Abstract
The coral-dwelling damselfish Dascyllus reticulatus shows heterosexual cohabitation on branching corals and has been considered to maintain a haremic mating system, where a few males monopolize mating within the group. However, details of the group structure have not been investigated. To clarify the individual-level group structure of D. reticulatus, we conducted field observational surveys on a damselfish population on reefs of Kuchierabu-jima Island, southern Japan. Relatively large D. reticulatus inhabited corals with long branches and wide gaps with a female-biased sex ratio, and they maintained haremic groups where the largest male monopolized mating. In contrast, small adults and juveniles cohabited in higher individual densities on short-branch corals, with no bias in individual sex ratio. Only nine of 26 adult males in the short-branch coral groups showed mating activities. Nineteen of 37 adult females in the short-branch coral groups spawned, and their spawning frequency was lower than that of the females on the long-branch coral. Thus, we observed two contrasting social compositions and mating activities within harem-like cohabitation groups that depended on body size and sheltering coral structures. We observed inter-harem moves by large non-breeding individuals from the short-branch corals to the long-branch corals, implying a conditional use of the two types of groups related to body size. Our observations present a new example of multiple forms of groups in haremic reef fishes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Social structure and mating systems are essential to understanding the mating strategies of reef fishes (Thresher 1984; Kuwamura 1996, 1997). Empirical field data have been used to define the patterns of spatial relationships and mating relationships to classify the mating systems of reef fishes: monogamy, harem polygyny, male-territory-visiting (MTV) polygamy, non-territorial polygamy, and polyandry (review in Kuwamura 1996, 1997). Polygamous mating systems, including harem polygyny and MTV polygamy, are broadly present in reef fishes (Robertson and Warner 1978; Warner and Robertson 1978; Thresher 1984; Moyer 1990).
Strong correspondence between mating system and social structure has been confirmed in various polygamous fishes. Females of MTV polygamy groups usually visit male mating territories during mating periods, where female mate choice often results in mate change (e.g., Warner and Schultz 1992; Kuwamura et al. 2000). In cases of harem polygyny, females stably spawn with cohabiting territorial dominant males. Therefore, heterosexual cohabitation, dominance relationships and mating monopolization are commonly confirmed as characteristics of social structure in haremic fishes (e.g., Kuwamura 1984; Sakai and Kohda 1997; Kadota et al. 2011).
Pomacentridae (damselfishes) is a common reef fish group characterized by a reproductive mode with parental care of demersal eggs (Nelson et al. 2016). The pomacentrids show a wide range of mating systems, including monogamy (Amphiprion: Fricke and Fricke 1977; Acanthochromis: Thresher 1985) and MTV polygamy (Stegastes: Karino and Nakazono 1993; Abudefduf: Keenleyside 1972). The genus Dascyllus is a coral-dwelling species group with a polygamous mating system (Fricke 1980; Fricke and Holzberg 1974; Schwarz and Smith 1990; Godwin 1995). In addition, other than species with gonochoristic sexualities, sequential hermaphroditism (i.e., sex change) has been confirmed in some pomacentrid species (Fricke and Fricke 1977; Moyer and Nakazono 1978b), including Dascyllus (Schwarz and Smith 1990; Kuwamura et al. 2016). Thus, damselfishes represent a wide ecological diversity in mating systems and sexualities.
Dascyllus reticulatus is a small planktivorous damselfish that commonly occurs on coral reefs of the eastern Indian Ocean and western Pacific and prefers branching corals for shelter and mating (Allen 1991; Godwin 1995; McCafferty et al. 2002). Dascyllus has intraspecific variation of group structure depending on population density, including multiple gregarious male groups in dense populations (Fricke 1977, 1980; Schwarz 1980; Coates 1982). Harem-like cohabiting groups typically occur in relatively low-density populations (Fricke 1977, 1980). Harem-like cohabiting groups of Dascyllus damselfishes often have strongly biased sex ratios towards females, with size-related protogynous sexuality where the largest individual functions as male and the remaining individuals are female (D. aruanus: Fricke and Holzberg 1974; Coates 1982; Kuwamura et al. 2016; D. marginatus: Holzberg 1973; D. reticulatus: Schwarz 1980). Moreover, a socially controlled protogynous sexuality with dominance relationships depending on individual body size within a colony group has also been suggested in the genus (Sale 1970; Fricke and Holzberg 1974; Coates 1982; Asoh 2003, 2004; Asoh and Yoshikawa 2003).
Dascyllus reticulatus is believed to maintain a harem polygyny based on the spatial cohabitation patterns of males and females (Schwarz and Smith 1990; Asoh 2005), despite a lack of data on social structures and mating system. Observational surveys are key approaches to confirm social structure and mating systems, but they have hardly been conducted for harem-like cohabiting groups of Dascyllus damselfishes. In the present study, we conducted an observational field survey on a relatively low-density population of D. reticulatus to describe the social structure and mating system of harem-like cohabiting groups. We found the coexistence of two distinctive group types within the harem-like cohabiting groups. Here, we report details of group structure of D. reticulatus in nature as a new example of multiple haremic group formation.
