Abstract
While male mate choice behaviour has been reported in many taxa, little is known about its plasticity and evolutionary consequences. In the damselfly Ischnura senegalensis, females exhibit colour dimorphism (gynomorph and andromorph). The body colour of gynomorphs changed ontogenetically in accordance with sexual maturation, while little change occurred in andromorphs. To test the male mate choice between sexually immature and mature females of both morphs, binary choice experiments were conducted. Virgin males that were reared separately from females after emergence did not show significant preference between sexually immature and mature females for both morphs, indicating that virgin males were unable to discriminate female reproductive status. On the other hand, males that had experienced copulation with gynomorphs preferred sexually mature gynomorphs to sexually immature ones. However, males that had experienced copulation with andromorphs could not discriminate between sexually immature and mature andromorphs, probably due to the absence of significant ontogenetic change in their thoracic colour. Therefore, female body colour is an important cue for males in discriminating between sexual maturation stages. Learned mate discrimination depending on copulation experience might help males to detect potential mates effectively and avoid sexually unreceptive immature female. We finally discuss the adaptive significance of the ontogenetic colour change in females.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Because females in most animal species make a greater investment in each offspring than males (Trivers 1972), selection would seem to favour the choice of males by females in situation of direct (e.g. Boggs 1995) or indirect (e.g. Bussière et al. 2008) benefit. Therefore, the main focus of studies on intersexual selection has been female mate choice behaviour (Andersson 1994). However, male mate choice behaviour is also widespread in many taxa (e.g. Bateman and Fleming 2006; Bonduriansky 2001; Clutton-Brock 2007). When females vary in quality, males are expected to be choosy (Amundsen 2000), resulting in male mate choice in relation to mate-encounter rate and parental investment (Kokko and Johnstone 2002). Because the number of matings and the amount of reproductive investment of males are limited due to both energy and time constraints, males exhibit pre- and/or post-copulatory mate choice depending on female qualities such as body size (Jones et al. 2001; Rutowski 1982), symmetry (Hansen et al. 1999), willingness to mate (Andersson et al. 2000; Van Gossum et al. 2001a; McLennan 1995) and so on. Such male mate choice is suggested to lead to the evolution of secondary sexual characteristics in females (Amundsen and Forsgren 2001; Clutton-Brock 2009) and of mating systems (Bonduriansky 2001), consequently resulting in sexual dimorphism. Therefore, in order to understand the evolution of sexual traits in females, the detailed behavioural property of male mate choice, such as innate preference and its plasticity, must be proposed.
In some coenagrionid damselflies, few females leave the waterside during the sexually immature stages (Hinnekint 1987). Due to their relatively long flying season, females at sexually immature and mature stages co-exist in the same grasslands near water, where sexually mature males search for suitable mating partners (Fincke 1987). Sexually mature females mate repeatedly throughout their reproductive stages (Robertson 1985; Takahashi and Watanabe 2009), while sexually immature females refuse male mating attempts (Hammers et al. 2009). They might try to escape from the males or to show mate refusal posture in response to the males (Cordero et al. 1998; Gosden and Svensson 2009). Therefore, mating attempts with sexually immature females are wastes of time for males, resulting in a decrease in their mate searching efficiency (Stoks and De Bruyn 1998). Males should discriminate the maturation stage of females before trying to mate in order to avoid sexually immature females.
Ontogenetic colour changes in relation to sexual maturation are widespread traits in odonate species (Corbet 1999). In coenagrionid damselfies, both sexes achieve sexual maturity at about a week after emergence when they start reproductive activity (Cooper et al. 1996). Because body colour is the most important visual cue for males in mate recognition in Odonata (Gorb 1998), differences in body colour between sexually immature and mature females might help males to discriminate the sexual maturity of females.
