Abstract
Predation threat-associated behavioral response was studied in Rana temporalis tadpoles to discover the importance of predators’ visual and chemical cues (kairomones and diet-derived metabolites of consumed prey) in evoking antipredator behavior. The caged predators (dragonfly larvae) fed on prey tadpoles or insects (Notonecta spp.) and water conditioned with the predators provided the threat stimuli to the tadpole prey. The predators’ visual cues were ineffective in evoking antipredator behaviors in the tadpole prey. However, exposure to caged tadpole-fed predators or water conditioned with tadpole-fed predators elicited predator avoidance behavior in the tadpoles; they stayed away from the predators, significantly reduced swimming activity (swimming time and distance traveled), and increased burst speed. Interestingly, exposure to water conditioned with starved predators did not elicit any antipredator behavior in the prey. Further, the antipredator responses of predator-experienced tadpoles were significantly greater than those exhibited by predator-naïve tadpoles. The study shows that R. temporalis tadpoles assess predation threat based exclusively on chemical cues emanating from the predators’ dietary metabolites and that the inclusion of conspecific prey items in the diet of the predators is perceived as a threat. The study also shows that antipredator behavior in these tadpoles is innate and is enhanced during subsequent encounters with the predators.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Assessing predation risk and developing necessary antipredator defense strategies are critical in optimizing one’s survival and fitness. Sensing predator presence before the actual encounter may offer selective advantage to animals because it would reduce the risk of predation (Lima and Dill 1990; Ferrari et al. 2010). Failure to respond to a potential predator may be fatal, and unnecessary antipredator behavior may have direct energetic costs as well as costs associated with reduced opportunities to feed or reproduce (Lima and Dill 1990). Therefore, sensory information obtained about a predator may be useful for a prey organism to accurately assess the potential risk, and also reduce energetic costs (Lima and Dill 1990). In aquatic ecosystems, many prey animals, including amphibian tadpoles, use olfactory or chemical signals to assess predation risk (Petranka et al. 1987; Kats et al. 1988; Kiesecker et al. 1996, 1999; Chivers and Smith 1998; Kats and Dill 1998; Mathis and Vincent 2000; Hickman et al. 2004; Saidapur et al. 2009; Ferrari et al. 2010). Chemical cues are important in aquatic environments since visual information may be obscured in water that is turbid or densely vegetated. During predation, chemicals released either by injured prey or the predator can be used by the prey to assess and avoid predation risk (Wisenden 2000). Previous studies from various animal taxa show that prey may detect the predator based on the alarm cues from injured conspecific prey (Wilson and Lefcort 1993; Chivers and Smith 1998; Kats and Dill 1998; Summey and Mathis 1998; Bryer et al. 2001; Kiesecker et al. 2002), or kairomones of predator origin (Wisenden 2000; Gyssels and Stoks 2006; Pohnert et al. 2007), or cues released from dietary metabolites of predator (Mathis and Smith 1993; Wilson and Lefcort 1993; Chivers et al. 1996; Laurila et al. 1997; Chivers and Mirza 2001; Kiesecker et al. 2002; Schoeppner and Relyea 2009a; Ferrari et al. 2010). Thus, information received by prey about the predation risk is quite complex. Hence, more definitive experiments are needed to understand the relative role of each of the chemical cues, kairomones, dietary cues, and alarm cues arising during prey–predator interaction.
The ability of prey species to detect their predators without prior experience is known in several taxa (Veen et al. 2000; Goth 2001; Barros et al. 2002; Berejikian et al. 2003; Hawkins et al. 2004). Studies on amphibian tadpoles have shown that antipredator behavior is innate (Laurila et al. 1997; Gallie et al. 2001; Mathis et al. 2003; Sharma et al. 2008; Saidapur et al. 2009). However, very few studies have addressed the question of whether inherited antipredator behavior is improved or modified by subsequent exposure to predator cues (Semlitsch and Reyer 1992).
