Abstract
Whereas mating behaviors and social structure have been studied extensively in monogamous hermaphroditic gobiid species, such studies are relatively limited for polygamous gobiid species. To investigate the reproductive strategy of polygamous gobies, mating groups of the common fusegoby Fusigobius neophytus were observed on reefs of Kuchierabu-jima Island, southern Japan. Males established mating nests on flat-rock surfaces within their territorial home ranges on sandy rubble flats. Females maintained independent home ranges outside the male home ranges during nonreproductive periods, but they shifted their home ranges to overlap with male ranges and actively visited male mating nests during their reproductive periods (1–3 days at ca. 7-day intervals). Females often changed mates during their serial mating. The mating system used by the common fusegoby fits with the definition of male-territory-visiting polygamy. The sex ratio within the study population was female-biased. Nest-holding males were significantly larger than females and were polygynous (mating with up to eight females). These characteristics fit well with the prediction of protogyny by the size-advantage model. Some of the females were observed to undergo functional sex changes to nest-holding males. In addition, small floating males demonstrated sneaking behavior. None of the floating males were derived from females that had changed sex, suggesting a diandric life-history pathway for F. neophytus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Gobiidae are a highly diverse group of bony fishes in terms of taxonomy (ca. 210 genera with at least 1950 species) and morphology (adult body size ranging from 8 mm to 50 cm) (Nelson 2006). Numerous field studies have contributed to our extensive biological knowledge of gobiid fishes, including the predominance of benthic gobiid species that exhibit demersal egg spawning with parental care (Miller 1984). The social and mating systems of gobiids are important aspects of the ecology of teleost fishes on reefs and have contributed greatly to our understanding of their reproductive strategies, including mate choice, alternative tactics, and sexuality (Warner 1975; Thresher 1984; Berglund 1997; Petersen and Warner 2002; Cole 2010). Extensive field studies have focused on the social and mating systems of gobies, and they have been found to present two main types of mating systems: monogamy and polygamy (Table 1).
Monogamy has been reported in at least 18 gobiid species, including many coral-dwelling hermaphroditic (sex-changing) species (e.g., Gobiodon and Paragobiodon) and species that maintain burrows in sandy habitats (Amblyeleotris and Valencienna) (Table 1). Polygamy has been reported in at least 14 species, particularly those that inhabit enclosed spaces such as rock cavities, reef crevices, and holes (Table 1). Female movements and activities as well as male responses to female behaviors are known to be important influences on the structure of the mating system employed by a species of interest (Emlen and Oring 1977; Warner 1984; Moyer 1991; Berglund 1997; Kuwamura 1997). However, the small spaces used as habitats by gobiids and their secretive nature means that spatial and mating relationships among individuals are unclear in many gobiid species (Table 1).
Harem polygyny and male-territory-visiting polygamy (MTV polygamy) are mating systems that are widely found in reef fishes (Thresher 1984; Warner 1984; Moyer 1991; Berglund 1997). In the former system, a dominant male and several females usually cohabit within the male’s territory, with the male monopolizing mating opportunities within the group (Kuwamura 1997). Monandric protogynous sexuality (i.e., where the males are derived from sex-changing females) is known to be widespread in haremic fishes (Warner 1975, 1984; Munday et al. 2006). The latter system, MTV polygamy, is characterized by the establishment of mating territories that females may visit (often from outside) for spawning (Kuwamura 1997). As a result, females may change mates according to mate choice (Berglund 1997; Kuwamura 1997). Diandric sexuality, i.e., the coexistence of males derived from sex-changed females as well as primary males (non-sex-changers), is common in MTV polygamous fishes such as wrasses (Labridae) (e.g., Robertson and Warner 1978; Warner and Robertson 1978; Warner 1984). The protogyny displayed by these polygamous mating groups agree well with the predictions of the size-advantage model: a sex change from female to male will be selectively favored in a mating system where large males monopolize the mating to the detriment of the smaller males (Warner 1975, 1984, 1988). In addition, in polygamic fishes, small primary males often employ alternative mating tactics such as group spawning and/or sneaking (Warner 1984; Kuwamura et al. 2009).
Polygamy has been observed in at least 14 species (Table 1), and extensive field studies have been conducted in four gonochores (Asterropteryx semipunctatus, Bathygobius fuscus, Microgobius gulosus, and Pomatoschistus microps) and three hermaphrodites (Coryphopterus nicholsi, Lythrypnus dalli, and Trimma okinawae) (Table 1). The ratio of hermaphroditic species to all polygamous goby species (0.5; 4 of 8 species that had their sexuality studied) contrasts markedly with the corresponding ratio for monogamous gobies (0.9; 10 of 11 species that had their sexuality studied; Table 1). While monogamous gobies are known to show protogyny or bidirectional sex changes, such sex change pattern has also been reported for hermaphroditic polygamous gobies (reviewed in Table 1), and the adaptive significance of changing sex as a mating strategy has been evaluated (Cole 1983; Sunobe and Nakazono 1993; St. Mary CM, 1994; Manabe et al. 2007; review in Cole 2010). In addition to hermaphroditic sexuality, the appearance of small males was reported for the polygamous species C. nicholsi and L. dalli, although there was no direct evidence of sneaking maneuvers by small males (Cole 1983; Drilling and Grober 2005). Thus, there are no clear examples of diandry in gobiids. The high occurrence ratio of sneaker males in polygamic goby species (0.7; 8 of 12 species that had their mating behavior studied) also contrasts strikingly with that of monogamous goby species, for which sneaking males are not observed (Table 1). Thus, we predict that diandric sexuality, as observed in other reef fish such as labrids, serranids, and pomacanthids (Sadovy de Mitcheson and Liu 2008), may even occur in polygamous gobies.
