Abstract
In this study, the effect of phosphates flotation wastes (PFW) and phosphogypsum (PPG) on organic biomass degradation, compost quality, and plant growth was examined. Our results showed that PFW and PPG improve composting mechanisms, accelerate organic substance degradation, and increase fertilizing elements (P2O5, K2O, MgO, CaO). The low level of C/N ratio in the finished composts reflects the good quality of the prepared composts. The effect of PFW and PPG additives on plant growth was evaluated using chickpea plants. Addition of PFW and PPG enhanced significantly chickpea plant growth and development, compared to the control, especially when added simultaneously. These results indicate that PFW and PPG can be utilized in compost formulations, and as fertilizers in agricultural practices to improve chickpea plant growth and development, while at the same time reducing the environmental and health risks associated with their disposal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Composting is one of the best-known techniques used in biological stabilization of organic wastes, which can then be used as a good source of nutrients and as soil conditioners in agricultural systems [1]. Composting is labor-intensive and time-consuming, making it unattractive for entrepreneurship prospects. However, recent days have witnessed a renewal of interest in composting, because of headway in composting technology. Many techniques, such as biological composting, co-composting using additives, addition of microbial inoculums and rapid composting using accelerators, have made composting more streamlined, and offered avenues for waste management strategies. Additives are typically mixtures of various amounts of different mineral fertilizers, or easily available forms of carbon, microorganisms, enzymes and pH-balancing reactants that are meant to improve microbial activity if these additives are in contact with the waste product [2].
Phosphate production industry generates huge quantities of byproducts, including rocks, phosphate flotation wastes (PFW) and phosphogypsum (PPG). These wastes are deposited or stockpiled with the mine sites and constitute a serious potential source of pollution and health risks. Morocco, with its largest share of the world’s phosphate reserves, is the leading exporter of phosphate and its derivatives. The country account for 14% of world production with 28 million tons in 2012 [3] and therefore generate large quantities of PFW and PPG. The Moroccan Cherefian Phosphate estimates that 200 hectares is the surface of wastes piles generated from industrial production of phosphates in Morocco. The recycling of the phosphate wastes could be a useful approach to limit their negative impacts and create opportunities for domestic value-adding.
It has been reported previously that PPG might be effective in reducing NH4 emissions during cattle manure composting by increasing SO42− content of the compost [4, 5]. In addition, Kammoun et al. [6] indicated that addition of PPG at a rate of 10% of total compost mixture (dry weight) decreased NH3 emissions significantly during pig manure composting. Moreover, PPG does not have any negative effect on compost maturity [4, 7, 8]. We have previously characterized at the physico-chemical levels PFW and showed that it contains high level of clay (45%). This high level of clay content present in PFW promote humidification and leads to the creation of clay–humus complex, making it suitable for composting processes [9].
The objective of the present study is to examine the effect of PFW and PPG on compost organic matter degradation, compost maturity and quality, and to determine their effect on the growth and development of chickpea plants.
Materials and methods
Compost mixtures preparation
Cattle manure was sorted manually from a farm in the city of Chichaoua, Morocco, phosphogypsum (PPG) and phosphate flotation waste (PFW) (Fig. 1) were obtained from the Moroccan phosphate plant in the city of Khouribga, Morocco. Twenty sub-samples (5 kg each) were taken from different locations and mixed rigorously using a riffle splitter. The size of PFW particles were less than 40 µm, and sample preparation for chemical analysis was conducted according to the experimental flow chart presented in (Fig. 2). Cattle manure, and straw samples were air-dried at room temperature, disaggregated in a ceramic pestle and mortar and sieved through a 2 mm sieve, and the < 2 mm fraction was used for analyses.
Phosphate flotation waste sample preparation for chemical analysis [9]
Three different phosphate-supplemented composts were examined in this study, they were; PFW30: PFW/manure/straw (3:6:1), PPG30: PPG/manure/straw (3:6:1), PFW15 + PPG15: PFW/PPG/manure/straw (1.5:1.5:6:1). A mixture of manure/straw (7.5:2.5) was used as a control.