It has been previously found that males have no innate mating preference for female morphs in Enallagma spp. (Fincke et al. 2007). They change their mating preference in accordance with their prior experience (Van Gossum et al. 2001b). In Ischunra senegalensis, Takahashi and Watanabe (2010b) revealed that virgin males had no preference in a binary choice experiment between dead female morphs, and that mated males preferred to mate with female morphs with which they had previously mated. Mating experience rather than the experience of encounters with females and rejection by females affected the sequential mating preference of males (Takahashi and Watanabe 2008). However, little is known about the effect of copulation experience on the discrimination between sexually immature and mature females. In the present study, we quantified the ontogenetic colour changes in females of I. senegalensis, a species in which females exhibit heritable colour dimorphism (Takahashi and Watanabe, unpublished), appearing as either gynomorphs or andromorphs. We also investigated innate mate choice between sexually immature and mature females, and learned mate choice in relation to their copulation experience. We then discuss the evolution of ontogenetic colour change in females from the viewpoint of the plasticity of male mate choice.
Materials and methods
Insect
Ischnura senegalensis is a non-territorial damselfly inhabiting the edges of pond in the warm-temperate zone of Japan. Sexually mature males attempt to mate with females whenever they encounter them without any courtship behaviour (Takahashi and Watanabe 2010b). The females mate multiply throughout their life, and each copulation lasts more than 3 h (Takahashi and Watanabe 2009). While the males show monomorphism, the females exhibit colour dimorphism (andromorph and gynomorph) which is determined by one autosomal locus with two alleles with sex-limited expression (Takahashi and Watanabe, unpublished) as is the case of other female dimorphic damselflies (Johnson 1964, 1966).
Preparation of test specimen
In order to obtain the test specimens, sexually mature females were collected in the field in Ibaraki Prefecture in the warm-temperature zone of Japan, and artificial oviposition was conducted in the laboratory. Females were placed individually into a Petri dish (φ90 mm) with a piece of wet filter paper as an oviposition substrate for several days without food. Eggs laid on the filter paper were kept in a Petri dish filled with water at room temperature, and most of them hatched 10–11 days after the oviposition. The first instar larvae were moved to large plastic containers (8 cm × 12 cm, height 5 cm) with submerged polypropylene meshes (5 cm × 5 cm, weave size 1 mm) as their perching sites. They were fed on live brine shrimps (Altemia sp.) every day. When they had grown to approximately 3 mm in body length, they were placed individually in plastic bottles (φ3.5 cm, height 5.8 cm) in order to prevent cannibalism. Medium-sized larvae (>5 mm) were fed on live Tubifex spp. as their main food for 3 months until emergence. A twig about 10 cm in length was placed in each bottle as a support for the emergence of the final instar larva.
An individual identification code was marked on the right hind-wing of each newly emerged adult using a fine felt-tipped pen. The sexes were separated in respective flying cages (40 cm × 40 cm × 50 cm) made of wooden frames covered by polypropylene mesh (weave size 1 mm). In order to decrease the risk of cannibalism, fewer than ten adults were reared in each cage. Adults fed on fruit flies, Drosophila spp., which had been cultured. Four to five days after emergence, females showed reproductive activity such as copulation and oviposition, indicating that they achieved sexual maturity (see Takahashi and Watanabe 2010c).
Measurements of body colour
Digital photos were taken of sexually immature (1–3 days old) and mature females (5–9 days old) of each morph that emerged in the laboratory. Within each period, body colour was relatively stable. According to the procedure of Joop et al. (2007), each individual was scanned at a resolution of 300 dpi using an EPSON image scanner (GT-7000U) with the same settings, i.e., the same illuminating condition, and the digital photos obtained were imported into Adobe Photoshop 7.0 (Adobe Systems, USA). An area for measurement was selected on the mesothorax that did not include the black area of the mesonotum. Mean red, green and blue (RGB) values were measured in a square of 200 pixels (10 × 20 pixels). Our data were not transformed to hue–saturation–brightness (HSB) values, because these values are adapted for human eyes (Fleishman and Endler 2000).
Colour should be evaluated based on the property of visual perception of the object (male damselfly, in this case), but the property of the visual perception of this species (or closely related species) has not been clarified. For various species including damselflies (Joop et al. 2007), in which there is no information on the property of the visual perception, RGB analyses have been traditionally used, and are suggested to be qualitatively correct methods to assess colour (Villafuerte and Negro 1998). Although RGB analysis is not the best way, we are convinced that the evaluation of colour using RGB is one of the possible methods to compare the body colour.