Materials and methods
Study area
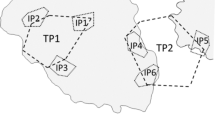
We conducted underwater surveys on reefs in Nishiura Bay, Kuchierabu-jima Island (30°28′N, 130°10′E), Kagoshima, southern Japan. The island fronts onto the Kuroshio Current in a biogeographically subtropical region, and over 600 fish species inhabit the reefs (Gushima and Murakami 1976; Kimura et al. 2017). We set up a study area of 50 × 100 m on the flat reef zone at depths of 4–8 m in the bay (Fig. 1).
Study area (50 m × 100 m) for observational surveys of harem-like groups of Dascyllus reticulatus on reefs of Kuchierabu-jima Island. A total of seven (A, B, C, D, E, F, G) and six (A2, C2, D2, E2, H, I) branching corals harboring harem-like cohabiting groups of D. reticulatus were present within the study area at the start of the study in 2016 and 2017, respectively. Inter-group moves and extra-group spawning are indicated by solid lines and dotted lines, respectively. The thickness of the arrow reflects the number of individuals (n values are shown in parentheses). Pale arrow indicates a failed case of movement. Outlines of reef substrates are drawn as pale lines
Field observations
The field observational survey was conducted daily using SCUBA from June to October 2016 and June to November 2017. On reefs of Kuchierabu-jima Island, breeding activity of D. reticulatus starts from June and ends around early November (Sakanoue, unpublished data). We could not conduct surveys during the winter season and early spring (December to April) because of stormy wave conditions. Water temperature ranged from 22.4 to 32.3 °C.
At the start of each study period, we captured all D. reticulatus within the study area using hand nets and screen nets. There were 56 individuals in 2016 (density 0.011 individuals/m2) and 88 individuals in 2017 (0.018 individuals/m2). Captured individuals were carefully transferred to the laboratory for the following procedures. While being anesthetized with diluted clove oil (0.05%), each individual was measured [standard length (SL)] to the nearest 0.1 mm using calipers and sexed by microscopic observation of the shape of the urogenital papilla (long and conical papilla indicate males, short and thick papilla indicate females, and undeveloped small papilla indicate juveniles, Thresher 1984; Mizushima et al. 2000). In addition, we confirmed gamete production by gently pressing the abdomen (Fricke 1980). A small individual (SL < 35 mm) having undeveloped small papilla and being unable to discharge gametes by the operation was defined as a juvenile. We injected a visible implant elastomer tag (Northwest Marine Technology Inc., Shaw Island, WA, USA) subcutaneously into the lateral body for individual discrimination. We did not mark individuals less than 20 mm in SL. All captured fish were released at the place of capture the next day. In order to accurately confirm the individual composition of each group and the functional sex of individuals, we recaptured, measured and sexed all individuals again 2 months into the study periods (i.e., September) and at the end of the study periods.
Dascyllus damselfishes form groups on branching corals, including Acropora, Pocillopora and Stylophora (Allen 1975; Scott 2008). In this study, D. reticulatus formed groups on Pocillopora eydouxi, P. elegans and Acropora aff. divaricata. In 2016, we found seven branching isolated corals (A, B, C, D, E, F, G in Fig. 1). In 2017, we found six branching isolated corals (A2, C2, D2, E2, H, I in Fig. 1). We measured the size of each shelter coral. The branch length and the inter-branch width of each shelter coral colony were measured randomly 5 times at once and the average value was calculated. We observed two conspicuous patterns in branch lengths (Fig. 2). Pocillopora eydouxi had thick long branches (branch length: median = 10.6 cm, range = 8.4–11.9 cm; inter-branch width: median = 6.8 cm, range = 6.2–7.9 cm, n = 4; groups A, A2, G and I in Fig. 1), which provided wider gaps. The other two corals, i.e., P. elegans (n = 3; groups E, E2 and F in Fig. 1) and Acropora aff. divaricata (n = 6; groups B, C, C2, D, D2 and H in Fig. 1) commonly developed fine short branches, which provided narrow gaps between branches (branch length: median = 2.7 cm, range = 2.2–3.0 cm; inter-branch width: median = 2.5 cm, range = 1.9–4.2 cm, n = 9; Table 1). We combined these two corals’ data together as the short-branching corals in the present study. In comparing branch length and inter-branch width, P. eydouxi had significantly longer branches and wider gaps than the other two short-branching corals (Mann–Whitney U test, U = 0, P < 0.01 in both). Whereas, in comparing coral colony sizes, there was no significant difference in the volumes of the coral colonies between the long-branch ones (median = 0.11 m3, range = 0.09–0.12 m3, n = 4) and short-branch ones (median = 0.05 m3, range = 0.03–0.18 m3, n = 9; Mann–Whitney U test, U = 11, P = 0.3; Table 1). We defined individuals that used the same sheltering coral as a single group. We called groups on Pocillopora eydouxi “long-branch groups”, and those on P. elegans and Acropora aff. divaricata “short-branch groups.”