The present study on Rana temporalis tadpoles was undertaken to discover (1) the relative role of visual and chemical cues in predator detection, (2) the source of the predators’ chemical cues that elicit antipredator behavior in prey tadpoles, and (3) whether antipredator behavior in these tadpoles is innate or acquired, and whether it is modified on subsequent encounters with predator cues. The larvae of the dragonfly, Pantala flavescens, which co-occur with R. temporalis tadpoles in natural water bodies, were used as the predator in this study.
Materials and methods
Eggs from four clutches of the bronze frog, R. temporalis, were collected from a stream in the Western Ghats near Anmod village, Karnataka State, India, in November. After transportation of eggs to laboratory, they were placed separately in plastic bowls (42 cm diam. and 16 cm deep) containing 10 L of aged (dechlorinated) tap water. After hatching, approximately 125 tadpoles each from 4 clutches were reared together in a glass aquarium (75 × 45 × 15 cm) with 15 L of aged tap water. Two such mixed rearing aquaria were maintained. Upon reaching the feeding stage (Gosner stage 25), they were fed with boiled spinach. Tadpoles of stages 27–28 were used in all experiments.
The last instar larvae of the dragonfly P. flavescens (~30 mm in length) that served as the predator were reared individually, to avoid cannibalism, in plastic bowls (14 cm diam. and 7 cm deep) with 200 mL of aged tap water. They were fed with either prey (R. temporalis) tadpoles (Gosner stages 25–26) or insects (Notonecta spp. 5 mm length) or starved before trials depending upon the experimental design.
Methods of experiment 1: predator detection in R. temporalis tadpoles
This experiment was conducted to discover whether R. temporalis tadpoles detect predators based on chemical or visual cues or both. A glass aquarium (90 × 30 × 15 cm) served as the test tank. A central line perpendicular to the long axis and a line parallel to it on both its sides were drawn at 5-cm distances on the outer surface of the tank bottom (Fig. 1). The central (10 cm × 30 cm) zone was used to release the test tadpoles (prey). The opposite ends of the test tank, stimulus zones, housed the predators (n = 2) in either a mesh cage wrapped with cheese cloth (providing chemical cues) or a transparent glass beaker (providing visual cues). The test tank was cleaned prior to each trial and filled with water to a depth of 3 cm. A single test tadpole was introduced in an open-ended mesh cage (10 cm diam.) placed at the center of the test tank and allowed to acclimate for 5 min as well as to perceive chemical/visual cues of predators. It was then released by gently lifting the cage and its behavior recorded. Each test tadpole was used only once. No food was provided to the test tadpoles or the predators during the trials.
The test tank used for predator detection trials in the tadpoles of Rana temporalis. Circle in the center of the test tank indicates the location of the release of the test tadpoles through a mesh cage. Circles in the end zones indicate areas where the containers (either a glass beaker or a mesh cage), which were either empty or housed predators (dragonfly larvae, Pantala flavescens), were kept. Zone A/B = 40 cm length; releasing zone = 10 cm length
The time spent by prey tadpoles in different zones of the test tank was recorded for 10 min. Our assumption was that, when test tadpoles detect predators or predation threat, they would spend more time in the zone away from the one housing predators. On the other hand, failure to detect such a threat will result in a random movement of test tadpoles in the test tank regardless of the predators’ visual and/or chemical cues. In trials with predators fed on insects or conspecific prey tadpoles, we assumed that the strength of predation threat would vary depending upon the dietary cues. If so, the degree of defense behavior would also vary. Each test comprised of 25 trials. The position of predators in the test tank was reversed between trials. A given set of predators was only used in three consecutive trials. The following tests were conducted.
End-bias tests
These tests were conducted to rule out bias of prey tadpoles towards any side of the test tank or the containers used for housing predators, i.e. mesh cage wrapped with cheese cloth or transparent glass beaker. Three sets of tests were conducted: (1) tests with stimulus zones housing glass beakers containing water to the level that matched the water level in the tank; (2) tests with stimulus zones housing mesh cage wrapped with cheese cloth, and (3) tests with a beaker and a mesh cage wrapped with cheese cloth placed at the opposite stimulus zones. A test tadpole chosen arbitrarily was released from the central zone of the test tank. The time spent by it in each zone in a given trial was recorded. For each set of tests, 25 trials were conducted using a new tadpole each time and after cleaning the test tank before each trial.
