Abstract
The inadequate treatment of waste tire is currently a major environmental and economical issue. In this study, the effective solubilization of waste tire into diethyl ether was attained by the thermal treatment in organic solvent at 200–300 °C. Not only aromatic, but also aliphatic alcohols were effective on the solubilization. Among aliphatic alcohols, 1-heptanol was the most effective solvent on the solubilization and reached near 70% in the thermal treatment at 200 °C under inert atmosphere. Model compound study containing sulfur–sulfur bond suggests the disulfide–alcohol exchange reaction leading devulcanization of the waste tire.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The amount of waste tire increase in 2018 was 1 million tons in weight in Japan [1] and increases globally [2]. Recycling of the waste tire [3] is worldwide of growing importance due to increasing raw material costs, diminishing resources, and growing awareness of environmental issues and sustainability [4].
The main recycling methods of waste tires were classified into burning [5] for energy recovery [4], pyrolytic conversions to gas [6], oil and chemicals [7,8,9,10,11,12,13,14,15], and material recycling by transforming used rubber [6] into products including tires [16, 17]. Although pyrolysis is regarded as one of the promising routes [16], co-pyrolysis of waste tire with biomass is also considered as influential method [18] to attain effective conversion of tires [19,20,21,22,23,24,25].
As an environmentally friendly recycling process for waste tire treatment, mechanochemical procedures are also gaining attention [26, 27] and reclamation of crushed tire scrap by mechanochemical procedure was reported [28]. However, they could not avoid reverse reaction because of the reactions without solvent [29].
We have been working on the chemical recycling of polymeric wastes containing tire in high-temperature fluids [30,31,32,33,34]. In the thermal treatment of them, we have confirmed that waste plastics including thermosetting resin such as phenol resin decomposed into their monomeric compounds [35,36,37] and solvent plays important roles chemically and physically [38, 39]. Liquid phase treatment of polymeric materials under mild conditions is green methods for effective and realistic transformation of wastes into valuable products [40].
In this study, to obtain fundamental information on the thermal/physical reaction of waste tires orienting life-time recycling, two kinds of tires were treated in high-temperature fluid using a 10 ml tubing bomb reactor.
Experimental
Materials
Two waste tire samples were used in this study. Waste tire A was prepared by cutting the tire into the small cubic particle as shown in Fig. 1a. Outer and inner parts were characterized separately after smashing to powders. Their elemental analysis values were different between inner part (C, 83.0%; H, 8.1%; N, 1.0%; S, 2.0%) and outer part (C, 58.6%; H, 7.4%; N, 0.3%; S, 1.4%). Tire contains inorganic fillers such as carbon black and silica (SiO2). Actually, the presence of silica was confirmed by X-ray fluorescence (XRF) analysis of the tire. Elemental analysis values indicated that outer part contains more silica than inner part.
Independently another waste tire as shown in Fig. 1b was cut as flakes and mixed fully, and used as waste tire B (C, 85.8%; H, 7.7%; N, 0.4%; S, 1.2%).
Solvents were used as received without further purification. They were Reagent Grade.
Characterization
Thermal characterization of tire samples before and after the thermal treatment was clarified by using Seiko SSC 5200 apparatus (TG/DTA). About 3–7 mg of the sample was measured at a heating rate of 10 °C/min under nitrogen atmosphere from RT to 600 °C or 1000 °C depending on the sample.
The organic products after the reaction were identified qualitatively by gas chromatography–mass spectrometry (JEOL, JMS—T100GC) and were analyzed quantitatively by gas chromatography with a flame ionization detector (FID) (GC-14A, Shimadzu, Column SE-30, OV-1).