In our preliminary survey, we confirmed that spawning of D. reticulatus in each colony group occurred in the morning, especially between 07:30 and 09:30. To survey the mating activities of D. reticulatus, we conducted behavioral observation during the morning (07:00–12:00 h) for a total of eight colony groups. To calculate reproductive success, we used groups that we were able to continuously monitor throughout the study period (nearly half of the Dascyllus individuals disappeared in September 2016 due to heavy disturbance by typhoons). In total, we observed two groups (A and E) in 2016 and six groups (A2, C2, D2, E2, H, and I) in 2017. Among these eight groups, three and five were long-branch groups (A, A2 and I) and short-branch groups (C2, D2, E, E2 and H; Table 2), respectively. Duration of the observation period for each group ranged 10–120 min depending on the mating activity of each group. To obtain comparative data of reproductive activity among groups (individuals), the morning behavioral observations were repeatedly conducted for 36–69 days in each group (Table 2). We recorded the time and place of male courtship displays (signal-jumps, Sale 1971), spawning behaviors, and parental egg care behavior. Before spawning, females often visited a male’s mating nest in a coral or on an adjacent rock surface (Tanaka 1999). Spawning events were visually confirmed by the presence of eggs on the nest. In this study, males who established mating nests and showed mating and egg care were called “breeding males.” Females confirmed to have spawned were called “spawning females.” To evaluate reproductive success, we calculated spawning frequency: “total number of spawning events (times)”/“observation period (days).” To evaluate the stability of mating partnerships, we calculated the total number of mating partners per individual. Spawning participation prevalence was calculated as “number of breeding males (or spawning females)”/“total number of adult individuals.”
Statistical analyses
The data did not meet parametric assumptions, so we used nonparametric tests for statistical analyses. We used Mann–Whitney U and Wilcoxon signed-rank tests to compare between short-branch and long-branch groups. The binomial proportion test (expected frequency 0.5) was used to compare sex ratios in the population. For correlation analyses between individuals’ body lengths (SL mm) and spawning frequencies (times/day), Spearman’s rank correlation coefficient test was used. P-values less than 0.05 were considered statistically significant. Statistical calculations were conducted using R 3.2.2 (R Core Team 2015).
Results
Difference in group composition and shelter coral type
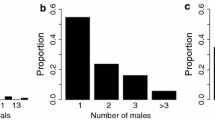
We observed multiple males cohabiting within the same colony group 9 times, and we observed solo males 4 times (Table 1). The occurrence ratio of multiple male groups was not significantly different between the long-branch groups and short-branch groups (Fisher’s exact probability test, P = 0.4). The sex ratio of the long-branch groups (8 males and 18 females; Table 1) was significantly different from a 1:1 ratio (binominal test for proportion, P = 0.04), while the ratio was not significantly different than 1:1 in the short-branch groups (37 males and 47 females; Binominal test, P = 0.3; Table 1). The individual densities of the short-branch groups (median = 240 individuals/m3, range = 39–300 individuals/m3, n = 9) were significantly higher than that of the long-branch groups (median = 58 individuals/m3, range = 25–100 individuals/m3, n = 4; Mann–Whitney U test, U = 3, P < 0.05; Table 1).
Males in long-branch groups (SL median = 60.8 mm, SL range = 51.6–72.1 mm, n = 8) were significantly larger than those in the short-branch groups (SL median = 42.0 mm, SL range = 34.8–55.5 mm, n = 37; Mann–Whitney U test, Z = 6, P < 0.001; Fig. 3). Similarly, females in long-branch groups (SL median = 53.7 mm, SL range = 47.1–61.8 mm, n = 18) were significantly larger than those in short-branch groups (SL median = 38.3 mm, range = 31.0–53.8 mm, n = 47; Mann–Whitney U test, Z = 7, P < 0.001; Fig. 3). Overall, mature individuals in long-branch groups (SL median = 55.7 mm, SL range = 47.1–72.1 mm, n = 26) were significantly larger than those in short-branch groups (SL median = 40.5 mm, SL range = 31.0–55.5 mm, n = 84; Mann–Whitney U test, Z = 7, P < 0.001; Fig. 3). Focusing on sexual differences within groups, males were significantly larger compared to females in both group types (Mann–Whitney U test, long-branch groups U = 16, short-branch groups Z = 3, P < 0.01 for both; Table 1).
All four long-branch groups were comprised of only mature individuals (adult individual ratio: all 1.0, n = 4; Table 1). In contrast, the short-branch groups included juvenile and adult individuals (adult individual ratio: median = 0.67, range = 0.55–0.88, n = 9; Table 1). The adult vs juvenile ratios were significantly higher in the long-branch groups compared to those of the short-branch groups (Mann–Whitney U test, U = 0, P < 0.01; Table 1). However, the net number of mature individuals was not different between the two groups (Mann–Whitney U test, U = 13, P = 0.4 ; Table 1).