Response to predator’s visual cues
In this test, P. flavescens larvae (n = 2) were placed in a transparent glass beaker at one end of the test tank so as to provide visual cues but not their water-borne chemical cues. A beaker containing only water was placed at the opposite end. We hypothesized that if R. temporalis tadpoles detect predators based on visual cues, they would spend more time in the zone away from the predator.
Response to predator’s chemical cues
In this test, the predators (n = 2) were placed in a mesh cage wrapped with cheese cloth at one end of the test tank so as to provide their chemical but not visual cues. We hypothesized that, if test tadpoles detect the water-borne chemical cues arising from the predator, they would spend more time away from the zone housing the predator. Three sets of trials were conducted in this experiment. In the first set, predators fed with insects and in the second set those fed with R. temporalis tadpoles were placed at one end of the test tank in a mesh cage. The opposite end housed an empty cage. It is assumed that, if the test tadpoles spend more time away from the predator zone, it would mean that they exhibit predator detection from chemical cues arising from the predator. In the third set of trials, the predators were housed at opposite ends of test tank, one end housing a conspecific tadpole-fed predator and the other end housing an insect-fed predator. It is assumed that, if the test tadpoles spend more time away from both end compartments, it would mean that they detect predators primarily based on the chemical cues of predator origin. On the other hand, if the test tadpoles avoid a particular zone, it would suggest that predator detection is based on the dietary cues released by the predator.
Response to predator’s visual versus chemical cues
This test was conducted by providing both visual and chemical cues simultaneously to clearly establish the relative importance of each of these cues. In this test, predators (n = 2) fed either with tadpoles or insects were housed in a mesh cage wrapped with cheese cloth placed at one end and in the glass beaker placed at the opposite end. In this test, we hypothesized that test tadpoles would spend more time in the zone housing predators in a glass beaker than in the zone housing tadpole-fed predators in the mesh cage. Further, in tests involving insect-fed predators at both ends (either in the glass beaker or in the mesh cage wrapped with cheese cloth), we assumed that the test tadpoles will not exhibit predator avoidance behavior. In all the above tests, data on the time spent by test tadpoles in stimulus zones A and B were compared by the Wilcoxon paired sign rank test.
Results of experiment 1
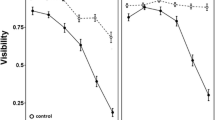
In the end-bias tests, tadpoles moved freely throughout the test tank. They showed no bias towards any particular side of the test tank. The placement of the glass beaker or mesh cage made no difference to the test tadpoles. They moved randomly throughout the test tank. Hence, data from all sets of end-bias tests were pooled and are presented in Table 1.
In trials with the visual cues of predators at one end zone, the test tadpoles moved randomly and freely throughout the test arena. The time spent by them near or away from the predators did not differ (Table 1). In trials providing chemical cues of insect-fed predators, the test tadpoles moved randomly throughout the test arena. There was no significant difference in the time spent in the zone with insect-fed predators or in the opposite predator-free zone (Table 1). In contrast, in trials with tadpole-fed predators, the test tadpoles spent a significantly greater amount of time in the zone away from the predator zone (Table 1). Likewise, in trials with both insect-fed and tadpole-fed predators (placed at the opposite ends in cages), the tadpoles spent a significantly greater amount of time near the zone housing the insect-fed predators (Table 1). Further, in another set of trials with tadpole-fed predators, the test tadpoles spent a significantly greater amount of time in the zone providing visual rather than chemical cues of predators. In trials with insect-fed predators in the glass beaker at one end and in the mesh cage at the opposite end, the test tadpoles moved randomly in the test tank (Table 1).
Methods of experiment 2: do tadpoles of R. temporalis detect predator’s kairomones?
