Abstract
The aim of the present study was to evaluate whether the interferon-induced helicase (IFIH1) Ala946Thr (rs1990760 A>G) polymorphism is associated with susceptibility to systemic lupus erythematosus (SLE) and dermatomyositis (DM) or polymyositis (PM) in the Japanese population. The study population consisted of 243 SLE patients, 125 DM/PM patients, and 268 healthy controls from Japan. A Taqman single nucleotide polymorphism genotyping assay was designed for rs1990760 by Applied Biosystems. There were no significant differences between SLE and DM/PM patients and healthy controls regarding the frequency of each genotype and allele. However, the frequency of the AA genotype and the A allele tended to be higher in PM patients with interstitial lung disease (ILD). Additionally, when comparing the AA and AG + GG genotypes at rs1990760, the AA genotype was significantly more frequent in PM patients with ILD than in healthy controls [odds ratio, 3.23 (95% confidence interval, 1.06–9.81); P = 0.04] or in PM patients without ILD [odds ratio, 5.40 (95% confidence interval, 1.37–21.26); P = 0.027]. Our observations suggest that the G allele protects against the onset of ILD and that the AA genotype is a risk factor for lung injury in PM patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The interferon-induced helicase (IFIH1), also known as melanoma differentiation associated-gene 5 (MDA5), is a member of the retinoic acid-inducible gene I-like helicase (RLH) family [1, 2]. The expression of genes encoding RLHs is strongly induced by type 1 interferon (IFN). RLHs interact with dsRNAs through their helicase domain. Specifically, IFIH1 responds to infection with picornaviruses. IFIH1 comprises two N-terminal caspase-recruitment domains (CARDs). The N-terminal CARDs are responsible for activating downstream signaling pathways that mediate dsRNA-induced type I IFN production [1].
Inference of the role of type I IFN in systemic lupus erythematosus (SLE) comes from the induction of autoimmunity during IFN therapy and the presence of circulating inducers of type I IFN in SLE patients [3, 4]. Thus, type I IFN has been one of the factors attributed to the pathogenesis of SLE. The increased bioavailability of type I IFN contributes to peripheral tolerance breakdown through the activation of immature myeloid dendritic cells [4].
Clinically, amyopathic dermatomyositis (C-ADM) is typified by skin lesions with amyopathy or hypomyopathy [5], and was recently reported to be complicated by rapidly progressive interstitial lung disease (ILD), especially in those patients with anti-CADM-140 antibodies [6]. Recently, the anti-CADM-140 antibodies in patient sera were shown to specifically react with MDA5 protein, confirming the identity of MDA5 as the CADM-140 autoantigen [7]. Additionally, infection with Coxsackie virus, a picornavirus, was reported to be one of the contributing factors in the pathogenesis of juvenile dermatomyositis (DM) [8].
One recent study has confirmed that the IFIH1 Ala946Thr (rs1990760 A>G) polymorphism is associated with susceptibility to type 1 diabetes and Graves’ disease [9, 10]. Another study has revealed that IFIH1 Ala946Thr shows a highly significant association with SLE in the US and Sweden [11]. However, the IFIH1 Ala946Thr polymorphism has not yet been investigated in the context of DM and polymyositis (PM). Taken together, we examined the association between the IFIH1 Ala946Thr polymorphism and SLE and DM/PM for the first time in the Japanese population.
Patients and methods
Patients and controls
The study population consisted of 243 SLE patients, 125 DM/PM patients and 268 healthy controls from Japan. The population of the present study was recruited from our institution. Collection of data and blood samples from patients and controls was undertaken in accordance with the ethical committee in our institution, and informed consent was obtained from all subjects according to the Declaration of Helsinki. The mean age and female frequency were 36.7 years old and 95.1%, 53.1 years old and 78.3%, and 42.7 years old and 94.1% in SLE patients, DM/PM patients and healthy controls, respectively. The mean age and female frequency were matched between SLE patients and healthy controls, although the mean age and male frequency were higher in DM/PM patients than in healthy controls. SLE and DM/PM were diagnosed according to the American College of Rheumatology Association classification and Bohan and Peter’s criteria, respectively [12, 13]. Additionally, SLE patients were classified into four subsets: SLE without neuropsychiatric abnormality (NP), SLE with NP, SLE without nephritis, and SLE with nephritis. DM/PM patients were also classified into four subsets: DM without interstitial lung disease (ILD), DM with ILD, PM without ILD and PM with ILD. ILD was defined as high-resolution computed tomography (HRCT) of the chest that included one or more of the following features: isolated ground-glass opacities, honeycombing and the concurrent presence of ground-glass attenuation, and traction bronchiectasia and/or bronchiolectasis.