Difference in reproductive activities
We observed a total of 61 spawning events by 28 mating pairs during the daily continuous surveys of the six groups in 2017 (Table 2). In only three cases (4%) did females spawn with males outside their original groups, all females in the short-branch groups (Table 2). In mating pairs, males were always larger than females (Wilcoxon signed-rank test, T = 0, P < 0.05, n = 28; Fig. 4). Spawning events occurred on 1–2 successive days (i.e., mating days) in each spawning cycle (median = 1, n = 38), and duration between spawning cycles (i.e., non-spawning period) ranged from three to 21 days (median = 9, n = 26). The occurrence frequencies of the mating days in the observation period were not significantly different between long-branch groups (median = 8.7%, range 5.6–15.0%, n = 3) and short-branch groups (median = 5.8%, range = 3.0–16.4%, n = 5; Mann–Whitney U test, U = 6, P = 0.7; Table 2). The frequencies of spawning in each group were also not significantly different between two group types (long-branch group: median = 0.23 times/day, range = 0.08–0.35 times/day, n = 3; short-branch group: median = 0.10 times/day, range = 0.03–0.28 times/day, n = 5; Mann–Whitney U test, U = 4, P = 0.3; Table 2). Thus, there were no differences in mating activities between the groups.
Mating pair combinations of Dascyllus reticulatus of harem-like cohabiting groups on reefs of Kuchierabu-jima Island. Data of two long-branch groups (A2 and I) and four short-branch groups (C2, D2, E2 and H) during 2017 season are used. Solid circles indicate male–female combination data in the long-branch groups (n = 7), and open circles indicate those in the short-branch groups (n = 21). The dotted line indicates 1:1 body size. SL standard length
Most female individuals in the long-branch groups spawned at least once during our observational survey (spawning participation prevalence 90%: 9 of 10 individuals; Tables 2, 3). In contrast, only 19 of 37 female individuals (51.3%) spawned in the short-branch groups (Tables 2, 3). The spawning participation prevalence ratio was significantly lower in the short-branch groups compared to those in the long-branch groups (Fisher’s exact probability test, P = 0.03). In addition, the spawning frequency of female individuals in the long-branch groups (median = 0.058 times/day, range = 0.00–0.125 times/day, n = 10) was significantly higher than that of the short-branch groups (median = 0.015 times/day, range = 0.00–0.09 times/day, n = 37; Mann–Whitney U-test, Z = 3, P = 0.001; Fig. 5). Even when excluding non-spawning females (long-branch groups: n = 1, short-branch groups: n = 18), females in the long-branch groups (median = 0.058 times/day, range = 0.028–0.125 times/day, n = 9) showed higher spawning frequency than those of short-branch groups (median = 0.017 times/day, range = 0.015–0.09 times/day, n = 19; Mann–Whitney U-test, U = 31, P = 0.007; Table 3).
In each group type, spawning frequency was not significantly correlated with female body size (Spearman correlation coefficient: long-branch groups: rs = − 0.21, P = 0.54, n = 10; short-branch groups: rs = 0.01, P = 0.9, n = 37; Fig. 5). When combining all group data, however, a significant correlation was confirmed, reflecting the contrasting results between the two group types (Spearman correlation coefficient, rs = 0.38, P = 0.01, n = 46).
Breeding males maintained mating relationships with multiple females in both group types (long-branch groups: median = 3 females, range 2–4, n = 3; short-branch groups: median = 3, range 1–7, n = 9; Mann–Whitney U-test, U = 12, P = 0.8; Table 4). The spawning participation prevalence ratio was not significantly different between the long-branch groups (3 of 4 males) and the short-branch groups (9 of 26 males; Fisher’s exact probability test, P = 0.3; Table 2). In the long-branch groups, the largest male in each group monopolized all spawning events (Tables 2 and 4). In the short-branch groups, one or three males monopolized all spawning events (Tables 2 and 4), but they were not always the largest males (group H, D2 and E2; Table 4).
The spawning frequency of males in the long-branch groups (median = 0.15 times/day, range = 0–0.35, n = 4) were higher than those in the short-branch groups (median = 0.00 times/day, range = 0.00–0.13, n = 24; Mann–Whitney U test, Z = 2, P = 0.04). Within the short-branch groups, the correlation between spawning frequency and male body size was significant (Spearman correlation coefficient, rs = 0.41, P = 0.04, n = 24). As a result of contrasting differences in body size composition between the two group types, the spawning frequency of males was significantly correlated with male body size when considering all data (Spearman correlation coefficient, rs= 0.54, P = 0.003, n = 28, Table 4).
Inter-group moves
Inter-group moves, or temporary intrusions to other groups, were observed in six individuals (four males, one female, and one juvenile; Fig. 1); all were non-breeding individuals belonging to the short-branch groups that moved or intruded into a long-branch group (A2; Fig. 1).
