Abstract
There is growing evidence of correlated behaviours across time, situations and/or contexts (behavioural syndromes) in animals, and their link with fitness makes these studies mandatory to understanding the species’ behavioural and evolutionary ecology. Whereas the role of the social environment on behavioural syndromes is receiving increasing attention, experimental studies testing whether environmental fluctuations govern sex-dependent behavioural syndromes are scarce. I performed an experiment to test for the existence of sex differences in activity syndromes through a changing social environment. Males and females of Bosca’s newt (Lissotriton boscai) were faced with three social situations perceived through chemical cues (own odour, no odour and same-sex conspecific odours) to measure their activity levels. Comparisons of the activity levels showed that both males and females discriminated the odourless stimuli from newt odour (either their own or the conspecific stimuli). Whereas activity levels were positively correlated between their own and the odourless stimuli in both sexes, the association between the odourless and conspecific stimuli was positive in males but decoupled in females. This is the first experimental evidence of sex differences in activity syndromes of wild-caught animals in response to social changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The study of behavioural syndromes in animals, suites of correlated behaviours across time and/or contexts, has been receiving an increasing attention in recent years (Sih et al. 2004a). The importance of the study of behavioural syndromes, personalities, copying styles, and temperaments in animals is rooted in their link with fitness components, and therefore with our understanding of the evolution, ecology, and conservation of species (Sih et al. 2004a, b; Réale et al. 2007; Smith and Blumstein 2008). Behavioural correlations have been documented in a variety of taxa in some behavioural traits but not in others, posing interesting questions on how such patterns have generated or decoupled, or why not.
Most studies of behavioural syndromes have focused on the repeatability of mating behaviours in birds (Bell et al. 2009), and attention to the role of the social environment on behavioural correlations has been paid only recently (Rohr and Madison 2003; Aragón et al. 2006a; Cote and Clobert 2007; Magnhagen and Bunnefeld 2009). Furthermore, sex differences in behavioural repeatability is relatively unexplored and mainly focused on a reproductive context in birds (Bell et al. 2009). There is only one study explicitly designed to test for sex differences in behavioural correlations regarding the social environment in birds (Schuett and Dall 2009). It has been demonstrated that environmental factors can play an important role in how behavioural syndromes are originated (Bell and Sih 2007), and it has been known that a variety of selective forces can shape male and female traits differently across taxa (Bernal et al. 2007; Clutton-Brock 2007; Van Damme et al. 2008). In concordance, previous findings suggest that environmental fluctuations might be responsible for antagonistic selection regarding sex on a bird personality trait (Dingemanse et al. 2004).
It is known that several species of newts can assess the social environment through the presence of conspecific chemical cues and respond accordingly to the perceived potential intraspecific competition and/or mating opportunities (Verrell 1983; Rohr et al. 2005; Aragón 2009a). Also, sex differences in response to potential changes in predation risk assessed from conspecific chemical cues have been previously documented in newts (Rohr et al. 2002). Moreover, the existence of behavioural syndromes in urodelians has been documented (Rohr and Madison 2003; Sih et al. 2004a). Individuals of the Bosca’s newt (Lissotriton boscai) are able to discriminate the social environment through chemical cues (Aragón et al. 2000; Aragón 2009a), and males and females react differently to social information from same-sex conspecifics (Aragón 2009b). Taking this background into account, the aim of this study was to examine whether males and females of the Bosca’s newt differ in behavioural syndromes with regard to the social environment assessed through chemical cues from same-sex individuals. I examined a simple case within behavioural syndromes (Sih and Bell 2008), the existence of correlations of the same behavioural trait measured in different social situations.
Materials and methods
Study species
Lissotriton boscai is a small newt endemic to the Iberian Peninsula. It inhabits shallow streams of slow-running clear water with aquatic vegetation, and is one of the most aquatic newts (Montori and Herrero 2004; Brea et al. 2007). Active adults can be observed in the water all year except for mid-summer when they become terrestrial for estivation, and the breeding period encompasses most of the aquatic phase (Caetano 1982). Sexual dimorphism in this species is less evident than in other newt species, which appears to be a consequence of divergent selective pressures (Aragón 2009b; Montori and Herrero 2004). Male–male competition by sexual interference is intense in L. boscai (Mouta-Faria 1995) whereas competition for food among females is higher than among males (Aragón 2009b).