Fusigobius neophytus is a reef-associated small fish that reaches around 6 cm in total length and is distributed in tropical and subtropical waters of the Indo-Pacific Ocean (Nakabo 2002). This goby usually occurs on sandy bottoms and rubble zones of reefs. In our preliminary observations of reefs of Kuchierabu-jima Island, southern Japan, the goby was found to establish home ranges in open spaces and to mate polygamously, making it suitable for a field observational survey of the spatial and mating relationships associated with polygamous mating. Fusigobius neophytus has been histologically confirmed to possess hermaphroditic gonads, and this species has been suggested to be protogynous (Cole 1990, 2010), although it has never been observed to undergo a functional sex change. We also found small males in the study population. Therefore, we predicted that that F. neophytus is diandric. The aim of the present study was to confirm this prediction, so spatial and mating patterns, mating behaviors, and functional sex were surveyed for F. neophytus in nature. We also compared the mating strategy of F. neophytus with those of other polygamous gobies.
Materials and methods
Study area
We conducted an underwater survey of a sandy zone with coral rubble in Nishiura Bay, Kuchierabu-jima Island (30°28′N, 130°10′E), south of Kyushu, Japan. The island fronts onto the Kuroshio Current in a biogeographically subtropical region, and over 200 fish species inhabit its reefs (Gushima and Murakami 1976). We set up a 20 m × 30 m study area on a sandy rubble bottom at depths of 1–3 m in the bay.
Field observations
The field observational survey was conducted daily using SCUBA during three study periods: June–October 2007, June–October 2008, and May–September 2009. We could not conduct any surveys during the winter season and early spring (November–April) because of stormy wind and wave conditions along the northern coast of the island, including the study area. Water temperature ranged from 23.5 to 30.7 °C during the study periods.
At the start of each study period, a total of 53, 53, and 47 individuals of F. neophytus were present within the study area in 2007, 2008, and 2009, respectively. All individuals were measured and sexed at the start and end of each study period. We used a hand net and a solution of quinaldine (1 %) to capture the fish. Captured individuals were anesthetized with clove oil (0.05 %), and their total lengths (TL) were measured to the nearest 0.1 mm using calipers in the laboratory. The sex of each individual was distinguished by observing the morphological structure of the genital papilla following anesthetization; this papilla is long and posteriorly tapered in males and bulbous with several processes at the papilla opening in females, as in other gobies (e.g., Sunobe and Nakazono 1993; Kuwamura et al. 1994). Observed spawning behaviors were also used to identify the sex of individuals in the field survey (see below). To distinguish individuals, we injected a visible fluorescent elastomer tag (Northwest Marine Technology Inc., Shaw Island, WA, USA) subcutaneously into the lateral body of each captured individual under anesthetization. Whenever new individuals (unmarked) appeared within the study area, we captured them and conducted the same measurement and marking procedure. Individuals less than 20 mm in TL were excluded from this because marking was difficult to perform on those individuals. All captured fish were released at the place of capture on the following day after resting in an aquarium overnight. Based on these individual recapture data, we analyzed the body size distribution, growth patterns, and sex ratio in the study population.
Most males of F. neophytus established mating nests on the surface of a round flat-topped rock (ca. 30 cm in diameter) in sandy open areas close to rocky substrates (Fig. 1); these males are called “nest-holding males” herein. In contrast, some male individuals did not maintain mating nests, and they are called “floating males” in the present study (see “Results”). To survey the mating activities of F. neophytus, we conducted census observations between 03:30 h and 09:00 h (10–60 min for each census) at spawning time to check whether eggs were present in each identified mating nest (n = 13) and to record the time, location, and presence of spawning pairs within the study area from June 10 to August 19, 2007. Close-up photographic data (C4040Z, Olympus Inc., Tokyo, Japan) were used to count eggs and to assess the developmental stages of the eggs in nests. We analyzed the stability of mating pair combinations using 32 days of continuous data (June 10–July 11, 2007, a period when individual disappearances and drastic shifts in home ranges did not occur). In addition, we observed spawning behavior for 240–300 min during August 20–September 14, 2007 (26 days) in order to record mating sequences.
To survey the home range distribution pattern and social behavior of F. neophytus, we set up a focal observation area (3 m × 3 m) in the center of the study area, where three nest-holding males and eight females were present (sex ratio 0.375). We drew the map on polyester tracing films and recorded the path of each individual by drawing on the films within the focal observation area during the evening (17:00–19:00 h). The home range of each individual was defined as the area within the most peripheral path during 120 min of observation (four 30-min observations). During the course of the study, we observed that females drastically changed their behavioral pattern depending on their mating activity. Therefore, we analyzed the behavioral data of the gobies and distinguished two periods within each spawning cycle based on female behavioral activity: the reproductive period (5–7 days during which mating behavior was exhibited) and the nonreproductive period (the subsequent 2–5 days). We analyzed the home range overlap rate among individuals, which was calculated as [100 (%) × overlapping area within a home range (cm2)/home range area (cm2)] in each of the two periods of the spawning cycle.