Three non-breeding males of group H (SLs = 42.0, 45.0, and 46.0 mm) successfully moved into group A2, but a non-breeding male (SL = 43.0 mm) failed after being attacked by the largest A2 male (SL = 63.5 mm) and immediately returned to group H. The non-breeding male became a breeding male in group H afterward (Table 4). The two emigrating males that succeeded in moving to A2 also suffered attacks by the larger A2 males in the shelter corals, and their status remained non-breeding until the end of the study (November 2017), except for one individual (SL = 46.0 mm), who obtained a spawning opportunity with a newly recruited small female, once. Thus, emigrating males did not easily obtain breeding status after moving to the long-branch groups. These four non-breeding males that conducted inter-group moves were significantly larger than the other non-breeding males in group H (SL median = 39.9 mm, SL range = 35.7–43.3, n = 6; Mann–Whitney U-test, U = 3, P = 0.04).
A juvenile from group C2 (SL = 33.2 mm) successfully moved and became a member of group A2 on September 2017 and became a mature female around the time of the inter-group move (no spawning within the observation period, but maturation was confirmed by sexing in the laboratory at end of the survey). A non-breeding female from group H (SL = 35.7 mm) also successfully became a member of group A2 (no spawning was confirmed by the end of study). In addition, three females showed temporary intrusions for spawning (extra group spawning; Fig. 1, Table 3). All three females (SLs = 32.2 and 36.1 mm in group D2 and 36.0 mm in group H) were accepted into group C2 and spawned once with the largest male (SL = 41.0 mm; Table 4). There were no failed inter-harem moves or temporary intrusions by females or juveniles, though emigration attempts were very limited.
Discussion
Social and environmental factors are known to promote intraspecific variation in the mating and social systems in some reef fishes (e.g., Pomacanthidae Moyer et al. 1983; Serranidae Yogo 1987; Shapiro 1988; Labridae Shapiro 1991; Karino et al. 2000). Intraspecific variation of social structure depending on population density has been observed in Dascyllus damselfishes, including multiple-male gregarious groups in dense populations (Fricke 1977, 1980; Schwarz 1980; Coates 1982). Harem-like cohabiting groups often occur in relatively low-density populations (Fricke 1977, 1980). This was the case for D. reticulatus in our current study. However, in addition to these population-level variations, we observed unique variation in the social structure of harem-like groups of Dascyllus damselfish within a population.
The coral-dwelling damselfish D. reticulatus showed two contrasting types of harem-like colony groups with different social structures and mating activities depending on body size and coral types (Table 5). In groups established on corals with long branches, the largest male maintained stable haremic mating relationships with cohabiting females (Table 5). In haremic fishes, the largest male often monopolizes mating with stably cohabiting females by excluding other males from mating sites (Robertson 1972; Moyer and Nakazono 1978a; Kuwamura 1984; Sakai and Kohda 1997; Kadota et al. 2011). D. reticulatus males of the long-branch groups seem to meet this condition by monopolizing mates and maintaining stable mating relationships with cohabiting females. Though social interactions among males were infrequently observed, we observed aggressive attacks by the largest male in one group toward intruding males from an adjacent group, implying a territoriality of large males of D. reticulatus in the long-branch groups. Thus, the social structure and mating relationships of the long-branch groups of D. reticulatus appear to be similar to many examples of haremic fishes. This is the first empirical evidence of harem groups in Dascyllus, as predicted by Schwarz and Smith (1990) and Asoh (2005). We would expect harem groups of D. reticulatus to occur in conditions where corals have long branches to accommodate colonies with large individuals.
In contrast, groups on short-branching corals showed social and mating conditions that were different from typical examples of harems (Table 5). Some males were reproductively active and maintained mating relationships with several females like haremic fishes. However, a lot of non-breeding adult individuals cohabited within the groups. Thus, mating opportunities and habitat resources were not monopolized in the short-branch groups. Our results suggest that not all harem-like cohabiting groups of D. reticulatus have identical social structures and mating activities.
Male body sizes were larger than those of females within groups and within mating pairs regardless of group types, suggesting that the principal mating group may be harem-like even in the short-branch groups. If so, what factors differentiate social structures between long-branch groups and short-branch groups? It is possible that high individual density conditions in short-branch groups promote a disorganized social structure. There were no differences in the number of adult individuals between the two group types; the high densities within short-branch groups were caused by juvenile cohabitations (Table 1). Large group sizes and cohabiting individual densities within a group often cause inactive reproduction in some individuals and sometimes result in protogynous sex changes in females (Yogo 1987; Sakai 1997). The sexuality of hermaphroditic fishes is socially controlled by interactions with local group members, especially dominant individuals (Robertson 1972; Warner 1988; Munday et al. 2006). In harem groups of Centropyge angelfish, decreased spawning frequency in females was observed when social interactions with dominant males were infrequent (Sakai 1997), which may be similar to the conditions in the short-branch groups in D. reticulatus. The low spawning participation prevalence ratio and the low spawning frequencies observed in the short-branch groups may be due to inadequate social dominance within the group.