Reaction of waste tires A and B
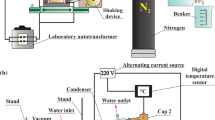
Thermal treatment of waste tires in high temperature in organic solvent was carried out using a 10 ml tubing bomb reactor as shown in Fig. 1c in which an infrared image furnace was used as the heater. In the typical reaction, 0.1 g of powdered tire sample and 1 ml of solvent were introduced in the 10 ml reactor. The thermal treatment was carried out at 150–430 °C for assigning time. After the thermal treatment, obtained contents in the autoclave were mixed with dissolving solvent such as diethyl ether and filtered by using glass filter. The obtained solid residue was dried and the dried residue was weighed to calculate solubilization as follows:
Solubilization (wt %) = 100 (0.1 − residue (g))/0.1.
Results and discussion
Characterization of waste tire samples
Waste tire A was separated into two parts, outer part and inner part. They show different thermal properties as shown in weight loss curves in Fig. 2. In the case of the outer part, sharp Differential Thermo-Gravimetry (DTG) peak was observed at 445 °C. The peak corresponds mainly to the weight loss of Styrene–Butadiene Rubber (SBR) [41, 42]. Shoulder peak was also observed at 380 °C which corresponds to the weight loss of Natural Rubber (NR) and/or Isoprene Rubber (IR). Even after the heating to 1000 °C, 30% was remained as the residue. It is carbonization products and carbon black. In the thermal characterization of the inner part of the waste tire A, one clear DTG peak at 415 °C was observed. The peak corresponds to the weight loss of Butadiene Rubber (BR) and Butyl Rubber (IIR), and the peak temperature in inner part was lower than that of the peak in the outer part. The difference of the TG peaks between inner and outer parts of the tire results in the difference of the rubber components between inner and outer parts.
Thermal characteristics of the waste tire B show two DTG peaks at 446 °C and 373 °C, as shown in Fig. 3. The thermal characteristic of the waste tire B was as expected, because the waste tire B (flakes) contains all part of the tire.
Reaction of outer part of waste tire A
Outer part of the waste tire A was treated in various solvent, because outer part is more stable thermally than inner part. In the thermal treatment in water at 430 °C for 2 h, solubility reached 56.8%. Water is superior solvent on the decomposition of polymeric materials at over 400 °C, because the high-temperature water promotes the cleavage of carbon–carbon bond via oxidation [32]. The production of monomeric compounds such as benzene and toluene were confirmed in the GC profile of the soluble matter in diethyl ether, as shown in Fig. 4a. Methanol was the better solvent than water and solubility increased to 68.7% at 430 °C for 2 h. At over 240 °C, sub- or supercritical methanol is expected, and high physical and chemical participation of methanol on the solubilization was considered. The same decomposition products with those in water were confirmed.
Tetrahydronaphthalene (tetralin) is well-known hydrogen-donor solvent. In the treatment of waste tire A in tetralin at 430 °C for 2 h, solubility reached to 77.5%. Production of naphthalene was confirmed by GC. It suggests the hydrogen donation of tetralin to radicals. In the reaction in tetralin, conversion into monomeric compounds was also confirmed by GC, as shown in Fig. 4c.
To avoid the carbonization reactions occurred at high temperature, outer part of the waste tire A was treated at 300 °C for 2 h. Solubilizations in water and methanol were 7.2 and 10.8%, respectively. It suggests that they were not effective solvents in the treatment at 300 °C, because the cleavage of carbon–carbon bond was scarcely occurred.
Aromatic solvents were effective and solubilization reached to 99.8% in the treatment in tetralin. In this treatment, no production of naphthalene was confirmed indicating the occurrence of no hydrogen-donor reaction. Thermal characteristics of the filtrate at 600 °C indicated the presence of the residue, suggesting that the filtrate solution contains dispersed and suspended tire components such as silica and carbon black. Importance of physical interactions among the composition units in the tire matrix is well known. Such non-covalent interactions among the composition units [43, 44] might be broken by tetralin.
m-Cresol and phenol were also effective solvents on the solubilities and the values reached 75.2 and 73.8%, respectively. In these solvents, the small cubic particles were broken, and the residue was obtained as coarse particles. Sulfur-containing compounds were found in the filtrate. It suggests the occurrence of chemical reaction of sulfur-containing compounds at 300 °C, because bond dissociation energy of sulfur–sulfur bond is smaller than those of carbon–carbon bonds.