Statistical analyses
Nonparametric tests were used for statistical analyses because most of the data in the present study did not fulfill the assumptions of parametric tests with respect to normality and homogeneity of variances. For multiple comparisons, we used the Kruskal–Wallis test with the Steel–Dwass post hoc test. In comparisons between two groups, the Mann–Whitney U test or the Wilcoxon signed-rank test were used. For correlation analyses, Spearman’s rank correlation coefficient test was used. The binomial proportion test (expected frequency 0.5) was used for statistical analyses of the sex ratio in the goby population. Statistical calculations were conducted using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA).
Results
Sex ratio and body size composition
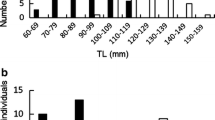
A female-biased sex ratio was consistently maintained in the study population throughout the 3-year study period [male/female (sex ratio), 16/37 (0.43), 14/39 (0.35), and 10/37 (0.27) at the start of study in 2007, 2008, and 2009, respectively; binomial test, all P < 0.001; Fig. 2].
Size classes of Fusigobius neophytus on reefs of Kuchierabu-jima Island. Data for individuals occurring in the study area at the start of each yearly study period are shown in 5-mm size classes (size was taken to be total length, TL) (n = 53, 53, and 47 in June in 2007, 2008, and 2009, respectively). Open bars females, solid bars nest-holding males, shaded bars floating males
Most males that appeared in the study area maintained mating nests (nest-holding males, n = 15, 13, and 9 in 2007, 2008, and 2009, respectively; Fig. 2). Floating males without mating nests occurred at much lower frequencies [6.3 % (n = 1), 7.1 % (n = 1), and 18.2 % (n = 2) of males in 2007, 2008, and 2009, respectively; Fig. 2].
The TL of the nest-holding males (median 60.4 mm, range 52.8–79.4 mm, n = 37) was significantly larger than that of females (median 50.5 mm, range 27.2–68.9 mm, n = 112) and that of floating males (41.6–51.0 mm, n = 4) (Kruskal–Wallis test, df = 2, K = 74.2, P < 0.001, Steel–Dwass post hoc test, P < 0.01 in each case; Fig. 2). No significant difference in total length was found between females and floating males (Steel–Dwass post hoc test, P > 0.05; Fig. 2).
Home range and spatial relationships
Nest-holding males did not overlap their home ranges with others in either period of the spawning cycle (overlap ratio 0.0 %, n = 3 for each period; Fig. 3). We only observed one contest between nest-holding males outside the focal observation area; in that case, the larger nest-holding male chased the smaller one away.
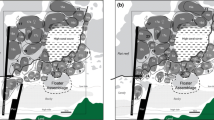
Home range distribution patterns of Fusigobius neophytus within the focal observation area (3 m × 3 m) during the reproductive period (left) and the nonreproductive period (right). Each home range was drawn based on 120 min of observation data in June–July 2009. The home ranges of five females (thin lines) and three nest-holding males (thick lines) are shown. Individual name codes indicate the sex of the individual (M male, F female). Total lengths (mm) are given in parentheses. The outlines of some bottom substrates are shown as pale lines
The home ranges of females overlapped considerably during both the reproductive period (median 13.6 % of the female home range, range 0.0–75.1 %, n = 5) and the nonreproductive period (median 24.6 % of the female home range, range 2.8–67.9 %, n = 5). Behavioral interactions occurred among females on rare occasions during both the reproductive period (n = 3) and the nonreproductive period (n = 4). In all cases, larger females chased away smaller ones.
During the nonreproductive period, the home ranges of females seldom overlapped with those of nest-holding males (median 0.0 % of the female home range, range 0–2.1 %, n = 5, Fig. 3) due to the defensive behavior of the egg-guarding males around nests (see below). In contrast, during the reproductive period, the home ranges of the females were located closer to the nests of nest-holding males (Fig. 3), resulting in an increase in the overlap ratio with nest-holding males (median 9.9 % of the female home range, range 3.0–17.1 %, n = 5; Fig. 3).
During the reproductive period, the areas of the home ranges of the nest-holding males (median 973 cm2, range 538–1546 cm2, n = 3) were not significantly different from those of females (1556 cm2, 290–6841 cm2, n = 8; Mann–Whitney U test, U = 7, P = 0.3). Likewise, there was no difference in home range area between the nest-holding males (median 346 cm2, range 237–516 cm2, n = 3) and females (1086 cm2, range 163–10060 cm2, n = 8) during the nonreproductive period (Mann–Whitney U test, U = 4, P = 0.1). The home range areas during the reproductive period and the nonreproductive period were not significantly different for these 11 individuals (Wilcoxon signed-rank test, T = 20, P = 0.3, n = 11).
Mate fidelity
In the continuous 32-day survey, in which a total of 54 spawning events by 12 males and 25 females were observed during four reproductive periods, there was no significant correlation between individual body size and spawning frequency in either sex (Spearman correlation coefficient: for females r s = 0.1, P = 0.6, n = 25; for males r s = 0.3, P = 0.3, n = 12; Table 2). For the mating pair combinations, there was no significant correlation between male body size and female body size (Spearman correlation coefficient, r s = 0.12, P = 0.4, n = 54). However, when we focused on relatively large females (>50 mm TL; F1–F11 in Table 2), a significantly positive correlation was found between the body sizes of the mating pair (r s = 0.47, P = 0.03, n = 24), suggesting that size-matching between a mating pair only tends to occur among large individuals.
Of the 18 females that spawned more than once within the 32-day survey, four (22 %; F6, F7, F13, and F25) changed their mate (Table 2). Conversely, the other 14 females (77.8 % of 18) repeatedly spawned with the same partner. Of the 10 males that had multiple mating opportunities within the 32-day survey, 7 mated with 2–5 different females (Table 2). The spawning frequency of these seven males (median 5 times, range 2–15, n = 7) was significantly higher than that of the other three males that mated with the same female individual during the survey (median 2 times, range 2–3, n = 3; Mann–Whitney U test, U = 2, P = 0.049; Table 2).