Procedure of binary choice experiment
Because males achieve sexual maturity 4–5 days post-emergence (Takahashi and Watanabe, unpublished), binary choice experiments between sexually immature and mature females were conducted for 6- to 9-day-old (sexually mature) males in the laboratory. Since the male’s mate choice behaviour was affected by the activity of females, such as mate refusal behaviour and face-to-face hovering (e.g. Cordero et al. 1998; Cordero Rivera and Andrés 2001), it was necessary to use immotile specimens in the binary choice experiment for testing the effect of female colour on the male mate choice (Takahashi and Watanabe 2009, 2010b). In our experiments, the virgin females of each morph in sexually immature (1–3 days old) and mature stages (5–9 days old) were placed in a freezer for 2 min to inhibit their activity, and dying females were used as the test specimens. Their abdomens were straightened, and their wings were kept closed. Two specimens were pinned with a micro-needle (φ0.18 mm) on the upper frame in the experimental cage (30 × 30 × 30 cm wooden frames covered by polypropylene mesh, weave size 1 mm) which was placed by a window and exposed to the sun. To evaluate the mating preference between sexually immature and mature females, both types of females were pinned next to each other (3 cm apart). The site of each specimen was changed in every trial. Since the body colour of the specimen deteriorates several hours after killing, dying specimens were used within 4 h after the refrigeration. We confirmed that there were no colour changes in the dying specimens.
To investigate an innate mating preference, a binary choice experiment was conducted using a virgin male that was reared separately from females after emergence. In the morning (0700–0900 hours), the male was introduced into an experimental cage in which a pair consisting of a sexually immature gynomorph and a sexually mature gynomorph, or a sexually immature andromorph and a sexually mature andromorph, was presented. In each binary choice experiment, every male attacked the dying specimen presented immediately upon being introduced into the experimental cage. Although males normally attack a female from behind, form a tandem, and then copulate with her in the natural condition, each binary choice experiment was stopped when the male attacked one specimen, i.e., it dashed to a female and tried to form a tandem with it. The males’ attacking behaviour in the direction of a specimen was judged as an indicator of the male choice.
In order to determine the effect of the copulation experience of the male on its ability to discriminate between sexually immature and mature females, a single virgin male was introduced into the experimental cage with a new virgin sexually mature female, either a gynomorph or an andromorph, in the morning. All virgin males enclosed with a single virgin female in the morning repeatedly tried to attack it, and each female accepted the males after several mating attempts. All copulation lasted for about 3–4 h, and terminated around noon. Just after the removal of the coexisting female at noon, the binary choice experiment between a sexually immature and a sexually mature gynomorph or between a sexually immature and a sexually mature andromorph was conducted for the males. The specimens in the binary choice experiments were newly prepared from the rearing cage. In the experiment, each male attacked the dying specimen immediately upon being introduced into the cage.
Statistical analyses
All statistical analyses were performed using R version 2.9.0 (R Development Core Team 2009). All values are presented as mean ± SE. Differences in RGB value between sexually immature and mature individuals were analyzed by the Mann–Whitney U test. Before the U test, we confirmed the plausibility of homoscedasticity by performing F tests at the probability of p > 0.05. The outcomes of the binary choice experiments were analyzed by performing binominal tests. The α-level in binomial tests was adjusted by Bonferroni correction to account for multiple comparisons (p = 0.05/2 = 0.025). Changes in mating preference of males (difference in the proportion of males that chose each presented female between virgin and experienced males) were analyzed by Fisher’s exact test. All statistical tests were two-tailed.