Reaction of waste tire B
In the thermal treatment of waste tire A, participation of the solvent with the tire proceeds from the surface of the cubic particles. To avoid heterogeneous effects of solvents, tire flakes as waste tire B were used to evaluate the solvent ability.
To make clear the effective solvent on the solubilization, waste tire B was treated in various solvent at 300 °C avoiding carbonization reaction.
Flakes were more reactive than cubic particles and solubility reached 37.3% in the reaction in methanol. m-Cresol was more effective solvent than methanol, and the solubility reached 75.0%, as shown in Table 1. However, in the reactions in methanol and m-cresol, monomeric compounds were not observed in GC patterns of the products, as shown in Fig. 4d, e. Furthermore, solubility around 90% was obtained in the treatments in tetralin and 1-methylnaphthalene. Production of naphthalene was not confirmed in the reaction in tetralin, suggesting that the hydrogen donor was not important also in the thermal treatment of flake samples at 300 °C. Tetralin and 1-methylnaphthalene were superior solvent physically to disperse and suspend tire components such as silica and carbon black.
Thermal treatments were carried out at 200 °C to elucidate the effects of solvents on the solubilization of tire. At this temperature, no solubilization was observed in the reaction without solvent even by the treatment for 6 h. As shown in Figs. 5a and 3, thermal characteristics of the residue treated without a solvent were similar with that before the treatment.
It was confirmed that 42.7 and 56.4% of waste tire B was solubilized in the thermal treatment at 200 °C for 6 h in methanol and m-cresol, respectively. Thermal characteristics of the residue obtained in the thermal treatment in m-cresol are shown in Fig. 5d. The peaks at 365 °C and 437 °C correspond to the weight loss of Natural Rubber (NR) and/or Isoprene Rubber (IR) and Styrene–Butadiene Rubber (SBR), respectively. The ratio of NR/IR components to SBR was low compared to that before the treatment. It indicated that more NR and/or IR component was converted than SBR to filtrate.
Also 41.4% and 86.8% were solubilized in the thermal treatment in tetralin and 1-methylnaphthalene at 200 °C. Filtrates of them were black suspension of tire components. Certainly, in the thermal treatment in 1-methylnaphthalene, the ratio of NR and/or IR components to SBR as shown in Fig. 5f was larger than that obtained in m-cresol.
To make clear the role of m-cresol, 1-heptanol whose number of carbons is seven was used as the solvent. As shown in Table 1, high solubility as 66.8% was observed in the thermal treatment at 200 °C for 6 h. Longer treatment time, 24 h, was not effective to proceed further solubilization. However, thermal characteristics of the residue obtained in 1-heptanol indicated that NR and/or IR component reacted gradually with an increase in the reaction time as shown in Figs. 6a–d from 0.5 to 24 h.
Reaction of waste tire B in aliphatic alcohol
The number of carbons of 1-heptanol is the same with that of m-cresol; however, 1-heptanol is non-aromatic compound and acidity of 1-heptanol is fairly lower than that of m-cresol. These facts suggested that ionic reaction and π–π interactions were not important on the solubility.
Waste tire B was treated in 1-heptanol at 200 °C varying the treatment atmosphere and the post-treatment solvent as shown in Table 2. THF was the most effective dissolving solvent and solubility increased to 64.3% even by the treatment for 2 h. In these reaction conditions, solubilities between under argon and air were within experimental error and the effects of oxygen on the solubility were small.
Thermal treatment of waste tire B was carried out using various aliphatic alcohols, as shown in Table 3. Solubility increased with an increase in carbon number and maximum solubility; 64.3%, was observed in the treatment in 1-heptanol. Solubility at 250 °C was 25.0% in the treatment in 1-heptanol at 150 °C and increased to 76.8% in the treatment at 250 °C.