Single nucleotide polymorphism (SNP) genotyping
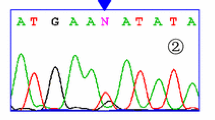
DNA was extracted from peripheral blood using standard methods. A Taqman SNP genotyping assay was designed for rs1990760 by Applied Biosystems. The sequences of the allele-specific probes were 5′-TAGTCGGCACACTTCTTTTGCAGTG-3′ (reporter dye, VIC) and 5′-TTTGTTTTCTCTTACAATGTAAAGT-3′ (reporter dye, FAM). Allelic PCR products were analyzed on the ABI Prism 7900HT Sequence Detection System using SDS 2.1 software (Applied Biosystems, Foster City, CA, USA). The quality value used for the auto-calling system was set to 95%. The genotype distribution conformed with the Hardy–Weinberg equilibrium in the controls.
Statistical analysis
The case–control study was analyzed using the chi-squared test on 2 × 2 and 2 × 3 contingency tables for genotype and allele frequency, with Fisher’s exact test used when appropriate. The odds ratio (OR) and 95% confidence interval (CI) were calculated. P < 0.05 was considered to indicate statistical significance.
Results
Genotype and allele frequencies for rs1990760 in SLE and DM/PM patients
Frequencies of the genotypes and alleles of the IFIH1 Ala946Thr (rs1990670) SNP are shown in Table 1. The frequencies of both genotypes and alleles were not significantly different among SLE and DM/PM patients and healthy controls. However, the frequencies of the AA genotype and the A allele tended to be higher in the subset of patients with PM with ILD.
Comparison between AA and AG + GG genotypes for rs1990760 in DM/PM patients and healthy controls
The frequencies of both the AA and AG + GG genotypes in DM/PM patients and healthy controls, under a recessive model for the A allele of rs1990760, are shown in Table 2A. The frequencies of both genotypes were not significantly different between the entire group of DM/PM patients and healthy controls. However, the AA genotype was significantly more frequent in PM with ILD patients than in healthy controls [OR, 3.23 (95% confidence interval, 1.06–9.81); P = 0.04]. Additionally, when comparing the AA and AG + GG genotypes for rs1990760 in PM patients with and without ILD, the AA genotype was significantly more frequent in patients with ILD than in those without ILD [OR, 5.40 (95% confidence interval, 1.37–21.26); P = 0.027].
On the other hand, the frequencies of both the AA + AG and GG genotypes in DM/PM patients and healthy controls, under a dominant model for the A allele of rs1990760, are shown in Table 2B. The frequencies of both genotypes were not significantly different between the group of DM/PM patients and healthy controls.
Discussion
The present study is the first to report the association of a SNP within IFIH1 with SLE and PM/DM in the Japanese population. Another study has revealed that IFIH1 shows a highly significant association with SLE in the US and Sweden [11]. Similarly, IFIH1 has previously been associated with type 1 diabetes and Graves’ disease [9, 10]. However, IFIH1 was not significantly associated with SLE in the present study. This sample size provides powers of 44.4 and 30.0% (as calculated using the analysis software R) to detect an association with an OR of 1.5 under the dominant model in SLE and DM, respectively. The lack of statistical power and racial differences may have contributed to the lack of association in the present study.
IFIH1 polymorphisms have not been investigated in DM/PM patients to date. IFIH1 was not significantly associated with DM/PM as a whole in the present study. IFIH1 is also known as MDA5 [1, 2]. Anti-MDA5 antibodies are often seen in cases of C-ADM with rapidly progressive ILD [7]. However, IFIH1 was not associated with DM with ILD in the present study. Unexpectedly, the AA genotype and the A allele tended to be found with higher frequencies in the PM with ILD subset. Additionally, when comparing the AA and AG + GG genotypes under a recessive model for the A allele of rs1990760, the AA genotype was significantly more frequent in patients in the PM with ILD subset than in those in the PM without ILD subset or healthy controls. On the other hand, the frequencies of both the AA + AG and GG genotypes under the dominant model for the A allele were not significantly different between the group of PM patients and the healthy controls, although the A allele was previously described as being a dominant model of inheritance in type 1 diabetes and Graves’ disease [9, 10]. These results suggest that the G allele protects against lung injury in PM patients.