Spawning success of females and males
When the females approached nest-holding males during the reproductive period, the males performed courtship behaviors to the approaching females. The nest-holding males swam around the females, fully expanded all of their fins toward the approaching females (jerk display), and led them to the mating nests. With the females staying close to the nests, the males swept sand onto the surfaces of the flat-rock mating nests using their anal fins (nest-sweeping behavior) and exhibited spawning-like behaviors, rubbing their abdomens against the rocky surface. Soon after receiving these courtship displays, the females laid eggs in a single layer in the mating nests on the rock surface (Fig. 1).
Spawning occurred between 03:11 and 08:40 h (it was usually initiated in the dark) between early June and late October. Most of the females spawned only once during each reproductive period (98 % of 171 spawnings). Two females spawned twice (with different males) within 1 day. The median value of the interval between serial spawning by females was 7 days (range 6–8 days, n = 50). The median duration of a mating sequence of a female was 151 min (range 126–175 min, n = 6). Females released 1480–3870 eggs in each spawning (median 2116 eggs, n = 8). Number of eggs was significantly correlated with female TL (Spearman’s rank correlation coefficient, r s = 0.82, P = 0.02, n = 7; Fig. 4).
Spawning success of Fusigobius neophytus in relation to total length. Top the number of eggs spawned by females in each reproductive period (n = 7 females). Bottom the total number of eggs in male nests in each reproductive period (n = 16 males). Simple linear regression lines are shown to indicate trends (r 2 = 0.63 and 0.07 for females and males, respectively)
Nest-holding males guarded eggs alone for 4–8 days (median 6 days, n = 65). The egg-guarding males frequently performed chasing attacks and biting behaviors towards fishes that approached the mating nest, regardless of whether those fishes were conspecifics (mostly females, n = 15) or other gobiid species (Asterropteryx semipunctata, Fusigobius inframaculatus, Gnatholepis anjerensis, Gnatholepis scapulostigma, and Istigobius decorates; n = 14). When larger piscivorous fishes approached the nests (e.g., Grammistes sexlineatus, Gymnothorax isingteena, Labracinus cyclophothalma, Pterois lunulata, P. volitans, and Synodus ulae; n = 9), the egg-guarding males left the mating nests and hid in holes or crevices in the adjacent rocky substrate. However, predation on goby eggs and adults was not observed during the study. Spawning for each nest-holding male occurred a median of 2 days (range 1–8 days; n = 13) after egg-hatching.
Nest-holding males spawned with 1–5 females within 1 day (median 1 female, n = 133), and mating occurred on 1–3 successive days. Overall, nest-holding males spawned with 1–8 females within each reproductive period (median 1, n = 107), resulting in a median of 5015 eggs (range 1274–15,056 eggs, n = 38 mating nests). The total number of eggs in the mating nest was not significantly correlated with male TL (Spearman’s rank correlation coefficient, r s = 0.3, n = 16 males, P = 0.3; Fig. 4). The spawning success (number of spawned eggs) of males was significantly higher than that of females in each reproductive period due to the extra mating opportunities afforded by polygyny to males (Mann–Whitney U test, U = 18, n = 7 and 23, P = 0.01; Fig. 4).
Protogynous sex change to nest-holding males
A morphological transition of the shape of the genital papilla was observed in a total of 24 female individuals in the 3-year survey (21 % of 112 females). Among these individuals, four soon disappeared without showing any male sexual behavior, while the other 20 showed nest-holding male sexual behavior. Among these 20 individuals, female function was confirmed in seven (based on spawning records) before they commenced mating as nest-holding males, i.e., they underwent a functional protogynous sex change (Table 3). A protandrous (male to female) sex change was never observed in any of the study individuals.
Among the seven functional sex changers, five occurred coincidentally with the disappearance of their last mating partners (F3, F4, F6, F10, and F26 in Table 3) and the other two occurred in the presence of mating partners (F8 and F20 in Table 3). In three cases (F3, F4, and F6; Table 3), the sex change was completed within a breeding season, 62–70 days after their last spawning event as a female. In the other four cases (F8, F10, F20, and F26; Table 3), a winter nonbreeding season (when no observational data were taken) was included within the process, and 289–347 days elapsed between the last spawning as a female and the start of mating as a nest-holding male. Some sex changers initiated jerk displays and nest-sweeping behaviors on a flat-rock surface several weeks before receiving eggs in their mating nests (median 16 days, range 5–33 days, Table 3).
The TLs of the seven females that underwent a functional sex change had significantly increased by the start of mating as nest-holding males (Wilcoxon signed-rank test, T = 0, n = 7, P < 0.05; Table 3), indicating the presence of a process that promotes a larger body size in males. At the start of the study (June 2007), female individuals that subsequently underwent a sex change had a significantly greater TL (median 56.5 mm, range 48.6–60.6 mm, n = 12) than those that remained female over the course of the study (median 50.3 mm, range 27.2–62.6 mm, n = 40; Mann–Whitney U test, Z = −3, P < 0.005), suggesting a possible effect of body size on sex change initiation in females.
Sneaking by floating males and change in tactics
Floating males (41.6–51.0 mm, n = 4) had a significantly smaller TL than sex-changing individuals that were destined to be nest-holding males (median 64.0 mm, range 51.1–70.4 mm, n = 20; Mann–Whitney U test, Z = −3, P < 0.01). There were no cases of females changing directly into floating males.