The composters were set up in 25 l plastic barrels containing different proportions of fresh wastes (based on dry volume). The components were mixed in a volumetric ratio, in a total volume of 200 L, the pile height was 165 cm with a bulk density of 20 g cm−3. The experiment was conducted in the city of Khouribga, Morocco and was repeated three times. The humidity of the prepared composts was determined by placing 3 g of the compost in infrared desiccators for 30 min. Humidity of the compost mixtures was maintained at 55% by addition of water, and aeration was achieved by mixing every week for 12 weeks. Maintaining proper aerobic condition is a crucial factor in the composting process [10].
Temperature and pH measurements
Temperature variation of the prepared composts was measured using a HI 8757 K-thermocouple thermometer (Hanna Instruments™, Italy). The pH of the composts was determined in a suspension of 1/10 weight/volume (w/v) compost/ water using a pH meter. Measurements were taken every week for 12 weeks.
Lignin extraction and quantification
Extraction of lignin from other organic polymers contained in the different composts (mainly cellulose and hemicelluloses) was conducted using acetic acid/formic acid/water mixture at 50–30–20 (volume/volume/volume) at a ratio of 1/12 (w/v) as previously described [11]. The soluble fraction was filtrated using GF/D Whatman glass microfiber filters. Water was added to the filtrate followed by centrifugation at 11,200×g for 10 min. The precipitated lignin was washed with bi-stilled water to a neutral pH, and then dried at 105 °C for 48 h [11].
Nutrients content (CaO, K2O, MgO, P2O5)
CaO content was measured by complexation in 0.3 N NaOH using atomic absorption spectrophotometer (AA-6200) at 465 nm, while K2O, MgO, and P2O5 contents were determined by perchloric acid attack using using AA6200 at 766.5 nm, 285.2 nm, and 880 nm, respectively.
NH4 emission and total nitrogen content
Ammonium N-NH4+ content was determined directly from 2 g of the compost mixtures by distillation in 10 ml of sodium hydroxide (10 N), using an RQ-flex reflectometer (Reflectoquant; Merck KGaA, Darmstadt, Germany). The emission of NH4 was measured by calculating the difference between NH4 content of the compost in the desired period and the initial concentration of NH4.
Total nitrogen was measured following titration with 10 ml of boric acid (1 N) and sulfuric acid (0.02 N) in the presence of nitrogen indicator Tashiro, using the following formula: N (%) = N' × (Ve − Vt) × 1.8/P, where N is total Kjeldahl nitrogen, Ve is the volume of the sample in ml, Vt is the volume of control in ml, N' is the normality of the sulfuric acid, and P weight of the test sample in mg.
Humification index
Humification index (HI) was measured to determine the maturity of the prepared composts. Soluble molecules (sugars and amino acids) were first eliminated from the different composts by rinsing with water. Extraction of hemic substances was accomplished in 0.1 N NaOH after agitation for 2 h. Extraction was repeated several times until a clear extract was obtained. The separation of humic and fulvic acids was performed by precipitation of the humic acids in an acid medium (pH 2; 4 °C; 24 h). Humic fractions were obtained after oxidation of the carbon by KMnO4 in an alkaline environment. The fractions were then separated by chromatographic techniques and identified by mass spectrometry (Hiden Analytical, UK).
Seed germination index
Seed Germination Index (GI) was measured to assess the phyto-toxicity of the different composting mixtures using seeds of Chickpeas (Cicer arietinum). Ten seeds were placed on a filter paper in petri dishes (10 cm in diameter) imbibed with 2 ml of composts aqueous extracts (1/10 w/v). Plant seeds in Petri plates were placed in the dark at 28 °C for 5 days. The number of germinating seeds and the GI was calculated using the following formula:
Plant growth
The effect of the different compost mixtures on plant growth was examined using seeds of chickpeas. Compound soil samples were obtained from a profundity of 0.2 m from the ground surface and screened throughout a 6 mm sieve. The soil was red loam and contained 4.33% gravel, 92.84% sand and 2.83% fine. Trays (0.44 × 0.32 × 0.14 m). Three chickpea plants were planted per pot and the experiment was repeated three times. Germinating chickpeas plants were watered periodically with deionized water. Plant dry weight was measured 4 weeks post germination.
Statistical analysis
Data were analyzed using one-way ANOVA-SAS Duncan's Multiple Range Test method in SAS (Statistical Analysis System) 8.2 for Windows.