Housing
Newts were captured in October–November 2009, at the beginning of the breeding period (Brea et al. 2007). I collected 30 adults (15 males and 15 females) from a stream located in Navia (Asturias province, northern Spain). Newts were transferred 2.5 km from the capture site and were individually housed in aquaria (20 × 30 cm) containing water (10 cm of depth). After gently drying individuals, I weighed them to the nearest 0.01 g (males: mean ± SE = 1.49 ± 0.04 g; females: mean ± SE = 2.04 ± 0.054 g) and measured their lengths to the nearest 0.5 mm (males: mean ± SE = 71.06 ± 1.13 mm; females: mean ± SE = 78.33 ± 1.02 mm). Newts were fed once a day by placing a piece of Lumbricus of similar size in the front of the newts’ snout, which was gulped down within a few seconds.
Behavioural assays
I performed an experiment to test whether newts responded differently to different social environments assessed through chemical cues, and whether behavioural correlations across social situations differ between sexes. Each individual responded to three treatments in a repeated measures design (Quinn and Keough 2002), and in a randomised and balanced order of presentation to avoid an order effect (Réale et al. 2007). Newts were held with the same water for 5 days before using odours in trials, which is sufficient time to allow the detection and discrimination of conspecific chemical stimuli contained in water (Aragón et al. 2000; Aragón 2009a). For each trial, the focal individual was transferred to an experimental aquarium (20 × 30 cm) and confined to an opaque cylinder (8 cm in diameter) in the middle of the aquarium during 1 min for habituation. Cylinders served as shelters where all newts remained at rest inside ensuring the same initial conditions through treatments. The time that newts spent active (mainly displacing through the aquaria, but also ascending to breathe or moving any part of their body) was recorded in experimental aquaria containing water (400 cl) with their own chemical signals, water without newt odour, and water with odour from other same-sex conspecific. All individuals were handled in the same way through treatments. For the conspecific odour treatment, focal individuals and their corresponding odour donors were size matched so that they were within 2 mm of the same size. To begin a trial, the opaque cylinder was gently removed, allowing the focal individual to move freely through the experimental aquarium for 10 min. I performed 90 trials [(15 males + 15 females) × 3 treatments] in the morning when individuals were fully active. No newt was tested more than once per 4 days (Aragón 2009b). The experiment was performed with water at 14°C, which was within the temperature range measured in their stream (13–14.5°C), and within the range observed during the entire aquatic phase of this species (Mouta-Faria 1995). All trials were recorded with a camcorder aligned perpendicularly to the experimental aquarium. After the experiment, newts were returned healthy to their capture point.
Statistical analyses
Treatment effect
To test for a differential response to the treatments, data were analysed with a repeated measures ANOVA with treatments as the within-subject factor, sex as the between-subject factor, and the time spent active as the response variable. The interaction between factors was included in the analyses to test whether the response to different treatments was dependent on sex. I then performed planned comparisons for each pairwise test (Quinn and Keough 2002), and significance was verified with the sequential Bonferroni adjustment of Rice (1989).
Behavioural syndromes
Whereas pairs of treatments that do not differ significantly might be considered as analogous situations, those significantly different can be considered as different social situations, and therefore suitable for examining behavioural syndromes regarding changes in the social environment. To test for behavioural correlations through a changing social environment, I performed ANCOVAs with the time spent active in one treatment as the response variable, sex as the between-subject factor, and the other treatment as a covariable. The interaction between the sex and the covariable was included to test for sex differences in the slopes of the regressions of the response variable on the covariable (Quinn and Keough 2002). In case of a significant interaction, I performed Pearson correlations between the response variable and the covariable for males and females separately. Normality was verified with Kolmogorov–Smirnov tests and homocedasticity across sexes and/or treatments were tested with Levene’s tests (Quinn and Keough 2002). Data were analysed with the software STATISTICA 7.0 (StatSoft 2004).