Results
Changes in body colour
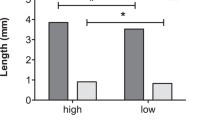
The thoracic colour of gynomorphs changed from light orange in immature to brown in mature stages. As shown in Fig. 1a, the content of each colour differed between maturation stages (Mann–Whitney U test: red: U = 2.0, n 1 = 27, n 2 = 31, p < 0.001; green: U = 85.0, n 1 = 27, n 2 = 31, p < 0.001; blue: U = 102.0, n 1 = 27, n 2 = 31, p < 0.001), indicating ontogenetic colour changes in gynomorphs. On the other hand, the thoracic colour of andromorphs was blue throughout their lives. No significant difference between sexually immature and mature individuals was found in any of the colour contents (Mann–Whitney U test: red: U = 372.0, n 1 = 34, n 2 = 24, p = 0.570; green: U = 357.0, n 1 = 34, n 2 = 24, p = 0.421; blue: U = 321.0, n 1 = 34, n 2 = 24, p = 0.170; Fig. 1b). Thus, little ontogenetic colour change occurred in andromorphs.
Ontogenetic changes in the brightness of red, green and blue in the thoraxes of gynomorphs (a) and andromorphs (b) of Ischunra senegalensis. The sample size of sexually immature gynomorphs, sexually mature gynomorphs, sexually immature andromorphs and sexually mature andromorphs were 27, 32, 34 and 24, respectively. Each asterisk indicates a significant difference between sexually immature and mature females at a probability of less than 0.001 (Mann–Whitney U test)
Mating preference
The males grasped and tried to form a tandem with the specimen. Some males hovered around the paired specimens just before they attacked them. Such behaviour suggested that the males recognized the paired specimens simultaneously and that they were able to freely choose one of the specimens in this experiment.
In the binary choice experiment between sexually immature and sexually mature females, each virgin male immediately attempted to mate with either specimen. Virgin males exhibited no preference between the sexually immature and mature gynomorphs (Table 1), suggesting that they cannot discriminate reproductive stages of gynomorphs. On the other hand, males that had copulated with gynomorphs significantly preferred sexually mature gynomorphs over sexually immature ones (Table 1). There was a significant difference in mating preference between virgin and experienced males (Fisher’s exact test, p = 0.032; Table 1). These results indicate that males improve their ability to discriminate between sexually immature and sexually mature gynomorphs throughout their mating experience.
In the binary choice experiments using andromorphs, virgin males exhibited no preference between the sexually immature and mature andromorphs (Table 2). The proportion of males choosing sexually mature females did not differ between the tests using andromorphs and gynomorphs (Fisher’ exact test, p = 1.0). This indicates that virgin males cannot discriminate reproductive stages of both morphs. Males that had experienced with andromorphs also showed a non-significant preference between sexually immature and sexually mature andromorphs (Table 2). In contrast to the case of gynomorphs, no significant difference in mating preference was found between virgin and experienced males (Fisher’s exact test, p = 0.595), though the proportion of experienced males that chose sexually mature females slightly increased. These results indicate that the effect of maturation in andromorphs on male mate choice is very week, and that mating experience with andromorphs did not improve the males’ ability to discriminate between immature and mature andromorphs.
Although the proportion of experienced males choosing sexually mature females in the test using gynomorphs (85.2%) was overwhelmingly higher than that in the test using andromorphs (66.7%), no statistically significant difference was found between these tests (Fisher’s exact test, p = 0.202). This might be due to a slight increase in the proportion of males that chose sexually mature females in the case of males experienced with andromorphs (Table 2).
Discussion
Because ontogenetic colour changes in accordance with sexual maturation are common in odonate species, body colour is one of the indicators of female sexual maturation (Corbet 1999). Cordero (1990) reported that in I. graellsii the thoracic colour of both female morphs changed, and they sexually matured in a week. However, in the present study, changes in thoracic colour in I. senegalensis were observed in gynomorphs but not in andromorphs (Fig. 1), suggesting that the thoracic colour in andromorphs does not correspond to sexual maturation. Gorb (1998) pointed out that thoracic colour is the most important cue for males in mate recognition in damselflies. Thus, it might be difficult for I. senegalensis males to discriminate between sexually immature and sexually mature andromorphs. In support of this prediction, males discriminated the maturation stages only of gynomorphs (Table 1). This implies that males discriminated reproductive status of females on the basis of their thoracic colour.