RIG-I comprises two N-terminal CARDs followed by a DExD/H box RNA helicase domain. RIG-I is a member of the RLH family, along with MDA5 and LGP2, based on the high similarities among their helicase domains [1, 2]. The IFIH1 Ala946Thr SNP does not reside in either the CARD or the helicase domain of the protein. However, this region of the protein is conserved among mammals and may have unknown functions or influence the active domains through effects on tertiary structure. The A allele of rs1990760 was a more frequently conserved amino acid than the G allele in healthy controls [9, 10]. Additionally, the frequency of the A allele was higher in type 1 diabetes and Graves’ disease than in healthy controls. These findings suggest that the A allele represents the functional allele for inflammation and autoimmune disease.
In general, autoreactive cytotoxic T cells mediate MHC I-restricted cytotoxicity against autoantigens expressed on muscle in PM [14]. On the other hand, DM is considered to be CD4+ T cell- and B cell-mediated muscle inflammation. The complement system is activated, resulting in the deposition on the membrane of an attack complex within muscle capillaries [14]. The cytokine profiles were different for PM and DM. Taken together, type 1 IFN may be more closely associated with autoreactive cytotoxic T cell inflammation of muscle and lung in PM. Additionally, the development of interstitial pneumonia, although uncommon, has been reported in association with IFN alpha therapy [15, 16]. These aspects may indicate that the A allele promotes IFN 1 and induces lung injury in PM patients, and that onset of ILD is frequent, especially in PM patients with the AA genotype.
In conclusion, although the IFIH1 Ala946Thr polymorphism was not associated with susceptibility to SLE or DM, the AA genotype may be a risk factor for the onset of ILD with PM in the Japanese population.
References
Takeuchi O, Akira S. MDA5/RIG-I and virus recognition. Curr Opin Immunol. 2008;20(1):17–22.
Yoneyama M, Kikuchi M, Matsumoto K, Imaizumi T, Miyagishi M, Taira K, et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol. 2005;175(5):2851–8.
Rönnblom L, Alm G, Oberg K. Autoimmune phenomena in patients with malignant carcinoid tumors during interferon-alpha treatment. Acta Oncol. 1991;30(4):537–40.
Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25(3):383–92.
Gerami P, Schope J, McDonald L, Walling H, Sontheimer R. A systematic review of adult-onset clinically amyopathic dermatomyositis (dermatomyositis siné myositis): a missing link within the spectrum of the idiopathic inflammatory myopathies. J Am Acad Dermatol. 2006;54(4):597–613.
Sato S, Hirakata M, Kuwana M, Suwa A, Inada S, Mimori T, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. 2005;52(5):1571–6.
Sato S, Hoshino K, Satoh T, Fujita T, Kawakami Y, Kuwana M. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease. Arthritis Rheum. 2009;60(7):2193–200.
Christensen M, Pachman L, Schneiderman R, Patel D, Friedman J. Prevalence of Coxsackie B virus antibodies in patients with juvenile dermatomyositis. Arthritis Rheum. 1986;29(11):1365–70.
Smyth D, Cooper J, Bailey R, Field S, Burren O, Smink L, et al. A genome-wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat Genet. 2006;38(6):617–9.
Sutherland A, Davies J, Owen C, Vaikkakara S, Walker C, Cheetham T, et al. Genomic polymorphism at the interferon-induced helicase (IFIH1) locus contributes to Graves’ disease susceptibility. J Clin Endocrinol Metab. 2007;92(8):3338–41.
Gateva V, Sandling J, Hom G, Taylor K, Chung S, Sun X, et al. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat Genet. 2009;41(11):1228–33.
Tan E, Cohen A, Fries J, Masi A, McShane D, Rothfield N, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–7.
Bohan A, Peter J. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292(7):344–7.
Dalakas M. Immunopathogenesis of inflammatory myopathies. Ann Neurol. 1995;37(Suppl 1):S74–86.
Tokita H, Fukui H, Tanaka A, Kamitsukasa H, Yagura M, Harada H, et al. Circulating KL-6 level at baseline is a predictive indicator for the occurrence of interstitial pneumonia during interferon treatment for chronic hepatitis C. Hepatol Res. 2003;26(2):91–7.
Okanoue T, Sakamoto S, Itoh Y, Minami M, Yasui K, Sakamoto M, et al. Side effects of high-dose interferon therapy for chronic hepatitis C. J Hepatol. 1996;25(3):283–91.
Acknowledgments
This study was supported in part by research grants from the Ministry of Health, Labour and Welfare in Japan.
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Gono, T., Kawaguchi, Y., Sugiura, T. et al. Interferon-induced helicase (IFIH1) polymorphism with systemic lupus erythematosus and dermatomyositis/polymyositis. Mod Rheumatol 20, 466–470 (2010). https://doi.org/10.1007/s10165-010-0311-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10165-010-0311-9