Results and discussion
Temperature and pH profile
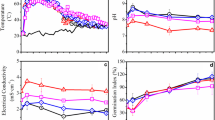
The temperature profiles of the prepared composts were comparable; the temperature increased gradually during the composting process and reached the thermophilic period (> 59 °C) by the third week, then decreased thereafter, indicating the decomposition of available organic materials by microorganism during the composting process [12]. The highest temperature value (69 °C) was recorded in PFW15 + PPG15 on the fourth week (Fig. 3a), followed by PFW30 and then PPG30. This rise in the temperature can be attributed to the breaking of carbon bonds by microorganisms present in the composts. It is therefore an indication of successful composting. At the end of the composting period, the temperature of the different compost mixtures was similar to the ambient temperature. It has been reported that the rate of humidity tends to decrease under the ascent of temperature, which results is losses of water in the form of vapor [13,14,15]. We maintained this parameter at a suitable level to ensure proper composting.
Evolution of temperature (a) and pH (b) during the composting period; “control” represents the control treatment, “PFW30” and “PPG30” represent the composts containing 30% initial raw materials (dry weight) of PFW and PPG, respectively, and “PFW15 + PPG15” represents the compost containing 15% PFW and 15% PPG
The initial pH of raw materials used in preparing the different composts is presented in Table 1. The pH of the different compost mixtures was measured during the process of decomposition to evaluate compost maturity and stability. The initial pH of the different compost mixtures ranged from 6.0 to 7.5, and increased rapidly during the first two weeks of composting process and reached 8.5 for PFW30, PFW15 + PPG15, and Control, and 7.5 for PPG30. The increase in pH coincides with the increase in temperature and is the result of proteins degradation and release of ammonia by microorganisms. However, the pH of PFW decreased significantly during the third week of composting, and remained stable at 7.25 after that. It is still not clear what causes this decrease in the pH.
After two weeks of composting, the pH of all composts decreased gradually but remained close to neutrality throughout the composting period, indicating that the different composts prepared were mature and of good quality (Fig. 3b). It has been reported that optimal pH values of composts should be between 6 and 8, value around neutrality are optimal for microorganisms’ biological activity, a good compost should remain close to neutrality throughout the composting process, as acidic pH affects the rate of respiration of microbes and decreases the rate of degradation [16]. Moreover, the high activity of microbes at thermophilic stage is achieved when the pH of the compost is alkaline [17].
P2O5, MgO, K2O and CaO contents
The physico-chemical properties of raw materials used in preparing the different composts and the nutritional statues of the different compost mixtures at the beginning of the trial are presented in Tables 1 and 2, respectively. Table 3 represents the evolutionary profiles of the physic-chemical parameters of the different composts used in this study. During composting, the P2O5 levels in PFW30, PPG30, and PFW15 + PPG15 were similar but significantly higher compared to the Control (Fig. 4a). In all treatments, P2O5 increased during the first 3 week of composting process and remained stable thereafter. At the end of the composting process, the level of P2O5 was four times higher than the control compost (Fig. 4).
Evolution of P2O5 (a), MgO (b), CaO (c) and K2O (d) contents during the composting period; “control” represents the control treatment, “PFW30” and “PPG30” represent the composts containing 30% initial raw materials (dry weight) of PFW and PPG, respectively, and “PFW15 + PPG15” represents the compost containing 15% PFW and 15% PPG
The initial percentages of MgO in the different composts ranged between 1 and 1.5% and were below the control treatment which had 1.8% of MgO (Fig. 4b). However, after six week of composting, the percentages of MgO in all treatments were similar, around 1.25%, until the end of the composting process (Fig. 4b).
The concentration of K2O at the start of the composting process varied between 1.1% and 1.4%. During the composting process, the percentage of K2O decreased in TPPG30 and increased in PFW15 + PPG15, but it did not change in PFW30. By the 6th week of composting process, the percentage of K2O in all treatments was similar and remained stable until the end of the composting process (Fig. 4c).
At the start of the composting, calcium concentration of PFW30, PPG30 and PFW15 + PPW15 treatments was significantly higher compared to the control (Fig. 4d). The concentration of CaO in phosphate waste—supplemented composted increased from the 3rd week to the 6th week of composting and was 42.32% in PFW30, 38.47% in PPG30 and 44.78% PFW15 + PPG15, but remained stable thereafter. At the end of the composting period, the content of CaO in the composts supplemented with PPF and/or PFW was 10 times higher than the Control.