Experiment 1 showed that tadpoles of R. temporalis display antipredator behavior to predators fed with conspecifics but not to those fed with insects. This implies that chemical cues arising from dietary metabolites containing conspecific tadpoles are detected by prey tadpoles and not the predators’ kairomones. In order to confirm whether the predators’ kairomones play any role at all in eliciting antipredator response in R. temporalis tadpoles, this experiment was conducted in January 2012. The eggs of R. temporalis were collected from the same place as those used in experiment 1. Approximately 125 tadpoles were reared in a glass aquarium (75 × 45 × 15 cm) with 15 L of aged tap water. Tadpoles of stages 27–28 were used for this experiment. The dragonfly larvae, P. flavescens (last instars), were collected from nature. Each larva was reared in a separate plastic bowl (14 cm diam. and 7 cm deep) with 200 mL of aged tap water.
Preparation of conditioned water
Dragonfly larvae starved for 96 h were placed individually in plastic bowls with 200 mL of aged tap water to exclude chemical cues of dietary origin. The water from these bowls served as conditioned water with kairomones, if any, released by the body of predators, and not contaminated with dietary or alarm cues.
Testing procedure
The responses of test tadpoles (predator-naïve) to water conditioned with chemical cues of predator’s kairomones were recorded by placing them in a glass test tank (28 × 15 × 15 cm) containing 600 mL of aged tap water. A Sony handycam was fixed above the test tank such that it covered the entire tank. The handycam was connected to a computer with the Ethovision Video Tracking System (Noldus Information Technology, The Netherlands) to track movement of the test tadpoles before (baseline) and after addition of conditioned water to the test tank. A test tadpole was introduced in the test tank and left undisturbed for 5 min. By using a burette placed ~1 cm above the water level, 50 mL of aged tap water was then added at the rate of ~1 mL/s. After transferring 50 mL of the chemical blank solution to the test tank, the movement of the test tadpole was tracked for 5 min using Ethovision to establish the baseline activity. After tracking the baseline activity, 50 mL of conditioned water (kairomones) was added using the same burette. Movement of the test tadpole was again tracked for a further 5 min. The tracks were saved on the computer to determine the activity pattern of the tadpoles before and after exposure to conditioned water. From the saved tracks, the maximum swimming speed (V max) or burst speed, the number of swimming spurts following spells of stationary periods, the time spent in swimming, and total distance traversed by a tadpole was obtained. A total of 50 trials were run using a new test tadpole each time. The test tank was washed thoroughly before each new trial and replenished with aged tap water. The data on the behavioral responses of tadpoles before and after the addition of the conditioned water with kairomones were compared using the Wilcoxon paired sign rank test.
Results of experiment 2
Table 2 shows V max, frequency of swimming spurts, total distance traversed, and time spent in swimming by R. temporalis tadpoles when exposed to chemical blank and water conditioned with predator’s kairomones. The V max, frequency of swimming spurts, total distance traversed, and time spent in swimming were comparable in tadpoles exposed to chemical blank and conditioned water (Table 2).
Methods of experiment 3: do predator experienced R. temporalis tadpoles show improved antipredator behavior upon subsequent encounter with predatory cues?
In experiment 1, the test tadpoles responded only to tadpole-fed predators by staying away from them, rather than from insect-fed predators, or to their visual cues. Prey tadpoles used in experiments 1 and 2 were predator-naïve. Hence, it was expected that these tadpoles would exhibit antipredator responses to water conditioned with predators fed on conspecific members. The present study was conducted to discover whether predator-experienced tadpoles would improve their antipredator behavior upon subsequent encounters with predatory cues. In this experiment, we exposed predator-naïve and predator-experienced tadpoles to water conditioned with predators fed on conspecific tadpoles, and tracked their antipredator activity by using the Ethovision video tracking system.
Preparation of conditioned water
Three dragonfly larvae (last instars) were placed in a plastic bowl (14 cm diam. and 7 cm deep) containing 600 mL of aged tap water along with 12 prey tadpoles (Gosner stages 27–28; SVL ~8 mm) around 0830 hours on the day before the test trials. During this period, the insect larvae fed on the prey tadpoles, and at around 1830 hours no tadpoles remained in the bowls. The following day (between 0930 and 1130 hours), predators were taken out from the bowls, and the water was filtered to remove suspended particles. The filtrate served as the conditioned water which contained water-borne chemical cues of predators from dietary metabolite origin. Since there were no injured tadpoles in the water in the bowls for more than 15 h, it is assumed that any alarm cues that might have been generated during the predation of the tadpoles had decayed. An earlier study has shown the labile nature of chemical cues involved in predator detection (Sharma et al. 2008).