Reaction of sulfur-containing compound
In the thermal treatment of waste tire B at 200 °C, participation of sulfur on the solubilization reaction of tire was suggested, because the bond dissociation energy of sulfur–sulfur bond is fairly smaller than that of carbon–carbon bond.
Diphenyl disulfide as the model compound of sulfur–sulfur bridge bond contained in the tire was treated in organic solvent at 300 °C for 2 h. In the treatment in m-cresol, the production of diphenyl sulfide was confirmed by GC–MS of the reaction products, as shown in Fig. 7. In the treatment in phenol, the production of diphenyl sulfide was also confirmed. By comparing the GC–MS profile with that obtained by the treatment in m-cresol, the production of the compound having S–O bond was supposed. The production of benzenethiol was confirmed by GC. In the treatment in tetralin, production of naphthalene was confirmed indicating the hydrogen-transfer reaction.
Disulfide (SS)–thiol (SH) exchange reaction is well known and the abundance of the S–S bond was determined using the exchange reaction [45]. The reaction products suggest the occurrence of the disulfide (SS)–alcohol (OH) exchange reaction, as shown in Fig. 8. The exchange reaction could cause S–S-bond scission resulting in the solubilization of the tire.
In the mechanochemical treatment of tire, reductive cleavage of S–S bond occurs because of the low-bond dissociation energy of sulfur–sulfur bond [46,47,48]. In the mechanochemical conditions, local high temperature is attained by the uneven temperature distribution. Such high temperature may cause bond scission reactions. On the contrary, liquid-phase treatment attains homogeneous cleavage of sulfur–sulfur bond.
Conclusions
Tire is heterogeneous composite materials and different thermal properties were observed between inner and outer parts. In the reaction of the outer part, high solubilization to monomeric compounds was attained in the hydrogen-donor solvent at 430 °C through stabilization after carbon–carbon bond cleavage reaction. Solubilization of waste tire flakes was attained by the thermal treatment at 300 °C in aromatic solvent such as tetralin and 1-methylnaphthalene. In these solvents, solubilization occurred without devulcanization of the tire. Certainly, the solubilized products are inappropriate for raw materials of recycled tire because of a possibility of insufficient properties of the recycled tire by the heterogeneous mixing.
The effective solubilization was attained even at 200 °C in aliphatic alcohol. In the thermal treatment in alcohol, solubilization reached at near 70% by the devulcanization reaction. The products bring the prompt material for recycling tire by mixing with virgin raw materials.
References
Tyre Industry of Japan (2019) Current status on scrap syre (used tyre) Recycling (published by The Japan Automobile Tyre Manufacturers Association Inc.) pp. 15–16. https://www.jatma.or.jp/media/pdf/tyre_industry_2019.pdf
Swapna VP, Stephen R (2017) Recycling of rubber in recycling of polymers: Methods, characterization and applications (ed. by R. Francis) Wiley-VCH Verlag GmbH & Co. KGaA, 141–161.
Rodriguez E, Gutierrez A, Palos R, Azkoiti MJ, Arandes JM, Bibao J (2019) Cracking of scrap tires pyrolysis oil in a fluidized bed reactor under catalytic cracking unit conditions. Effects of operating conditions. Energy Fuels 33:3133–3143
Noordermeer J, Dierkes W, Blume A, Hoek H, Reuvekamp L, Saiwari S (2017) Life-time recycling loops for elastomer products: state-of-the art. International Elastomer Conference, October 9–12, Cleveland, USA.