Results
There was a significant treatment effect in the time that newts were active (repeated measures ANOVA: F 1,56 = 4.83, P = 0.011; Fig. 1), and the interaction between sex and treatment was not significant (F 1,56 = 1.0430, P > 0.35). The activity level was significantly higher in the odourless water than in the conspecific water (F 1,28 = 10.82, P = 0.002) and than in their own odour (F 1,28 = 5.81, P = 0.022), whereas there were no significant differences between their own odour and the conspecific odour (F 1,28 = 0.82, P = 0.372). Variances did not differ significantly between sexes in any treatment nor across treatments within sexes (Levene’s tests: 0.23 < P < 0.92).
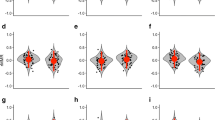
The activity level in the odourless water was significantly associated with that of their own odour (ANCOVA: main effect, F 1,26 = 18.14, P < 0.001; Fig. 2a), and there was no significant effect of the interaction (F 1,26 = 0.001, P > 0.97). The activity level in the conspecific water was significantly associated with that of the odourless treatment (ANCOVA: main effect, F 1,26 = 7.27, P = 0.01), and the interaction was significant (F 1,26 = 5.81, P = 0.02; Fig. 2b). Pearson correlations revealed a significant positive association between the conspecific odour and odourless treatments in males (r = 0.77, F 1,13 = 19.11, P < 0.001), but not in females (r = 0.05, F 1,13 = 0.03, P > 0.84).
Relationships between the time that Lissotriton boscai newts spent active in aquaria with no odour and a that with their own odour, b that with odour from other same-sex conspecifics during periods of 10 min. Solid lines are for males (a: y = 87.31 + 0.50x, b: y = 73.72 + 0.66x), dashed lines for females (a: y = 60.55 + 0.49x, b: y = 109.17 + 0.05x), and dotted lines represent those in which x equals y
Discussion
In this experiment, newts showed the lowest mean activity level in the presence of conspecific odours, the highest in the odourless water, and it was intermediate in the presence of their own odour. Individuals discriminated between the odourless water from their own or conspecific stimuli, but not between their own and conspecific stimuli. Similarly, another experiment with L. boscai males revealed that individuals avoid ponds with no conspecific odour while selecting those with their own or conspecific stimuli in similar proportions (Aragón et al. 2000). Consistently, other studies did not provide evidence for site defence or territoriality for the genus Lissotriton (Verrell and McCabe 1988), including L. boscai (Aragón 2009a). Even territorial species of salamanders out of the breeding period may be attracted to conspecific chemical cues when the other choice is a substrate with no odour (Verrell and Davis 2003). Previous studies with vertebrates showing conspecific attraction have suggested that individuals might assess site quality through conspecific chemical cues (Hurst et al. 1996; Luque-Larena et al. 2001; Aragón et al. 2006b). In this way, juvenile red-spotted newts, Notophthalmus viridescens, are attracted to chemical cues from same-age individuals to mitigate effects of environmental stress, and they also show higher activity levels in the treatment without newt odour than in the conspecific odour treatment (Rohr and Madison 2003). Furthermore, the activity of juvenile newts was positively correlated across treatments of moisture fluctuations, although the sex of individuals were unknown (Rohr and Madison 2003).
The relationship between the responses to the odourless stimulus and those to their own stimulus showed high repeatability in activity, at least in the short-term, independently of sex. On the other hand, the largest difference between treatments was detected between the odourless and the conspecific odour treatments, indicating unequivocally that these treatments represent two different situations for newts, which is a requirement to elucidate patterns of behavioural syndromes regarding changes in the social environment. Interestingly, in this case, a linear model showed a significant interaction effect between sex and the association through treatments, revealing sex differences in the relationship between social (conspecific odours) and asocial situations (odourless water). In fact, there was a strong positive association only in the case of males, whereas the correlation was decoupled in females. This is not a by-product of variance differences since they were homogeneous between sexes in all treatments and within sexes across treatments. In the same way, the sex differences obtained here cannot be attributed to differences in sample sizes as the number of males and females was equal.