In the long-branch groups, the sex ratio was biased towards females, which may result from the territoriality of the largest male preventing male emigration and maintaining social control on sex change in females. On the other hand, the sex ratio was not biased in the short-branch group, which is likely due to weak social dominance by large breeding males in the high-density conditions. A few individual non-breeding females in the short-branch groups changed sex into males (Sakanoue in prep), which supports the idea of there being insufficient social dominance conditions within the group. Observational data on behavioral interactions within groups are expected to clarify the differences in social relationships among cohabiting members between the two group types in future.
Small species of Dascyllus rarely move away from their shelter coral due to high predation pressure (Sale 1971; Fishelson et al. 1974). The distribution of branching corals suitable for D. reticulatus shelters were broadly scattered in the study area (Fig. 1), which would indicate considerable risks in leaving the sheltering corals. We observed predation on D. reticulatus by carnivorous fish, including groupers Cephalopholis urodeta and Variola albimarginata and a sandperch Parapercis millepunctata (Sakanoue unpublished data). When hiding, D. reticulatus places its body into the gap between coral branches. The gap in short-branching corals (around 2–3 cm branch length) is too small for individuals with SLs greater than 5 cm, requiring them to live in long-branching corals. In contrast, corals with long branches would be unsuitable or unpreferable for small adult or juvenile individuals because they may allow predators to intrude. In addition, the numerous narrow gaps and complicated labyrinth structure would allow a high density of small adult and juvenile individuals to cohabitate within a coral colony. Fricke (1980) surveyed D. marginatus in the Red Sea and showed that the size of coral determines group size and that individuals living in the genus Stylophora had body lengths that were greater than for those of individuals residing in the finer genus Acropora, which is similar to our observations for D. reticulatus. Thus, individual living space requirements may be a strong factor promoting the segregation of group composition based on different coral types.
The reproductive success of females is determined by the amount of eggs that can be made, so it increases according to body size and is not affected by mating system (Warner 1975), which is consistent with our observation of high spawning frequency in large-sized females in the long-branch groups. It is widely confirmed that large males display a high capacity and ability to care for eggs (Schmale 1981; Thresher and Moyer 1983; Peterson 1995). So, it would be beneficial for the females in the long-branch groups to spawn with large males. In contrast, females in the short-branch groups seemed to prefer mating with small males on narrow nest spaces between coral branches. For individuals settled on short-branching corals, inter-group moves may be a crucial pathway to obtaining better conditions for reproduction.
Only non-breeding individuals from the short-branch groups conducted inter-group moves. Emigrants had body sizes that were relatively large compared to the other non-breeding individuals in their original groups, which may be related to swimming ability for eluding attacks from larger males and predators during the moves. All inter-group moves were conducted toward an adjacent long-branch group, and no reverse-directive moves from long-branch groups occurred. The fact that no individuals moved from the long-branch coral to the short-branch coral suggests that the large-branching corals were preferable for large D. reticulatus individuals. As a conditional life-history pathway of D. reticulatus, we predict that individuals will first settle and stay on short-branch corals to prioritize survival when small, and then change groups after growth to achieve better reproductive status.
The previous studies on group composition of Dascyllus suggested the occurrence of harem groups in low-density populations (Fricke and Holzberg 1974; Coates 1982; Holzberg 1973; Schwarz 1980). However, none of them directly confirmed the sexualities of cohabiting individuals within groups. In the present study, we confirmed the presence of harem groups on long-branching corals. Furthermore, even within harem-like cohabiting groups, we also found another type of social group on short-branching corals mainly comprised of smaller adults and juveniles. In haremic reef fishes, the social structure of each group is organized basically in unitary form; each group is comprised of reproductively active adults and juveniles and is organized in size-based dominance relationships (e.g., Labridae: Kuwamura 1984, Pomacanthidae: Sakai and Kohda 1997, Cirrhitidae: Kadota et al. 2011). In the present study, D. reticulatus maintained two types of harem-like groups with different social structures and mating activities, and this is the first report of the coexistence of dual group forms in haremic fishes.
Hattori and Casadevall (2016) suggested that mating system variation (monogamy or harem polygyny) in the genus Dascyllus is determined by shelter size. In the case of D. reticulatus, gregarious groups have often been observed in densely distributed coral colonies, i.e., shelter rich conditions (Asoh 2005). Furthermore, as shown in the present study, the physical structures of branching corals also affected the group structure of D. reticulatus. However, sample sizes of the long-branch groups are limited in the present study. Therefore, further detailed examination of the social structure and mating system, including experimental approaches, are expected for verifying the causal effect of coral structure on alternative group forms and for revealing the adaptive significance of the dual group forms in the context of the survival and mating strategy of D. reticulatus.