Larsen MB, Schultz L, Glarborg P, S-Jensen L, D-Johansen K, Frandsen F, Henriksen U (2006) Devolatilization characteristics of large particles of tyre rubber under combustion conditions. Fuel 85:1335–1345
Lombardi L, Carnevale E, Corti A (2015) A review of technologies and performances of thermal treatment systems for energy recovery from waste. Waste Manage 37:26–44
Verma P, Jafari M, Bodisco TA, Rainey T, Ristovski Z, Brown R (2018) Diesel engine performance and emissions with fuels derived from waste tyres. Sci Rep 8:2457
Kumaravel ST, Murugesan A, Kumaravel A (2015) Tyre pyrolysis oil as an alternative fuel for diesel engines: a review. Renew Sustain Energy Rev 60:1678–1685
Williams PT (2013) Pyrolysis of waste tyres: a review. Waste Manage 33:1714–1728
Martinez JD, Puy N, Murillo R, Garcia T, Navarro MV, Mastral AM (2013) Waste tyre pyrolysis: a review. Renew Sustain Energy Rev 23:179–213
Antoniou N, Stavropoulos G, Zabaniotou A (2014) Activation of end of life tyres pyrolytic char for enhancing viability of pyrolysis: critical review, analysis and recommendations for a hybrid dual system. Renew Sustain Energy Rev 39:1053–1073
Quek A, Balasuburamanian R (2013) Liquefaction of waste tires by prolysis for oil and chemicals: a review. J Anal Appl Pyrol 101:1–16
Hita I, Arabiourrutia M, Olazar M, Bilbao J, Arandes JM, Castano P (2016) Opportunities and barriers for producing high quality fuels from the pyrolysis of scrap tires. Renew Sustain Energy Rev 56:745–759
Rudniak L, Machiniewski PM (2017) Modelling and experimental onvestigation of waste tyre pyrolysis process in a loboratory reactor. Chem Process Eng 38:445–454
Acevedo B, Fernandez AM, Barriocanal C (2015) Identification of polymers in waste tyre reinforcing fibre by thermal analysis and pyrolysis. J Anal Appl Pyroly 111:224–232
Fukuta M (2010) Industrial application status of rubber recycle technology. Toyotagouseigiho 52:13–19
Sienkiewicz M, K-Lipka J, Balas JA (2012) Progress in used tyres management in the European Union: a review. Waste Manage 32:1742–1751
Sathiskumar C, Karthikeyan S (2019) Recycling of waste tires and its energy storage application of by-products: a review. Sustain Materials and Technol 22:e00125
Onay O, Koca H (2015) Determination of synergetic effect in co-pyrolysis of lignite and waste tyre. Fuel 150:169–174
Abnisa F, Daud WMAW (2014) A review on co-pyrolysis of biomass: an optional technique to obtain a high-grade pyrolysis oil/. Energy Convers Manage 87:71–85
Abnisa F, Daud WMAW (2015) Optimization of fuel recovery through the stepwise co-pyrolysis of palm shell and scrap tire. Energy Convers Manage 99:334–345
Ucar S, Karagoz S (2014) Co-pyrolysis of pine nut shells with scrap tires. Fuel 137:85–93
Cao Q, Jin L, Bao W, Lv Y (2009) Investigations into the characteristics of oils produced from co-pyrolysis of biomass and tire. Fuel Process Technol 90:337–342
Duan P, Jin B, Xu Y, Wang F (2015) Co-pyrolysis of microalgae and waste rubber tire in supercritical ethanol. Chem Eng J 269:262–271
Xuesong Z, Lei H, Chen S, Wu J (2016) Catalytic co-pyrolysis of lignocellulosic biomass with polymers: a critical review. Green Chem 18:4145–4169
Okamoto H, Inagaki S, Onouchi Y, Furukawa J (1979) Reclamation of disposal rubber vulcanizates (I) Reclamation of crushed tire scrap by the mechano-chemical procedure with accelerators. Rubber Chem Technol 52:774–777
Onouchi Y, Inagaki S, Okamoto H, Furukawa J (1980) Reclamation of disposal rubber vulcanizates (II) Reclamation of crushed tire scrap with thiol and amine derivatives. Rubber Chem Technol 53:756–762