It has been shown that the social environment may differently modulate the expression of behavioural syndromes for different behavioural types in perches, such as bolder individuals changed their behaviour less between social and asocial situations than did shyer ones (Magnhagen and Bunnefeld 2009). Regarding sex differences in repeatability, studies on parental care in house sparrows revealed males to be more consistent than females (Schwagmeyer and Mock 2003; Nakagawa et al. 2007). In addition, a meta-analysis showed that adult males were more repeatable regarding mate preference than females (Bell et al. 2009). In agreement to the present study, zebra finches in a foraging context showed that males were not more exploratory but behaved more consistently across social and asocial situations than females. This finding holds despite individuals of both sexes influencing each other’s exploratory behaviour within a social situation where the stimuli were opposite-sex companions (Schuett and Dall 2009). In the present study, sex-dependent behavioural syndromes were modulated by a social situation where the stimuli were chemical signals from same-sex conspecifics but in the absence of the signallers, and therefore independently of the conspecific behaviour. Considering a cost–benefit scenario, several ultimate mechanisms have been proposed for differences in correlated behaviours, such as the net benefits of specialisation, the net benefits of consistency per se, and the social net benefits of predictability (Sih and Bell 2008). Although the scope of the present study does not elucidate which mechanisms are involved, it is likely that newts were faced with a treatment manipulation that generated sex differences in the cost–benefit ratio revealing in this way sex differences in behavioural syndromes.
It has been known that the patterns of behavioural correlations can vary among populations suggesting that differences in environmental factors can modulate these correlations (Bell 2005; Dingemanse et al. 2007). For instance, exposure of three-spined sticklebacks to predation risk variations can generate behavioural syndromes produced by both selection by predators and behavioural plasticity (Bell and Sih 2007). Interestingly, it has been shown that annual adult survival of great tits was related to a personality trait (exploration), that this relationship had opposite trends for males and females, and that these trends were reversed by environmental fluctuations (Dingemanse et al. 2004). Further studies are needed to examine whether sex differences in behavioural syndromes across social situations are generated by selection, plasticity or both.
References
Aragón P (2009a) Conspecific male chemical cues influence courtship behaviour in the male newt Lissotriton boscai. Behaviour 146:1137–1151
Aragón P (2009b) Sex-dependent use of information on conspecific feeding activities in an amphibian urodelian. Funct Ecol 23:380–388
Aragón P, López P, Martín J (2000) Conspecific chemical cues influence pond selection of male newts Triturus boscai. Copeia 2000:874–878
Aragón P, Meylan S, Clobert J (2006a) Dispersal status-dependent response to the social environment in the common lizard, Lacerta vivipara. Funct Ecol 20:900–907
Aragón P, Massot M, Gasparini J, Clobert J (2006b) Socially acquired information through chemical cues in the common lizard, Lacerta vivipara. Anim Behav 72:965–974
Bell AM (2005) Behavioral differences between individuals and two populations of stickleback (Gasterosteus aculeatus). J Evol Biol 18:464–473
Bell AM, Sih A (2007) Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus). Ecol Lett 10:828–834
Bell AM, Hankison SJ, Laskowski KL (2009) The repeatability of behaviour: a meta-analysis. Anim Behav 77:771–783
Bernal XE, Rand AS, Ryan MJ (2007) Sexual differences in the behavioral response of túngara frogs, Physalaemus pustulosus, to cues associated with increased predation risk. Ethology 113:755–763
Brea C, Galán P, Ferreiro R, Serantes P (2007) Preliminary data about reproductive biology of Bosca’s newt (Lissotriton boscai) and palmate newt (Lissotriton helveticus) under controlled and natural conditions. Munibe 25:170–179
Caetano MH (1982) Variabilité sexuelle de Triturus boscai (Lataste, 1879) dans le Parc National de Peneda-Gerês (Portugal). Amphib Reptil 3:99–109
Clutton-Brock TH (2007) Sexual selection in males and females. Science 318:1882–1885