Test subjects
A total of 25 tadpoles (Gosner stages 27–28) were placed in aquarium (40 × 40 × 10 cm) containing 5 L of aged tap water. These tadpoles were from the stock that was raised in the absence of any predators from the time of hatching. Eight such aquaria were maintained. An insect predator starved for 1 day was then introduced in four of these aquaria from 0900 to 1700 hours. On an average, the predator ate 3 ± 0.4 and injured 5 ± 0.7 tadpoles during the 8 h period with the prey tadpoles. The predator and the injured tadpoles were then removed. The uninjured but predator-experienced tadpoles were used for trials on the subsequent day. The test tadpoles of the remaining four aquaria (without the predators) served as predator-naïve test subjects.
Testing procedure
The responses of test tadpoles (predator-naïve and predator-experienced) to water conditioned with chemical cues of predator (dietary cues) were recorded by placing them in a glass test tank (28 × 15 × 15 cm) containing 600 mL of aged tap water. The testing procedure was the same as in experiment 2. A test tadpole (predator-naïve and experienced) was initially subjected to chemical blank water and then subjected to conditioned water (dietary cues). The movement of the test tadpoles (both naïve and experienced) before and after adding conditioned water was tracked using Ethovision. A total of 50 trials each were run using a new test tadpole each time from the predator-naïve and predator-experienced groups. The test tank was washed thoroughly before each new trial and replenished with aged tap water.
The data on behavioral responses of predator-naïve and predator-experienced tadpoles before and after the addition of the conditioned water with chemical cues of predator were compared using the Wilcoxon paired sign rank test. Behavioral responses exhibited by predator-naïve and predator-experienced tadpoles to conditioned water were compared using the Mann–Whitney U test.
Results of experiment 3
Upon exposure to conditioned water, both predator-naïve and predator-experienced tadpoles showed a significant increase in the burst speed (V max) but a significant decline in the number of swimming spurts, swimming time, and total distance moved when compared to their baseline activity (Table 3). However, predator-experienced tadpoles showed a significantly greater burst speed (U = 913.5, P < 0.020) than that exhibited by predator-naïve tadpoles similarly exposed to conditioned water. Further, the number of swimming spurts (U = 750.0, P < 0.001), time spent in swimming (U = 786.0, P < 0.001), and distance traversed (U = 817.0, P < 0.003) by the predator-experienced tadpoles were significantly lower than that exhibited by naïve tadpoles upon exposure to conditioned water.
Discussion
The present study shows that the visual cues of the predator (P. flavescens) do not evoke antipredator behavior in R. temporalis tadpoles suggesting little role for vision in predator detection, which is similar to that reported in other amphibian species (Stauffer and Semlitsch 1993; Kiesecker et al. 1996; Mathis and Vincent 2000; Hickman et al. 2004; Saidapur et al. 2009). Further, the present findings clearly show that tadpoles of R. temporalis exhibit antipredator behavior in the form of predator avoidance, reduced movements, and high burst speed (V max) specifically in response to chemical cues of conspecific tadpole-fed predators. No antipredator responses were elicited on encounters with the predator fed on insects (heterospecific prey), suggesting that R. temporalis tadpoles do not perceive the dragonfly larvae as a threat when they fed on heterospecifics. These findings are in conformity with that reported for tadpoles of R. aurora which exhibited antipredator behaviors in response to tadpole-fed newts but not to those fed on insects (Wilson and Lefcort 1993). Mathis and Smith (1993) showed that fathead minnows also exhibit antipredator behavior only to the predator pike fed on conspecifics but not to that predator fed on swordtails. In contrast, the tadpoles of R. temporaria and R. sylvatica exhibit antipredator behavior to chemical cues of a predator irrespective of its diet. However, both these species elicit a stronger antipredator response to tadpole-fed predators compared to that evoked by insect-fed predators (Laurila et al. 1997; Chivers and Mirza 2001). On the other hand, Petranka and Hayes (1998) did not observe any variation in antipredator behavior of R. sylvatica tadpoles in response to starved or tadpole-fed dragonfly larvae. Therefore, it appears that antipredator behavior in response to predator’s diet-based cues varies among different prey species of anuran tadpoles. This may be because the degree of perception of different chemical cues generated during predator–prey interactions may vary among different species.