Seghar S, Asaro L, R-Monnet M, Hocine NA (2019) Thermo-mechanical devulcanization and recycling of rubber industry waste. Resour Conserv Recycl 144:180–186
Guo X, Xiang D, Duan G, Mou P (2010) A review on mechanochemistry applications in waste management. Waste Manage 30:4–10
Sato Y, Kurahashi S (1995) Material recycling of used tires by liquid-phase cracking. J Japan Institute Energy 74:91–98
Suzuki Y, Tagaya H, Asou T, Kadokawa J, Chiba K (1999) Decomposition of prepolymers and molding materilas of phenol resin in subcritical and supercritical water under an Ar atmosphere. Ind End Chem Research 38:1391–1395
Tagaya H, Suzuki Y, Komuro N, Kadokawa J (2001) Reactions of model compound of phenol resin in sub- and supercritical water under an Ar atmosphere. J Mater Cycles Waste manag 3:32–37
Tagaya H, Shibasaki Y, Kato C, Kadokawa J, Hatano B (2004) Decomposition reactions of epoxy resin and polyetheretherketone resin in sub- and supercritical water. J Mater Cycles Waste Manag 6:1–5
Zhang L, Zhou B, Duan P, Wang F, Xu Y (2016) Hydrothermal conversion of scrap tire to liquid fuel. Chem Eng J 285:157–163
Shibasaki Y, Kamimori T, Tagaya H (2004) Decomposition reactions of plastic model compounds in sub- and supercritical water. Polymer Degrad Stability 83:481–485
Ikeda A, Katoh K, Tagaya H (2008) Monomer recovery of waste plastics by liquid phase decomposition. J Mater Sci 43:2437–2441
Okubo K, Sugeno T, Tagaya H (2015) Chemical recycling of poly(p-phenylene sulfide) in high temperature fluids. Polymer Degrad Stability 111:109–113
Chiba K, Tagaya H, Kobayashi T, Shibuya Y (1987) Solvent extract liquefaction of coal with fractionated anthracene oil and recycle solvent. Ind End Chem Res 26:1329–1335
Tagaya H, Ono T, Chiba K (1988) Evaluation of the solvent ability for coal liquefaction using a phenolic resin coal model. Ind End Chem Res 27:895–898
Sugeno T, Tagaya H (2015) The effects of solvents on the chemical decomposition of 8 foamed phenol resin in high temperature conditions. J Mater, Cycles Waste Manag 17:453–458
Danon B, Görgens J (2015) Determining rubber composition of waste tyres using devolatilisation kinetics. Thermochim Acta 621:56–60
Han J, Li W, Liu D, Qin L, Chen W, Xiang F (2018) Pyrolysis characterization and mechanism of waste tyre: a thermogravimetry mass spectrometry analysis. J Anal Appl Pyrolysis 129:1–5
Takanohashi T, Iino M (1995) Investigation of associated structure of upper Freeport coal by solvent swelling. Energy Fuels 9:788–793
Tagaya H, Sugai J, Onuki M, Chiba K (1987) Low-temperature coal liquefaction using n-butylamoine as a solvent. Energy Fuels 1:397–401
Matsumoto H, Kuninori T (1978) Determination of SH group, Japan Scientific Societies Press, pp 86–100.
Jana GK, Mahaling RN, Das CK (2005) A novel devulcanization technology for vulcanized natural rubber. J Appl Polymer Sci 99:2831–2840
Dopieralski P, R-Arino J, Anjukandi P, Krupocka M, Marx D (2017) Unexpected mechanochemical complexity in the mechanistic scenarios of disulfide bond reduction in alkaline solution. Nat Chem 9:164–170
Zhang X, Lu Z, Tian D, Li H, Lu C (2013) Mechanochemical devulcanization of ground tire rubber and its application in acoustic absorbent polyurethane foamed composites. J Appl Polymer Sci 127:4006–4013
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yamashita, D., Usui, K., Takahashi, T. et al. Chemical recycling of waste tire in high-temperature organic fluid. J Mater Cycles Waste Manag 22, 1249–1257 (2020). https://doi.org/10.1007/s10163-020-01017-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-020-01017-2