In coenagrionid damselflies, virgin males showed a no mating preference between species (Fincke et al. 2007), between female colour morphs, and between sexes (Takahashi and Watanabe 2010b). In the present study, males also showed non-significant preference between sexually immature and mature females in I. senegalensis. This indicates that virgin males were unable to distinguish sexually mature females from sexually immature females. Although the mate preferences of virgin males were slightly biased to mature females in both morphs, the effect of female maturity on the mating preference of the virgin males is too weak to be detected with our current sample size.
On the other hand, males experienced copulation with gynomorphs preferred sexually mature gynomorphs over immature ones. Males changed their mating preference in accordance with their copulation experience. In the field, males could avoid attempting to mate with sexually immature gynomorphs by forming a search image of sexually mature gynomorphs. Because mating attempts with sexually immature females are probably costly in terms of time wasted (Stoks and De Bruyn 1998), the search image established on the basis of copulation experience may help males to detect potential mates, and consequently to increase their mating success. Such mate choice is advantageous in species where sexually immature and mature females coexist at the breeding site, as in I. senegalensis.
Although the proportion of males that chose sexually mature females slightly increased, males experienced copulation with andromorphs showed no obvious preference between sexually immature and mature andromorphs. This is due to the absence of a significant ontogenetic change in thoracic colour in andromorphs (Fig. 1). These results imply that males were able to discriminate the reproductive status of gynomorphs on the basis of their thoracic colour. Although we can identify the age of andromorphs on the basis of the colour of the ventral side of the abdomen in I. senegalensis (Takahashi and Watanabe 2009), the colour of the ventral side of the abdomen may not be important for males as a cue for recognizing females, because they typically attempt to mate with females from the side or from behind (Gorb 1999). Therefore, if males are not able to learn to discriminate sexually immature and mature andromorphs in the field, the males might continue to attempt to mate with sexually immature andromorphs as well as sexually mature andromorphs in natural populations throughout their lives. In the present study, no significant effect of female maturation on mate choice of males experienced with an andromorph was detected due to the small sample size, but the proportion of males that chose sexually mature andromorph after experienced copulation with an andromorph slightly increased. This suggests that factors other than body colour, such as the degree of wing transparency, also help males to discriminate female reproductive stages.
Although experienced males were apt to avoid mating with sexually immature gynomorphs, sexually immature andromorphs may not be recognized by males as unsuitable individuals with which to mate. In the stickleback Culaea inconstains, males preferred females exhibiting a nuptial colour over females not exhibiting a nuptial colour. Thus, immature-specific body colour functioned to reduce the frequency of male mating attempts during pre-reproductive stages (Amundsen and Forsgren 2001). Because persistent pre-copulatory mating attempts, i.e., male harassment, are costly for females, they may often evolve various counter-strategies to avoid mating attempts (Arnqvist and Rowe 2005). In damselflies, male harassment strongly influences female fitness (Takahashi and Watanabe 2010a). Therefore, immature specific colour in gynomorphs may have evolved as a strategy by which females avoid male harassment during the pre-reproductive period. Takahashi et al. (unpublished) indicated that food intake in andromorphs is less than that in gynomorphs during their pre-reproductive period. In Odonata, ontogenetic colour changes frequently occur in Ischnura spp. (Fincke et al. 2005), where sexually immature and mature females coexist at the same site (Hinnekint 1987). These facts also suggest that immature specific colour may be a counter strategy against male mating harassment. The learned mate choice of males based on copulation experience may drive the evolution of age-specific colour in females.
The maintenance of multiple female morphs in damselflies has usually been explained in the context of sexual conflict. The mating harassment of sexually mature females is an important selection pressure (e.g. Svensson et al. 2005; Takahashi et al. 2010). However, it is an indisputable fact that immature females repeatedly suffer male mating attempts in the wild (Hammers et al. 2009). Because sexually immature andromorphs are predicted to be subject to more mating attempts by males compared with sexually immature gynomorphs due to male mate choice behaviour, each morph must pay a different cost during its sexually immature stage. Pre-copulatory mating attempts by males, i.e., male harassment, decrease the food intake of females and thus cause their egg production to decline (Takahashi and Watanabe 2010a). Therefore, non-random attempts to mate with sexually immature females must affect the fitness of each female morph, and potentially contribute to the maintenance of female polymorphism.