References
Allen GR (1975) Damselfish of the south seas. TFH, Neptune City
Allen GR (1991) Damselfishes of the world. Mergus, Melle
Asoh K (2003) Gonadal development and infrequent sex change in a population of the humbug damselfish, Dascyllus aruanus, in continuous coral-cover habitat. Mar Biol 142:1207–1218
Asoh K (2004) Gonadal development in the coral reef damselfish Dascyllus flavicaudus from Moorea, French Polynesia. Mar Biol 146:167–179
Asoh K (2005) Gonadal development and diandric protogyny in two populations of Dascyllus reticulatus from Madang, Papua New Guinea. J Fish Biol 66:1127–1148
Asoh K, Yoshikawa T (2003) Gonadal development and an indication of functional protogyny in the Indian damselfish, Dascyllus carneus. J Zool (Lond) 260:23–39
Coates D (1982) Some observations on the sexuality of humbug damselfish, Dascyllus aruanus (Pisces, Pomacentridae) in the field. Z Tierpsychol 59:7–18
Fishelson L, Popper D, Avidor A (1974) Biosociology and ecology of pomacentrid fishes around the Sinai Peninsula (northern Red Sea). J Fish Biol 6:119–133
Fricke HW (1977) Community structure, social organization and ecological requirements of coral reef fish (Pomacentridae). Helgolander wiss Meeresunters 30:412–426
Fricke HW (1980) Control of different mating systems in a coral reef fish by one environmental factor. Anim Behav 28:561–569
Fricke HW, Fricke S (1977) Monogamy and sex change by aggressive dominance in coral reef fish. Nature 266(5605):830–832
Fricke HW, Holzberg S (1974) Social units and hermaphroditism in a pomacentrid fish. Naturwissenschaften 61:367–368
Godwin J (1995) Phylogenetic and habitat influences on mating system structure in the humbug damselfish (Dascyllus, Pomacentridae). Bull Mar Sci 57:637–652
Gushima K, Murakami Y (1976) The reef fish fauna of Kuchierabu, offshore Island of southern Japan. J Fac Anim Husb Hiroshima Univ 15:47–56
Hattori A, Casadevall M (2016) Sex change strategies and group structure of damselfishes. In: Frederich B, Parmentier E (eds) Biology of damselfishes. CRC Press, Boca Raton, pp 55–83
Holzberg S (1973) Beobachtungen zur Ökologie undzum Sozialverhalten des Korallenbarsches Dascyllus marginatus (Pices, Pomacentridae). Z Tierpsychol 33:492–513
Kadota T, Osato J, Hashimoto H, Sakai Y (2011) Harem structure and female territoriality in the dwarf hawkfish Cirrhitichthys falco (Cirrhitidae). Environ Biol Fish 92:79–88
Karino K, Nakazono A (1993) Reproductive behavior of the territorial herbivore Stegastes nigricans (Pisces: Pomacentridae) in relation to colony formation. J Ethol 11:99–110
Karino K, Kuwamura T, Nakashima Y, Sakai Y (2000) Predation risk and the opportunity for female mate choice in a coral reef fish. J Ethol 18:109–114
Keenleyside MHA (1972) The behaviour of Abudefduf zonatus (Pisces, Pomacentridae) at Heron Island, Great Barrier Reef. Anim Behav 20:763–774
Kimura Y, Hibino Y, Miki R, Minetoma T, Koeda K (2017) Field guide to fishes of Kuchinoerabu-jima Island in the Osumi Group, Kagoshima, southern Japan. The Kagochima University Museum, Kagoshima
Kuwamura T (1984) Social structure of the protogynous fish Labroides dimidiatus. Publ Seto Mar Biol Lab 29:117–177
Kuwamura T (1996) An introduction to reproductive strategies of fishes. In: Kuwamura T, Nakashima Y (eds) Reproductive strategies in fishes, vol 1. Kaiyusha, Tokyo, pp 1–41
Kuwamura T (1997) The evolution of parental care and mating systems among Tanganyikan cichlids. In: Kawanabe H, Hori M, Nagoshi M (eds) Fish communities in Lake Tanganyika. Kyoto University Press, Kyoto, pp 59–86
Kuwamura T, Karino K, Nakashima Y (2000) Male morphological characteristics and mating success in a protogynous coral reef fish, Halichoeres melanurus. J Ethol 18:17–23
Kuwamura T, Suzuki S, Kadota T (2016) Male-to-female sex change in widowed males of the protogynous damselfish Dascyllus aruanus. J Ethol 34:85–88
McCafferty S, Bermingham E, Quenouille B, Planes S, Hoelzer G, Asoh K (2002) Historical biogeography and molecular systematic of the Indo-Pacific genus Dascyllus (Teleostei: Pomacentridae). Mol Ecol 11:1377–1392
Mizushima N, Nakashima Y, Kuwamura T (2000) Semilunar spawning cycle of the humbug damselfish Dascyllus aruanus. J Ethol 18:105–108
Moyer JT (1990) Social and reproductive behavior of Chaetodontoplus mesoleucus (Pomacanthidae) at Bantayan Island, Philippines, with notes on pomacanthid relationships. Jpn J Ichthyol 36:459–467
Moyer JT, Nakazono A (1978a) Population structure, reproductive behavior and protogynous hermaphroditism in the angelfish Centropyge interruptus at Miyake-jima, Japan. Jpn J Ichthyol 25:25–39
Moyer JT, Nakazono A (1978b) Protandrous hermaphroditism in six species of the anemonefish genus Amphiprion in Japan. Jpn J Ichtyol 25:101–106