Two floating males were observed to conduct sneaking (streaking) attempts (41.6 and 42.0 mm TL). They performed surreptitious spawning behaviors (sperm release) underneath a nest-holding male pairing with a spawning female. We also observed six similar sneaking attempts by floating males that failed; the floating males were attacked and chased away by the nest-holding males as they approached the mating nests.
Among the four floating males, the two sneakers (41.6 and 42.0 mm TL) disappeared 127 and 81 days, respectively, after they first appeared in the study area. The other two males (51.0 and 50.8 mm TL) started to maintain territorial home ranges to defend the nests from which the nest-holding males had disappeared. After taking over the mating nests, these two individuals spawned with females as nest-holding males 85 and 343 days, respectively, after they first appeared in the study area as floating males. At that time, the TLs of these formerly floating males reached 61.1 and 64.5 mm, respectively, and were not statistically significantly different from those of the other nest-holding males (Mann–Whitney U test, n = 37, Z = −0.6, P = 0.6), indicating that they underwent rapid growth before the start of mating as nest-holding males, as also seen for the sex changers.
Discussion
In many gobies, males play an important role in caring for the eggs, and the nests are usually established within enclosed spaces such as sandy burrows, caves, rock cavities, and empty shells (Miller 1984; Table 1). Males of the common fusegoby Fusigobius neophytus also maintain non-overlapping territorial home ranges, including mating nests where they care for demersal eggs. However, the male F. neophytus establishes a mating nest in an open space. Females of F. neophytus also maintain home ranges in the open space in order to visit mating nests from the outside (Fig. 3). Thus, unusually for a gobiid, F. neophytus exhibits social groups that show polygamous mating in the open habitat.
Mating pair cohabitation has been confirmed to occur in many monogamous gobies as well as in haremic polygynous gobies (Microgobius gulosus and Trimma okinawae) (Table 1). This was not the case for F. neophytus in the present study. In polygamous reef fishes, where both male and females potentially have multiple mating opportunities, females usually visit males and/or mating nests from outside male territories (Berglund 1997; Kuwamura 1997). In the small gobies Asterropteryx semipunctatus and Bathygobius fuscus, which both inhabit rocky bottoms, polygamous mating and considerable spatial segregation between the home ranges of the sexes have been reported as examples of male-territory-visiting polygamy (Taru et al. 2002; Manabe et al. 2009). In the present study, it was confirmed that F. neophytus shows considerable spatial segregation between the sexes, and that nest-holding males have multiple mating opportunities during each reproductive period. In addition, some females of F. neophytus were observed to change mates. Therefore, based on their spatial and mating relationships, it was concluded that the mating system of F. neophytus fits with the definition of male-territory-visiting (MTV) polygamy (for terminology, see Kuwamura 1997).
Female choice of males or mating sites has been confirmed to occur in a number of reef fishes that exhibit MTV polygamy (Robertson and Hoffman 1977; Karino et al. 2000; Kuwamura et al. 2009). In the case of F. neophytus, we found that large females tended to mate with large males, but there was no clear partnership trend in general (Table 2). This suggests that mate choice partly affects the spatial distribution pattern and mating relationships of this goby. In addition, females and floating males that became nest-holding males underwent rapid growth, implying that body size is important in male mating success via mate choice and/or male–male territorial competition. Moreover, our results indicated that new nest-holding males that were derived from sex-changing females spent several weeks waiting to receive eggs in their mating nests (Table 3), suggesting that there are considerable costs associated with the acquisition of mating opportunities as territorial nest-holding males. A more detailed investigation of the mating process should help to elucidate the contribution of mate choice to the mating system.
The mating system of a particular fish species profoundly affects its mating strategy and behavior (Robertson and Choat 1974; Warner 1984, 1988; Kuwamura and Nakashima 1998; Munday et al. 2006). Reef fishes demonstrating polygynous or polygamous mating broadly adopt protogynous sexuality as a mating strategy; the adaptive significance of this strategy is clearly explained by the size-advantage model, which theoretically predicts that a female to male sex change will be selectively favored in a mating system where large males monopolize the mating to the detriment of the smaller ones (Warner 1975, 1984). Protogyny (or a predominance of protogyny in a bidirectional sex change process) has been observed in the polygamous gobies Coryphopterus nicholsi and Lythrypnus dalli and the haremic polygynous goby Trimma okinawae (Cole 1982; St. Mary 1994, 1996; Sunobe and Nakazono 1990). This was the case for the polygamous goby F. neophytus in the present study. The adaptive significance of protogynous sexuality is also supported by the observation that nest-holding males enjoyed greater spawning success than females (Fig. 4), and is consistent with the size-advantage theory. Based on the histology of gonadal structures in F. neophytus, Cole (1990, 2010) strongly argued that protogynous sexuality occurs in the goby. The present study provides further evidence of functional sex changes in female F. neophytus in the wild.
In sex-changing fishes, including gobies, it has often been demonstrated that the disappearance of large dominant individuals triggers sex changes in subordinate individuals (i.e., social control of sex changes) (Munday et al. 2006; Cole 2010). In the case of F. neophytus, relatively large females underwent sex changes, and in some cases the timing of these sex changes was associated with the disappearance of nest-holding males, the mating partners of the females. Though social interactions among females or smaller subordinate individuals have also been suggested to play an important role in the social control of sex changes in various reef fishes (Munday et al. 2006; Cole 2010), this may be not the case for F. neophytus because of the rarity of social interactions among females. Therefore, social dominance relationships (especially among mating-related individuals) may trigger and mediate sex changes in F. neophytus. Further investigations should aim to clarify how sex changes are socially controlled in F. neophytus.