The existence of kairomones (predator odor) that elicit antipredator behavior in prey has been reported in a wide spectra of animals (Kats and Dill 1998; Ferrari et al. 2010). Kairomones are generally considered as the chemical signatures of predators. A few studies involving starved predators have shown that kairomones are unable to elicit antipredator behavior in some prey taxa (Crowl and Covich 1990; Stirling 1995; Schoeppner and Relyea 2009b). Few other studies have shown that kairomones also induce antipredator behavior (Hazlett and Schoolmaster 1998; Petranka and Hayes 1998; Van Buskirk and Arioli 2002; Gyssels and Stoks 2006). With a few exceptions, in most of the earlier studies on amphibian tadpoles, it was difficult to determine whether antipredator responses are because of recognition of the predator kairomones or to the chemicals originating from the predators’ diet (Petranka and Hayes 1998; Schoeppner and Relyea 2009b). The tadpoles of R. pipiens do not respond to cues of starved predators (Schoeppner and Relyea 2009b). In contrast, Petranka and Hayes (1998) reported that tadpoles of Bufo americanus and R. sylvatica exhibit strong antipredator behavior in response to chemical cues of starved predators. The present study shows the relative importance of predator-specific chemical cues or kairomones and predator diet-derived cues in eliciting antipredator defense behavior in R. temporalis tadpoles. Our findings show that tadpoles of R. temporalis specifically respond to chemical cues derived from predators fed on conspecific tadpoles. These tadpoles do not respond to predatory cues of starved predators. The findings therefore suggest that R. temporalis tadpoles do not respond to predator-derived kairomones. Perhaps they do not perceive such cues as a predation risk. On the other hand, dietary cues of predators preying upon conspecific prey rather than the kairomones seem to provide more reliable information on the vulnerability to predation. Hence, R. temporalis tadpoles exhibit antipredator defense strategies only when they perceive a real predation threat (i.e. their conspecifics falling prey). These findings support the threat sensitive hypothesis according to which prey species assess and adjust their behavior in accordance with the predation risk (Helfman 1989; Chivers et al. 2001; Ferrari et al. 2008).
The present study shows that the antipredator behaviors seen in R. temporalis tadpoles are of an innate nature since the predator-naïve tadpoles respond to water-borne chemical cues derived from the predators’ diet containing conspecific tadpoles. This finding is similar to that reported in other amphibian tadpoles (Laurila et al. 1997; Gallie et al. 2001; Mathis et al. 2003; Sharma et al. 2008; Fraker 2009). Further, the predator-experienced tadpoles of R. temporalis exhibit intense antipredator response upon subsequent exposure to predatory cues compared to that of predator-naïve tadpoles. It is likely that R. temporalis tadpoles remember their early encounter with a predator and hence improve their antipredator responses. Alternatively, the predator-experienced tadpoles used in the trials were those that survived despite their co-existence with the predators for 8 h, and may therefore indicate their superior quality over those that fall prey. Their survival may have been by virtue of their superior antipredator strategies in the arms race between prey and predator. If so, the high level of antipredator strategy observed in these tadpoles on exposure to conditioned water suggests that it is perhaps their innate quality that is superior to other members. Therefore, additional studies are needed to resolve this issue.
In summary, the present study shows that R. temporalis tadpoles perceive dietary-derived chemical cues of the predatory insect to assess the degree of predation threat. They exhibit antipredator behavior only if the diet includes conspecific prey items. Further, antipredator behavior is innate in these tadpoles. An enhanced antipredator behavior of predator-experienced tadpoles may suggest their superior quality and/or learning to respond to real predation threats through experience.