References
Amundsen T (2000) Why are female birds ornamented? Trends Ecol Evol 15:149–155
Amundsen T, Forsgren E (2001) Male mate choice selects for female coloration in fish. Proc Natl Acad Sci USA 98:13155–13160
Andersson M (1994) Sexual selection. Princeton University Press, New Jersey
Andersson J, Borg-Karlson AK, Wiklund C (2000) Sexual cooperation and conflict in butterflies: a male-transferred anti-aphrodisiac reduces harassment of recently mated females. Proc R Soc Lond B 267:1271–1275
Arnqvist G, Rowe L (2005) Sexual conflict. Princeton University Press, New Jersey
Bateman PW, Fleming PA (2006) Males are selective too: mating, but not courtship, with sequential females influences choosiness in male field crickets (Gryllus bimaculatus). Behav Ecol Sociobiol 59:577–581
Boggs CL (1995) Male nuptial gifts: phenotypic consequences and evolutionary implications. In: Leather SR, Hardie J (eds) Insect reproduction. CRC Press, New York, pp 215–242
Bonduriansky R (2001) The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol Rev 76:305–339
Bussière LF, Hunt JH, Stölting KN, Jennions MD, Brooks R (2008) Mate choice for genetic quality when environments vary: suggestions for empirical progress. Genetica 134:69–78
Clutton-Brock TH (2007) Sexual selection in males and females. Science 318:1882–1885
Clutton-Brock T (2009) Sexual selection in females. Anim Behav 77:3–11
Cooper G, Holland PWH, Miller PL (1996) Captive breeding of Ischnura elegans (Vander Linden): observations on longevity, copulation and oviposition (Zygoptera: Coenagrionidae). Odonatologica 25:261–273
Corbet PS (1999) Dragonflies: behaviour and ecology of Odonata. Harley, Colchester
Cordero A (1990) The inheritance of female polymorphism in the damselfly Ischnura graellsii (Rambur) (Odonata: Coenagrionidae). Heredity 64:341–346
Cordero Rivera A, Andrés J (2001) Estimating female morph frequencies and male mate preferences of polychromatic damselflies: a cautionary note. Anim Behav 61:F1–F6. doi:10.1006/anbe.2000.1572
Cordero A, Santolamazza Carbone S, Utzeri C (1998) Mating opportunities and mating costs are reduced in androchrome female damselflies, Ischnura elegans (Odonata). Anim Behav 55:185–197
Fincke OM (1987) Female monogamy in the damselfly Ischnura verticalis Say (Zygoptera: Coenagrionidae). Odonatologica 26:129–143
Fincke OM, Jödicke R, Paulson D, Schultz DT (2005) The evolution and frequency of female color morphs in Holarctic Odonata: why are male-like morphs typically the minority? Int J Odonatol 8:183–212
Fincke OM, Fargevieille A, Schultz TD (2007) Lack of innate preference for morph and species identity in mate-searching Enallagma damselflies. Behav Ecol Sociobiol 61:1121–1131
Fleishman LJ, Endler JA (2000) Some comments on visual perception and the use of video playback in animal behaviour studies. Acta Ethol 3:15–27
Gorb SN (1998) Visual cues in mate recognition by males of the damselfly, Coenagrion puella (L.) (Odonata: Coenagrionidae). J Insect Behav 11:73–92
Gorb SN (1999) Visual cues in mate recognition in the damselfly Ischnura elegans (Zygoptera: Coenagrionidae). Intl J Odonatol 2:83–93
Gosden TP, Svensson EI (2009) Density-dependent male mating harassment, female resistance and male mimicry. Am Nat 173:709–721
Hammers M, Sánchez-Guillén RA, Van Gossum H (2009) Differences in mating propensity between immature female color morphs in the damselfly Ischnura elegans (Insecta: Odonata). J Insect Behav 22:324–337
Hansen LTT, Amundsen T, Forsgren E (1999) Symmetry: attractive not only to females. Proc R Soc Lond B 266:1235–1240
Hinnekint B (1987) Population dynamics of Ischnura e. elegans (Vander Linden) (Insecta: Odonata) with special reference to morphological colour changes, female polymorphism, multiannual cycles and their influence on behaviour. Hydrobiology 146:3–31