Reef-fish populations with MTV polygamy often possess a diandric life history (i.e., the coexistence of primary and secondary males). Primary males have been males since sexual maturation, while secondary males are formed from females in a protogynous sex change (Robertson and Warner 1978; Warner and Robertson 1978; Nakazono 1979; Moyer 1991). In the present study, we observed sneaking attempts by small floating male F. neophytus, but there were no examples of females changing sex directly into floating males. Floating males of F. neophytus had a smaller body size than sex-changing females that became nest-holding males. Although direct evidence for the developmental process that leads to small males of F. neophytus is unavailable, our results suggest that floating males may be derived from primary males. Thus, as predicted, it appears that F. neophytus is diandric.
Small primary males of polygamous fishes are often reported to perform the sneaking tactic as a form of parasitic mating (Warner 1984; Nakazono 1979; Moyer 1991; Taborsky 2008). Sneaking has been reported in at least eight polygamous gobies, including the species (F. neophytus) observed in the present study (Table 1). In another hermaphroditic goby, C. nicholsi, Cole (1983) deduced the presence of small males in samples of individuals based on histological examination of the gonads. In another sampling study, St. Mary (1993) and Drilling and Grober (2005) also found small female-sized males in populations of L. dalli. In these gobies, however, the mating behaviors of the small males are yet to be revealed. Thus, the present study represents the first report of observations of the application of the sneaking tactic by a polygamous hermaphroditic goby, F. neophytus.
In these hermaphroditic gobies, the frequency of occurrence of small males is commonly low (5.4 % of the C. nicholsi population, Cole 1983; 0.6–4.5 % of L. dalli populations, Drilling and Grober 2005; 2.6 % of the F. neophytus population in the present study). In the present study, sneaking attempts by floating male F. neophytus were observed in only 1.2 % of 171 spawnings, suggesting that they represented a rather small contribution to male reproductive success. In the present study, two floating males of F. neophytus changed tactics to become nest-holding males by home range replacement, possibly due to the superior mating tactics and mating success of the nest-holder. Similar tactical changes by sneakers have also been observed in gonochoristic gobies (Manabe et al. 2009; Taru et al. 2002; Takegaki et al. 2012). Future research should aim to clarify the tactical advantages and life-history pathways of small floating males of F. neophytus, in addition to the factors determining their sexuality.
References
Amundsen T, Forsgren E (2001) Male mate choice selects for female coloration in a fish. Proc Natl Acad Sci USA 98:13155–13160
Behrents KC (1987) The influence of shelter availability on recruitment and early juvenile survivorship of Lythrypnus dalli Gilbert (Pisces: Gobiidae). J Exp Mar Biol Ecol 107:45–59
Berglund A (1997) Mating systems and sex allocation. In: Godin JGJ (ed) Behavioural ecology of teleost fishes. Oxford University Press, New York, pp 237–265
Cole KS (1982) Male reproductive behaviour and spawning success in a tempetate zone goby, Coryphopterus nicholsi. Can J Zool 60:2309–2316
Cole KS (1983) Protogynous hermaphroditism in a temperate zone territorial marine goby, Coryphopterus nicholsi. Copeia 1983:809–812
Cole KS (1984) Social spacing in the temperate marine goby Coryphopterus nicholsi. Mar Biol 80:307–314
Cole KS (1990) Patterns of gonad structure in hermaphroditic gobies (Teleostei: Gobiidae). Environ Biol Fish 28:125–142
Cole KS (2010) Reproduction and sexuality in marine fishes: patterns and processes. University of California Press, California
Drilling CC, Grober MS (2005) An initial description of alternative male reproductive phenotypes in the blue banded goby, Lythrypnus dalli (Teleostei, Gobiidae). Environ Biol Fish 72:361–372
Emlen ST, Oring LW (1977) Ecology, sexual selection and the evolution of mating systems. Sci 197:215–223
Gainsner A (2005) Parental care and reproductive behavior of the clown goby, Microgobius gulosus, with observations on predator interactions. Environ Biol Fish 73:341–356
Hesthagen IH (1977) Migrations, breeding, and growth in Pomatoschistus minutus (Pallas) (Pisces, Gobiidae) in Oslofjorden, Norway. Sarsia 63:17–26
Karino K, Kuwamuta T, Nakashima Y, Sakai Y (2000) Predation risk and the opportunity for female mate choice in a coral reef fish. J Ethol 18:109–114
Kuwamura T (1997) The evolution of parental care and mating systems among Tanganyikan cichlids. In: Kawanabe H, Hori M, Nagoshi M (eds) Fish communities in Lake Tanganyika. Kyoto University Press, Kyoto, pp 59–86
Kuwamura T, Nakashima Y (1998) New aspects of sex change among reef fishes: recent studies in Japan. Environ Biol Fish 52:125–135