References
Barros M, Boere V, Mello EL Jr, Tomaz C (2002) Reactions to potential predators in captive-born marmosets (Callithrix penicillata). Int J Primatol 23:443–454
Berejikian BA, Tezak EP, LaRae AL (2003) Innate and enhanced predator recognition in hatchery-reared chinook salmon (Oncorhynchus tshawytscha) juveniles. Can J Fish Aquat Sci 56:830–838
Bryer PJ, Mirza RS, Chivers DP (2001) Chemosensory assessment of predation risk by slimy sculpins (Cottus cognatus): responses to alarm disturbance, and predator cues. J Chem Ecol 27:533–546
Chivers DP, Mirza RS (2001) Importance of predator diet cues in responses of larval wood frogs to fish and invertebrate predators. J Chem Ecol 27:45–51
Chivers DP, Smith RJF (1998) Chemical alarm signaling in aquatic predator-prey systems: a review and prospectus. Ecoscience 5:338–352
Chivers DP, Wisenden BD, Smith RJF (1996) Damselfly larvae learn to recognize predators from chemical cues in the predator’s diet. Anim Behav 52:315–320
Chivers DP, Mirza RS, Bryer PJ, Kiesecker JM (2001) Threat-sensitive predator avoidance by slimy sculpins: understanding the importance of visual versus chemical information. Can J Zool 79:867–873
Crowl TA, Covich AP (1990) Predator-induced life-history shifts in a freshwater snail. Science 247:949–951
Ferrari MCO, Messier F, Chivers DP (2008) Can prey exhibit threat-sensitive generalization of predator recognition? Extending the predator recognition continuum hypothesis. Proc R Soc Lond B 275:1811–1816
Ferrari MCO, Wisenden BD, Chivers DP (2010) Chemical ecology of predator-prey interactions in aquatic ecosystems: a review and prospectus. Can J Zool 88:698–724
Fraker ME (2009) The effect of prior experience on a prey’s current perceived risk. Oecologia 158:765–774
Gallie JA, Mumme RL, Wissinger SA (2001) Experience has no effect on the development of chemosensory recognition of predators by tadpoles of the American toad, Bufo americanus. Herpetologica 57:376–383
Goth A (2001) Innate predator-recognition in Australian brush-turkey (Alectura lathami, Megapodiidae) hatchlings. Behaviour 138:117–136
Gyssels F, Stoks R (2006) Behavioral responses to fish kairomones and autotomy in a damselfly. J Ethol 24:79–83
Hawkins LA, Magurran AE, Armstrong JD (2004) Innate predator recognition in newly-hatched Atlantic salmon. Behaviour 141:1249–1262
Hazlett BA, Schoolmaster DR (1998) Responses of cambarid crayfish to predator odor. J Chem Ecol 24:1757–1770
Helfman GS (1989) Threat-sensitive predator avoidance in damselfish-trumpetfish interactions. Behav Ecol Sociobiol 24:47–58
Hickman CR, Stone MD, Mathis A (2004) Priority use of chemical over visual cues for detection of predators by graybelly salamanders, Eurycea multiplicata griseogaster. Herpetologica 60:203–210
Kats LB, Dill LM (1998) The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience 5:361–394
Kats LB, Petranka JW, Sih A (1988) Antipredator defenses and the persistence of amphibian larvae with fishes. Ecology 69:1865–1870
Kiesecker JM, Chivers DP, Blaustein AR (1996) The use of chemical cues in predator recognition by western toad tadpoles. Anim Behav 52:1237–1245
Kiesecker JM, Chivers DP, Marco A, Quilchano C, Anderson MT, Blaustein AR (1999) Identification of a disturbance signal in larval red-legged frogs, Rana aurora. Anim Behav 57:1295–1300
Kiesecker JM, Chivers DP, Anderson M, Blaustein AR (2002) Effect of predator diet on life history shifts of red-legged frogs, Rana aurora. J Chem Ecol 28:1007–1015
Laurila A, Kujasalon J, Ranta E (1997) Different antipredatory behavior in two anuran tadpoles: effects of predator diet. Behav Ecol Sociobiol 40:329–336
