potential benefits, even if there are chances of success in the near future. Nevertheless, the open-source model has vulnerabilities, for example, it lacks ways of ensuring quality and is still needing better ways to handle intellectual property.
This proposal has a difficult implementation since the pharmaceutical sector is placed at the end of the technological cycle, and not at the beginning, like the software sector.
According to the IGAE’s Analysis Work Report on the health expenses (http://www.map.es) of August 2005, the rise of the price of drugs and other technologies explains 46.1% of the rise of health expenses in Spain. Plus, a consumers’ growing role in the price fixing is foreseen. The solution would then be to combine the benefits for society with NCEs prices that are rewarding enough to proceed with an R&D process that is more demanding each time.
The pharmaceutical R&D has very high investment costs and very low production costs. Therefore, there could be a price system established in two parts, like the phone bill and the light bill: one for access, stable and low, and other variable, depending on the level of use.
We cannot forget the resistance of a patents system that, although defeated, generates a monopoly that allows the producer to fix prices, mostly high, and to be protected from the competition for a dozen years in order to facilitate innovation and regain the investment. However, in spite of governmental regulatory difficulties on the access for patients, there are delays in approvals, each time more restrictive indications, and lack of public reimbursement.
Lately, and curiously, rather than using the law to defend their patents, big firms often settle out of court. Several branded firms have tried to extend their lucrative monopolies by filing less rigorous secondary patents designed to block generics, and the courts are just catching up.
John Evans at Northwestern University [10] proposes this pricing model in two parts. The first part would be for the purchase of the NCE patent by the government through a rewards and subsidies system. The fixing of the amount would depend on the NCE’s effects on health, in terms of survival, quality of life and other reductions in health costs. If these were substantial, the quantity could be quite high. The second part would consist of the free offering of the new patent to any company that wanted to produce the new drug, at low prices, similar to the marginal production costs. This would be the part of the price in which we would promote an almost perfect competition.
This approach preserves public health systems financial viability over time. It would be guaranteed by the previous knowledge of the NCE's economic impact. The private initiative would not be threatened by the successive regulatory restraints that governments use to hold down the costs and it would still be rewarded by the price subsidies or patent funds system. The patients would have quick access to the forthcoming innovations thanks to a relevant role of the new drugs evaluation agencies, all in a global environment with the greatest coordination and reciprocity in their approval decisions. The economic side of evaluation is used in other phases of development, such as components selection, choice of clinical trial parameters and decisions on continuing or stopping the NCEs’ development. The effectiveness studies will be important, since they will allow us to realise if the clinical results required for approval are produced with the same intensity in the medical practice. This information will be crucial for determining the amount of reward or grant that the innovative company will have to be paid for its patent [11, 12].
Later in the clinical setting, doctors and managers in hospitals and primary care have to manage competing claims on their limited budgets. They have to decide what services to fund and what not to fund, as well as the extent of funding. Extra resources will not remove the fundamental need to make such choices because healthcare needs and wants will always outstrip the resources available.
Economic approaches to resource management at the local level have had limited success, partly because economists have failed to consider properly the practical challenges that managers and doctors face in making rational priority setting decisions. Perhaps programme budgeting and marginal analysis, which recognizes the need to balance clinical autonomy with financial responsibility could be a reasonable option.
Summarizing, it would be as in a relay race: it starts with the basic research in university laboratories or research centres, in open environments, and it follows with the entrance of pharmaceutical companies as organizations with enough experience and muscle to carry out the clinical research. On the next step, the evaluation agencies quickly and rigorously approve those innovations which are proved to have results on health. Later, the governments buy the patent through a transparent system of rewards and grants in order to, finally, put it on the market where a strong industrial sector would bet in an auction system. Sadly, there are global regulatory agencies (FDA and EMEA) with competences for approval in terms of efficacy and security, but there is no joint work experience between governments in the subject of price fixing, not even in the European Union, knowing that the pharmaceutical industry is losing competitiveness [13].
If we were able to conceal the three perspectives that matter, i.e., the one of concern to sellers, through fast registrations and a greater comeback for non-cosmetic investments which add real value to the improvement of health; the purchasers' one, through a greater transparency and feasibility in their decisions; and the users' one, through an early and secure access to technological innovation, then we will have made an important step in the right direction. The governments should, therefore, focus on the process of avoiding barriers to socially efficient innovations and in the regulation of priorities that guarantee the sustainability of public health systems.