Moyer JT, Thresher RE, Colin PL (1983) Courtship, spawning and inferred social organization of American angelfishes (Genera Pomacanthus, Holacanthus and Centropyge; pomacanthidae). Env Biol Fish 9(1):25–39
Munday PM, Buston PM, Warner RR (2006) Diversity and flexibility of sex-change strategies in animals. Trends Ecol Evol 21:89–95
Nelson JS, Grande TC, Wilson MVH (2016) Fishes of the world, 5th edn. Wiley, New Jersey
Peterson CW (1995) Male mating success and female choice in permanently territorial damselfishes. Bull Mar Sci 57:690–704
Robertson DR (1972) Social control of sex reversal in a coral reef fish. Science 177:1007–1009
Robertson DR, Warner RR (1978) Sexual patterns in labroid fishes of the western Caribbean: the parrotfishes (Scaridae). Smithson Contr Zool 255:1–26
Sakai Y (1997) Alternative spawning tactics of female angelfish according to two different contexts of sex change. Behav Ecol 8:372–377
Sakai Y, Kohda M (1997) Harem structure of the protogynous angelfish, Centropyge ferrugatus (Pomacanthidae). Environ Biol Fish 49:333–339
Sale PF (1970) Behavior of the humbug fish. Aust Nat Hist 16:362–366
Sale PF (1971) Extremely limited home range in a coral reef fish, Dascyllus aruanus (Pisces; Pomacentridae). Copeia 1971:324–325
Schmale MC (1981) Sexual selection and reproductive success in males of the bicolor damselfish, Eupomacentrus partitus (Pisces: Pomacentridae). Anim Behav 29:1172–1184
Schwarz AL (1980) Almost all Dascyllus reticulatus are girls! Bull Mar Sci 30:328
Schwarz AL, Smith CL (1990) Sex change in the damselfish Dascyllus reticulatus (Richardson) (Perciformes: Pomacentridae). Bull Mar Sci 46:790–798
Scott WM (2008) Damselfishes and anemonefishes. TFH, Neptune City
Shapiro DY (1988) Variation of group composition and spatial structure with group size in a sex-changing fish. Anim Behav 36:140–149
Shapiro DY (1991) Intraspecific variability in social systems of coral reef fishes. In: Sale PF (ed) The ecology of fishes on coral reefs. Academic Press, San Diego, pp 331–355
Tanaka Y (1999) Reproductive behavior, eggs and larvae of four Dascyllus species in the aquarium. J School Mar Sci Technol Tokai Univ 47:223–244
Thresher RE (1984) Reproduction in reef fishes. TFH, Neptune City
Thresher RE (1985) Distribution, abundance, and reproductive success in the coral reef fish Acanthochromis polyacanthus. Ecology 66:1139–1150
Thresher RE, Moyer JT (1983) Male success, courtship complexity and patterns of sexual selection in three congeneric species of sexually monochromatic and dichromatic damselfishes (Pisces: Pomacentridae). Anim Behav 31:113–127
Warner RR (1975) The adaptive significance of sequential hermaphroditism in animals. Amer Nat 109:61–82
Warner RR (1988) Sex change in fishes: hypotheses, evidence, and objections. Environ Biol Fish 22:81–90
Warner RR, Robertson DR (1978) Sexual patterns in labroid fishes of the western Caribbean: the wrasses (Labridae). Smithson Contr Zool 254:1–27
Warner RR, Schultz ET (1992) Sexual selection and male characteristics in the bluehead wrasse, Thalassoma bifasciatum: mating site acquisition, mating site defense, and female choice. Evolution 46(5):1421–1442
Yogo Y (1987) Hermaphroditism and the evolutionary aspects of its occurrences in fishes. In: Nakazono A, Kuwamura T (eds) Sex change in fishes. Tokai University Press, Tokyo, pp 1–47
Acknowledgements
We thank the people of Kuchierabu-jima Island for allowing the field survey. Dr. H. Hashimoto, Dr. T. Tomiyama, Dr. Y. Kimura, and colleagues at the laboratory of Biology of Aquatic Resources, Hiroshima University, are thanked for their helpful support of this study. We are grateful to Dr. T. Fujii (Kagoshima University) and Mr. T. Shimada (Japanese Society for Coral Taxonomy) for kind support in taxonomic identification of coral species. We would like to thank Editage (www.editage.jp) for English language editing. This study was supported by grants from JSPS KAKENHI (Grant Numbers 24570027, 15K07222 and 18K06419) and the Sasakawa Scientific Research Grant from The Japan Science Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed in the present study were in accordance with the guidelines for proper conduct of animal experiments and related activities by Hiroshima University (ID: A170410) and the guidelines for ethological studies by the Japan Ethological Society.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Sakanoue, R., Sakai, Y. Dual social structures in harem-like colony groups of the coral-dwelling damselfish Dascyllus reticulatus depending on body size and sheltering coral structures. J Ethol 37, 175–186 (2019). https://doi.org/10.1007/s10164-019-00584-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-019-00584-8