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Mathis A, Smith RJF (1993) Fathead minnows, Pimephales promelas, learn to recognize northern pike, Esox lucius, as predators on the basis of chemical stimuli from minnows in the pike’s diet. Anim Behav 46:645–656
Mathis A, Vincent F (2000) Differential use of visual and chemical cues in predator recognition and threat-sensitive predator-avoidance responses by larval newts (Notophthalmus viridescens). Can J Zool 78:1646–1652
Mathis A, Murray KL, Hickman CR (2003) Do experience and body size play a role in responses of larval ringed salamanders, Ambystoma annulatum, to predator kairomones? Laboratory and field assays. Ethology 109:159–170
Petranka J, Hayes L (1998) Chemically mediated avoidance of a predatory odonate (Anax junius) by American toad (Bufo americanus) and wood frog (Rana sylvatica) tadpoles. Behav Ecol Sociobiol 42:263–271
Petranka JW, Kats LB, Sih A (1987) Predator-prey interactions among fish and larval amphibians: use of chemical cues to detect predatory fish. Anim Behav 35:420–425
Pohnert G, Steinke M, Tollrian R (2007) Chemical cues, defense metabolites and the shaping of pelagic interspecific interactions. Trends Ecol Evol 16:198–204
Saidapur SK, Veeranagoudar DK, Hiragond NC, Shanbhag BA (2009) Mechanism of predator-prey detection and behavioral responses in some anuran tadpoles. Chemoecology 19:21–28
Schoeppner NM, Relyea RA (2009a) When should prey respond to consumed heterospecifics? Testing hypothesis of perceived risk. Copeia 2009:190–194
Schoeppner NM, Relyea RA (2009b) Interpreting the smells of predation: how alarm cues and kairomones induce different prey defences. Funct Ecol 23:1114–1121
Semlitsch RD, Reyer HO (1992) Modifications of antipredator defenses in tadpoles by environmental conditioning. J Anim Ecol 61:353–360
Sharma SS, Veeranagoudar DK, Shanbhag BA, Saidapur SK (2008) Activity of Sphaerotheca breviceps tadpoles in response to chemical cues of the predaceous Hoplobatrachus tigerinus tadpoles. J Ethol 26:303–307
Stauffer H, Semlitsch RD (1993) Effects of visual, chemical and tactile cues of fish on the behavioral responses of tadpoles. Anim Behav 46:355–364
Stirling G (1995) Daphnia behavior as a bioassay of fish presence or predation. Funct Ecol 9:778–784
Summey MR, Mathis A (1998) Alarm responses to chemical stimuli from damaged conspecifics by larval anurans: tests of three neotropical species. Herpetologica 54:402–408
Van Buskirk J, Arioli M (2002) Dosage response of an induced defense: how sensitive are tadpoles to predation risk? Ecology 83:1580–1585
Veen T, Richardson DS, Blaakmeer K, Komdeur J (2000) Experimental evidence for innate predator recognition in the Seychelles warbler. Proc R Soc Lond B 267:2253–2258
Wilson DJ, Lefcort H (1993) The effect of predator diet on the alarm response of red-legged frog, Rana aurora tadpoles. Anim Behav 46:1017–1019
Wisenden BD (2000) Olfactory assessment of predation risk in the aquatic environment. Philos Trans R Soc Lond B 355:1205–1208
Acknowledgments
The work was supported by grants from Department of Science and Technology (No. SP-SO/AS-38/2009) and University Grants Commission (SAP-DRS-II-No. F-3/32/2006), New Delhi. The study was conducted following the guidelines of ethical Committee of CPCSEA, New Delhi.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Mogali, S.M., Saidapur, S.K. & Shanbhag, B.A. Tadpoles of the bronze frog (Rana temporalis) assess predation risk before evoking antipredator defense behavior. J Ethol 30, 379–386 (2012). https://doi.org/10.1007/s10164-012-0335-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-012-0335-z