Johnson C (1964) The inheritance of female dimorphism in the damselfly, Ischnura damula. Genetics 49:513–519
Johnson C (1966) Genetics of female dimorphism in Ischnura demorsa. Heredity 21:453–459
Jones KM, Monaghan P, Nager RG (2001) Male mate choice and female fecundity in zebra finches. Anim Behav 62:1021–1026
Joop G, Gillen A, Mikolajewski DJ (2007) Colour polymorphism in female Coenagrion puella: differences in egg shape (Odonata: Coenagrionidae). Intl J Odonatol 10:71–80
Kokko H, Johnstone RA (2002) Why is mutual mate choice not the norm? Operational sex ratios, sex roles and the evolution of sexually dimorphic and monomorphic signalling. Philos Trans R Soc Lond B 357:319–330
McLennan DA (1995) Male mate choice based upon female nuptial coloration in the brook stickleback, Culaea inconstans (Kirtland). Anim Behav 50:213–221
R Development Core Team (2009) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.Rproject.org
Robertson HM (1985) Female dimorphism and mating behaviour in a damselfly, Ischnura ramburi: females mimicking males. Anim Behav 33:805–809
Rutowski R (1982) Epigamic selection as evidenced by courtship partner preferences in males of the checkered white butterfly, Pieris protodice. Anim Behav 30:108–112
Stoks R, De Bruyn L (1998) Unusual reproductive associations of Ischnura elegans. Notulae Odonatol 5:3–7
Svensson EI, Abbott J, Härdling R (2005) Female polymorphism, frequency dependence, and rapid evolutionary dynamics in natural populations. Am Nat 165:567–576
Takahashi Y, Watanabe M (2008) Male mate preference depending on the mating experience in the damselfly, Ischnura senegalensis (Rambur) (Odonata: Coenagrionidae). Jpn J Entomol 11:13–17 (In Japanese with English summary)
Takahashi Y, Watanabe M (2009) Diurnal changes and frequency dependence in male mating preference for female morphs in the damselfly Ischnura senegalensis (Rambur) (Odonata: Coenagrionidae). Entomol Sci 12:219–226
Takahashi Y, Watanabe M (2010a) Female reproductive success is affected by selective male harassment in the damselfly Ischnura senegalensis. Anim Behav 79:211–216
Takahashi Y, Watanabe M (2010b) Mating experience affecting male discrimination between sexes and female morphs in the damselfly, Ischnura senegalensis (Rambur) (Odonata: Coenagrionidae). Odonatologica 39:47–56
Takahashi Y, Watanabe M (2010c) Morph-specific fecundity and egg size in the female-dimorphic damselfly Ischnura senegalensis. Zool Sci 27:325–329
Takahashi Y, Yoshimura J, Morita S, Watanabe M (2010) Negative frequency-dependent selection in female color polymorphism of a damselfly. Evolution 64:3620–3628
Trivers RL (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man, 1871–1971. Aldine, Chicago, pp 136–179
Van Gossum H, Stoks R, De Bruyn L (2001a) Discriminative mate choice in relation with female maturation in Ischnura elegans (Odonata: Coenagrionidae). Intl J Odonatol 4:83–91
Van Gossum H, Stoks R, De Bruyn L (2001b) Reversible frequency-dependent switches in male mate choice. Proc R Soc Lond B 268:83–85
Villafuerte R, Negro JJ (1998) Digital imaging for color measurement in ecological research. Ecol Lett 1:151–154
Acknowledgments
This study was partially supported by a Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists (20-104) to Y.T.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Takahashi, Y., Watanabe, M. Male mate choice based on ontogenetic colour changes of females in the damselfly Ischnura senegalensis . J Ethol 29, 293–299 (2011). https://doi.org/10.1007/s10164-010-0257-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-010-0257-6