Kuwamura T, Yogo Y, Nakashima Y (1993) Size-assortative monogamy and paternal egg care in a coral goby Paragobiodon echinocephalus. Ethology 95:65–75
Kuwamura T, Nakashima Y, Yogo Y (1994) Sex change in either direction by growth-rate advantage in the monogamous coral goby, Paragobiodon echinocephalus. Behav Ecol 5:434–438
Kuwamura T, Sagawa T, Suzuki S (2009) Interspecific variation in spawning time and male mating tactics of the parrotfishes on a fringing coral reef at Iriomote Island, Okinawa. Ichthyol Res 56:354–362
Kvarnemo C (1995) Size-assortative nest choice in the absence of competition in males of the sand goby, Pomatoschistus minutus. Environ Biol Fish 43:233–239
Lassig B (1976) Field observations on the reproductive behaviour of Paragobiodon spp. (Osteichthys: Gobiidae) at Helon Island great barrier reef. Mar Behav Physiol 3:283–293
Lassig BR (1977) Socioecological strategies adopted by obligate coral-dwelling fishes. In: Taylor DL (ed) Proceedings of Third International Coral Reef Symposium. Rosenstiel School of Marine and Atmospheric Science, Miami, pp 565–570
Magnhagen C (1992) Alternative reproductive behaviour in the common goby, Pomatoschistus microps: an ontogenetic gradient? Anim Behav 44:182–184
Manabe H, Ishimura M, Shinomiya A, Sunobe T (2007) Field evidence for bi-directional sex change in the polygynous gobiid fish Trimma okinawae. J Fish Biol 70:600–609
Manabe H, Hagiwara K, Yonemori A, Fujiwara K, Shinomiya A (2009) Semi-lunar spawning cycle and mating tactics in the marine goby Asterropteryx semipunctata. Ichthyol Res 56:92–95
Manabe H, Toyoda K, Nagamoto K, Dewa S, Sakurai M, Hagiwara K, Shinomiya A, Sunobe T (2013) Bidirectional sex change in seven species of Priolepis (Actinopterygii: Gobiidae). Bull Mar Sci 89:635–642
Massironi M, Rasotto MB, Mazzoldi C (2005) A reliable indicator of female fecundity: the case of the yellow belly in Knipowitschia panizzae (Teleostei: Gobiidae). Mar Biol 147:71–76
Mazzoldi C, Rasotto MB (2001) Extended breeding season in the marbled goby, Pomatoschistus marmoratus (Teleostei: Gobiidae), in the Venetian Lagoon. Environ Biol Fish 61:175–183
Mazzoldi C, Rasotto MB (2002) Alternative male mating tactics in Gobius niger. J Fish Biol 61:157–172
Mazzoldi C, Seaggiante M, Ambrosin E, Rasotto MB (2000) Mating system and alternative male tactics in the grass goby Zosterisessor ophiocephalus (Teleostei: Gobiidae). Mar Biol 137:1041–1048
Mazzoldi C, Poltronieri C, Rasotto MB (2002) Egg size variability and mating system in the marbled goby Pomatoschistus marmoratus (Pisces: Gobiidae). Mar Ecol Prog Ser 233:231–239
Miller PJ (1984) The tokology of gobioid fishes. In: Potts GW, Wotton RJ (eds) Fish reproduction: strategies and tactics. Academic, London, pp 119–153
Mobley KB, Amundsen T, Forsgren E, Svensson PA, Jones AG (2009) Multiple mating and a low incidence of cuckoldry for nest-holding males in the two-spotted goby, Gobiusculus flavescens. BMC Evol Biol 9:6. doi:10.1186/1471-2148-9-6
Moyer JT (1991) Comparative mating strategies of labrid fishes. Monograph No. 1. The Watanabe Ichithyological Institute, Tokyo
Mück I, Wacker S, Myhre LC, Amundsen T (2013) Nest distribution affects behaviour and mating success in a marine fish. Behav Ecol Sociobiol 67:609–619
Munday PM (2002) Bi-directional sex change: testing the growth-rate advantage model. Behav Ecol Sociobiol 52:247–254
Munday PM, Caley MJ, Jones GP (1998) Bi-directional sex change in a coral-dwelling goby. Behav Ecol Sociobiol 43:371–377
Munday PL, Buston PM, Warner RR (2006) Diversity and flexibility of sex-change strategies in animals. Trends Ecol Evol 21:89–95
Nakabo T (2002) Fishes of Japan with pictorial keys to the species, Engl edn. Tokai University Press, Tokyo
Nakashima Y, Kuwamura T, Yogo Y (1995) Why be a both-ways sex changer? Ethology 101:301–307
Nakashima Y, Kuwamura T, Yogo Y (1996) Both-ways sex change in monogamous coral gobies, Gobiodon spp. Environ Biol Fish 46:281–288
Nakazono A (1979) Studies on the sex reversal and spawning behavior of five species of Japanese labrid fishes. Rept Fish Res Lab Kyushu Univ 4:1–64
Nelson JS (2006) Fishes of the world, 4th edn. Wiley, Hoboken
Petersen CW, Warner RR (2002) The ecological context of reproductive behavior. In: Sale PF (ed) Coral reef fishes: dynamics and diversity in a complex ecosystem. Academic, San Diego, pp 103–118
Privitera LA (2002) Reproductive biology of the coral-reef goby, Asterropteryx semipunctata, in Kaneohe Bay, Hawaii. Environ Biol Fish 65:289–310
Rasotto MB, Mazzoldi C (2002) Male traits associated with alternative reproductive tactics in Gobius niger. J Fish Biol 61:173–184
Reavis RH (1997) The natural history of a monogamous coral-reef fish, Valenciennea strigata (Gobiidae): 2. behavior, mate fidelity and reproductive success. Environ Biol Fish 49:247–257
Reavis RH, Barlow GW (1998) Why is the coral-reef fish Valenciennea strigata (Gobiidae) monogamous? Behav Ecol Sociobiol 43:229–237