The gathering of incentives between the regulatory agencies and the pharmaceutical industry must be realized through innovation and authorization stimulating systems that minimize risks and measure the benefits versus the existing alternatives in a realistic way [14].
There is a trend for claiming for consistent data through monitoring post-authorizing drugs in a systematic way, parting from the regional health systems clinical-administrative databases. Nobody suggests excluding the industry cooperation, which of course has an obvious interest in the physicians' and patients’ well-being and trust [15].
It is starting to be shown that the regulatory agencies put a type of distinctive “testing period” for NCEs during the first two years after entering the market. Manufacturers should rigorously assess security in the clinical environment [16–18].
Pharmaceutical and biotechnological industries, governments, physicians and patients need each other, but the current model shows some weaknesses for the strong development of new drugs. This entire discussion paper make us think in terms of new models and approaches, that eventually require answers to the following questions:
What is a fair price for a patent, how do we assess the true market value of patents, and who pays for the development failures?
The current lack of transparency, the difficulties in sharing successes and failures in R&D, the unclear rules in fixing prices for new drugs, the suspicion between private and public partnerships, the insufficient research on calculating social net benefits, and the effectiveness and efficacy of new drugs are all elements that should be discussed openly.
References
Ray, W.A., Stein, C.M.: Reform of drug regulation beyond and independent drug-safety board. N. Engl. J. Med. 354, 2 (2006)
Rovira, J.: Crisis en el modelo de investigación e innovación en medicación. Salud 2000(103), 30 (2005)
Fixing the drugs pipeline: Econ. Technol. Q. (2004)
Angell, M.: The truth about drug companies. Random House, New York (2004)
Grabowski, H.G., Wang, Y.R.: The quantity and quality of worldwide new drug introductions, 1982–2003. Health Affairs, pp. 452–460 (2006)
Coduras, A., del Llano, J.: Bioempresa: la oportunidad se acerca. Gestión Clínica y Sanitaria 27, vol. 8, No. 1, Primavera de (2006)
Acebillo, J., Artells, J.J.: La biomedicina como factor de creación de valor y crecimiento económico. Documento de trabajo no 20. Fundación Salud, Innovación y Sociedad. Barcelona, Julio de (2004)
Rodríguez-Monguió, R., Seoane Vázquez, E.C.: Análisis y alternativas para el sector farmacéutico español a partir de la experiencia de los EEUU. Fundación Alternativas. Documento de Trabajo 57/2004
Estudio económico de la industria farmacéutica en España: Disponible en: www.fgcsal.org (2002)
At F.D.A.: Strong drug ties and less monitoring. Nytimes.com. Cited 6 December 2004
Models that takes drugs: Econ. Technol. Q. (2005)
Solving the drug dilemma: Washingtonpost.com. Cited 22 August 2003
Foro Europeo de Política Farmacéutica: Disponible en: www.fgcsal.org
Peiró, S., Meneu, R.: Autorización y monitorización de medicamentos: reconciliar la protección a la innovación y a los pacientes. Gestión Clínica y Sanitaria, Primavera 7(1), 3–6 (2005)
Laupacis, A., Paterson, J.M., Mamdani, M., Rostom, A., Anderson, J.M.: Gaps in the evaluation and monitoring of new pharmaceuticals: proposals for a different approach. CMAJ 169, 1167–1170 (2003)
Pasty, B.M., Furberg, C.D.: COX-2 inhibitors—lessons in drug safety. N. Engl. J. Med. 352, 1133–1135 (2005)
Fontanarosa, P.B., Rennie, D., De Angelis, C.D.: Postmarketing survillance—lack of vigilante, lack of trust. JAMA 292, 2647–2650 (2004)
Wood, A.J., Stein, C.M., Woosley, R.: Making medicines safer—the need for an independent drug safety board. N. Engl. J. Med. 339, 1851–1854 (1998)
Acknowledgments
To Joaquím Camprubí, Vanessa Campo, Luis Angel Oteo, Juan Gervás, Paloma Fernandez-Cano, Alberto Oteo, Joan Rovira, Vicente Ortún, Ricard Meneu, Salvador Peiró, Álvaro Hidalgo, Guillem López y Timm Volmer, Felipe Martín for the stimulating comments to previous drafts.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
del Llano, J. Discussion point: should governments buy drug patents?. Eur J Health Econ 8, 173–177 (2007). https://doi.org/10.1007/s10198-006-0018-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-006-0018-1