Cote J, Clobert J (2007) Social personalities influence natal dispersal in a lizard. Proc R Soc Lond B 274:383–390
Dingemanse NJ, Both C, Drent PJ, Tinbergen JM (2004) Fitness consequences of avian personalities in a fluctuating environment. Proc R Soc Lond B 271:847–852
Dingemanse NJ, Wright J, Kazem AJN, Thomas DK, Hickling R, Dawnay N (2007) Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J Anim Ecol 76:1128–1138
Hurst JL, Hall S, Roberts R, Christian C (1996) Social organization in the aboriginal house mouse, Mus spretus Lataste: behavioural mechanisms underlying the spatial dispersion of competitors. Anim Behav 51:327–344
Luque-Larena JJ, López P, Gosalvez J (2001) Scent matching modulates space use and agonistic behaviour between male snow voles Chionomys nivalis. Anim Behav 62:1089–1095
Magnhagen C, Bunnefeld N (2009) Express your personality or go along with the group: what determines the behaviour of shoaling perch? Proc R Soc Lond B 276:3369–3375
Montori A, Herrero P (2004) Amphibia, Lissamphibia. In: Ramos MA et al (eds) Fauna Ibérica. Museo Nacional de Ciencias Naturales, CSIC, Madrid, pp 43–274
Mouta-Faria M (1995) A field study of reproductive interactions in Bosca’s newt, Triturus boscai. Amphib Reptil 16:357–374
Nakagawa S, Gillespie DOS, Hatchwell BJ, Burke T (2007) Predictable males and unpredictable females: sex difference in repeatability of parental care in a wild bird population. J Evol Biol 20:1674–1681
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ (2007) Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318
Rice WR (1989) Analysing tables of statistical test. Evolution 43:223–225
Rohr JR, Madison DM (2003) Dryness increases predation risk in efts: support for an amphibian decline hypothesis. Oecologia 135:657–664
Rohr JR, Madison DM, Sullivan AM (2002) Sex differences and seasonal trade-offs in response to injured and non-injured conspecifics in red-spotted newts, Notophthalmus viridescens. Behav Ecol Sociobiol 52:385–393
Rohr JR, Park D, Sullivan AM, McKenna M, Propper CR, Madison DM (2005) Operational sex ratio in newts: field responses and characterization of a constituent chemical cue. Behav Ecol 16:286–293
Schuett W, Dall SRX (2009) Sex differences, social context and personality in zebra finches, Taeniopygia guttata. Anim Behav 77:1041–1050
Schwagmeyer PL, Mock DW (2003) How consistently are good parents good parents? Repeatability of parental care in the house sparrow, Passer domesticus. Ethology 109:303–313
Sih A, Bell AM (2008) Insights for behavioral ecology from behavioral syndromes. Adv Study Behav 38:227–281
Sih A, Bell AM, Johnson JC, Ziemba RE (2004a) Behavioral syndromes: an integrative overview. Q Rev Biol 79:241–277
Sih A, Bell AM, Johnson JC (2004b) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378
Smith BR, Blumstein DT (2008) Fitness consequences of personality: a meta-analysis. Behav Ecol 19:448–455
StatSoft, Inc. (2004) STATISTICA. Data analysis software system, Version 7. http://www.statsoft.com
Van Damme R, Entin P, Vanhooydonck B, Herrel A (2008) Causes of sexual dimorphism in performance traits: a comparative approach. Evol Ecol Res 10:229–250
Verrell P (1983) The influence of the ambient sex ratio and intermale competition on the sexual behaviour of the red-spotted newt, Notophthalmus viridescens (Amphibia: Urodela: Salamandridae). Behav Ecol Sociobiol 13:307–313
Verrell PA, Davis K (2003) Do non-breeding, adult long-toed salamanders respond to conspecifics as friends or as foes? Herpetologica 59:1–7
Verrell PA, McCabe N (1988) Field observations of the sexual behaviour of the smooth newt, Triturus vulgaris vulgaris (Amphibia: Salamandridae). J Zool 214:533–545
Acknowledgments
I thank two anonymous reviewers for their helpful comments, and the “Cal Moreno” Field Station for the use of their facilities, and B. Carrera and A. Martínez for technical support. The experiment was performed with a license from the Consegería de Medio Ambiente, Ordenación del Territorio e Infraestructuras del Gobierno del Principado de Asturias (Spain).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Aragón, P. The response to the social environment reveals sex-dependent behavioural syndromes in the Bosca’s newt (Lissotriton boscai). J Ethol 29, 79–83 (2011). https://doi.org/10.1007/s10164-010-0224-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-010-0224-2