Robertson DR, Choat JH (1974) Protogynous hermaphroditism and social systems in labrid fish. In: Great Barrier Reef Committee (eds) Proceedings of Second International Coral Reef Symposium. Great Barrier Reef Committee, Brisbane, pp 217–225
Robertson DR, Hoffman SG (1977) The roles of female mate choice and predation in the mating systems of some tropical labroid fishes. Z Tierpsychol 45:298–320
Robertson DR, Warner RR (1978) Sexual patterns in labroid fishes of the western Caribbean. II: The parrotfishes (Scaridae). Smithson Contr Zool 255:1–26
Sadovy de Mitcheson Y, Liu M (2008) Functional hermaphroditism in teleosts. Fish Fish 9:1–43
St. Mary CM (1993) Novel sexual patterns in two simultaneously hermaphroditic gobies, Lythrypnus dalli and Lythrypnus zebra. Copeia 1993:1062–1072
St. Mary CM (1994) Sex allocation in a simultaneous hermaphrodite, the blue-banded goby (Lythrypnus dalli): the effects of body size and behavioral gender and the consequences for reproduction. Behav Ecol 5:304–313
St. Mary CM (1996) Sex allocation in a simultaneous hermaphrodite, the zebra goby Lythrypnus zabra: insights gained through a comparison with its sympatric congener, Lythrypnus dalli. Environ Biol Fish 45:177–190
Sunobe T, Nakazono A (1990) Polygynous mating system of Trimma okinawae (Pisces: Gobiidae) at Kagoshima, Japan with a note on sex change. Ethology 84:133–143
Sunobe T, Nakazono A (1993) Sex change in both directions by alternation of social dominance in Trimma okinawae (Pisces: Gobiidae). Ethology 94:339–345
Sunobe T, Nakazono A (1999a) Mating system and hermaphroditism in the gobiid fish, Priolepis cincta, at Kagoshima, Japan. Ichthyol Res 46:103–105
Sunobe T, Nakazono A (1999b) Alternative mating tactics in the gobiid fish, Eviota prasina. Ichthyol Res 46:212–215
Swenson RO (1997) Sex-role reversal in the tidewater goby, Eucyclogobius newberryi. Environ Biol Fish 50:27–40
Taborsky M (2008) Alternative reproductive tactics in fish. In: Oliveira RF, Taborsky M, Brockman HJ (eds) Alternative reproductive tactics: an integrative approach. Cambridge University Press, Cambridge, pp 251–299
Takegaki T (2000) Monogamous mating system and spawning cycle in the gobiid fish, Amblygobius phalaena (Gobiidae). Environ Biol Fish 59:61–67
Takegaki T, Nakazono A (1999) Reproductive behavior and mate fidelity in the monogamous goby, Valenciennea longipinnis. Ichthyol Res 46:115–123
Takegaki T, Svensson O, Kvarnemo C (2012) Socially induced tactic change in 2 types of sand goby sneaker males. Behav Ecol 23:742–750
Taru M, Sunobe T (2000) Notes on reproductive ecology of the gobiid fish Eviota abax at Kominato, Japan. Bull Mar Sci 66:507–512
Taru M, Kanda T, Sunobe T (2002) Alternative mating tactics of the gobiid fish Bathygobius fuscus. J Ethol 20:9–12
Thresher RE (1984) Reproduction in reef fishes. TFH, Neptune City
Warner RR (1975) The adaptive significance of sequential hermaphroditism in animals. Am Nat 109:61–82
Warner RR (1984) Mating behavior and hermaphroditism in coral reef fishes. Am Sci 72:128–136
Warner RR (1988) Sex change in fishes: hypotheses, evidence, and objections. Environ Biol Fish 22:81–90
Warner RR, Robertson DR (1978) Sexual patterns in the labroid fishes of the western Caribbeean. I: The wrasses (Labridae). Smithson Contr Zool 254:1–27
Whiteman EA, Côté IM (2003) Social monogamy in the cleaning goby Elacatinus evelynae: ecological constraints or net benefit? Anim Behav 66:281–291
Wong MYL, Munday PL, Buston PM, Jones GP (2008) Monogamy when there is potential for polygyny: tests of multiple hypotheses in a group-living fish. Behav Ecol 19:353–361
Yanagisawa Y (1982) Social behaviour and mating system of the gobiid fish Amblyeleotris japonica. Jpn J Ichthyol 28:401–422
Acknowledgments
We thank the people of Kuchierabu-jima Island for allowing the field survey, and Prof. Hiroaki Hashimoto and colleagues at the Laboratory of Biology of Aquatic Resources, Hiroshima University as well as Mr. Y. Masui (Blue7C) for their support of this study. We would also like to express our deep gratitude to two anonymous reviewers for their critical reading of our manuscript. This study was supported by grants from the Inamori Foundation and JSPS KAKENHI grants (Nos. 17770017, 24370006, 24570033, and 15K07222). This paper was written in memory of Prof. Kenji Gushima, who consistently and kindly supported us during the present study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical notes
All procedures performed in the present study were in accordance with the guidelines for proper conduct of animal experiments and related activities of Hiroshima University (ID: CD001737) and the guidelines for ethological studies of the Japan Ethological Society.
About this article
Cite this article
Tsuboi, M., Sakai, Y. Polygamous mating system and protogynous sex change in the gobiid fish Fusigobius neophytus . J Ethol 34, 263–275 (2016). https://doi.org/10.1007/s10164-016-0472-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-016-0472-x