N-NH4+ emission
N-NH4 emissions in all treatments increased sharply during the first two weeks of thermophilic composting, and decreased thereafter to values close to zero by the end of the composting process (Fig. 5). The increase in N-NH4 emission may be to the increased temperature and pH. Moreover, N-NH4+ emission in PPG30 remained low from the fourth week until the end of the composting process, compared to N-NH4+ emission in the other treatments. It has been reported that ammonia emission is the main form of N loss during composting [18]; which occurs at a rate much higher than NO3 emission.
NH4+ content loss of the composting piles during the composting period; “control” represents the control treatment, “PFW30” and “PPG30” represent the composts containing 30% initial raw materials (dry weight) of PFW and PPG, respectively, and “PFW15 + PPG15” represents the compost containing 15% PFW and 15% PPG
The highest emission of NH4 (90%) was observed in PPG15 + PFW15 during the first two weeks and in PFW30 during the first 3 weeks. While NH4 loses in PPG30 was similar than the control, about 50% loses (Fig. 5b). Losses via NH4 volatilization range from as low as 13% to as high as 70% of manure containing nitrogen [19]. The wide variations are due to differences in properties of the raw material used, environmental conditions and compost management practices [19]. López et al. [20], underlined that the proportion and quality of clay has an influence on the capacity of absorption of ammonium. NH4 retention can be also influenced by temperature, chemical additives and pH [21].
The Effect of PFW and PPG on maturity parameters of the composts
Effect of PFW and PPG on compost C/N ratio
The C/N ratio is an important indicator of compost quality. It has been indicated that a C/N ratio of less than 20 and even 15 characterizes mature composts [22,23,24]. In this experiment, C/N ratio in PFW30, PPG30, and PPW15 + PPG15 were similar but remained low compared to the Control. The COT% of the finished composts is 57%, 56%, 55% and 54% in PPW15 + PPG15, PFW30, PPG30, and Control, respectively. At the beginning of the composting process, the C / N ratio in all mixtures including the Control treatment was between 25 and 32 but decreased gradually with time until it stabilized at 13 by the end of the composting process (Fig. 6a), indicating that the composts prepared are mature.
C/N (a), Humification Index (b), and lignin content (c) changes in the composting piles during the composting period; “control’’ represents the control treatment, “PFW30” and “PPG30” represent the composts containing 30% initial raw materials (dry weight) of PFW and PPG, respectively, and “PFW15 + PPG15” represents the compost containing 15% PFW and 15% PPG
Humification index
Humification index (HI) was monitored in phosphate-supplemented composts. HI decreased gradually during composting with no significant difference between the treatments (Fig. 6b), and went from 1 to 0.75. However, the HI in PFW30 was slightly higher when compared to PFW30 and PFW15 + PPG15, probably due to the clay composition of PFW. It has been reported that clay protects the humus from destruction by microorganisms and so favors humification [25]. In addition, humic substances (HA and FA) improves the structure of clay-containing particles by assuring the elementary cohesion of particles by electrostatic connections and weak connections established between the organic molecules and the clays [21]. HI of the final composts obtained in this study was similar to those obtained in previous studies for mature composts [26, 27].
Lignin content
The degradation of lignin in the different compost was monitored during composting. There was significant difference between the different treatments and the Control. Lignin content decreased significantly during composting and went from 13% at the beginning of the composting process to 7.15% DM at the end (Fig. 6c).
The effect of PFW and PPG of seed germination and plant growth
It has been reported that amendment of immature compost in a soil has negative effects on germination, growth and development of plants, and that the best indicator of maturity of compost remains its phytotoxicity [28]. In addition, stable compost does not necessarily mean that it is mature as it can still have an inhibitory or phytotoxic effect on plant growth [29, 30]. In this context, the effect of PPW and PPG on seed germination was examined to determine the potential phytotoxicity of the phosphate additives. Addition of PPW and PPG alone or in combination had no observable effect on chickpea seed germination. The GI index profiles in all treatments including the control were similar. The GI increased rapidly during the first two weeks of the composting process and reached 100% after four weeks (Fig. 7a), indicating that addition of PPW and PPG has no negative effect on seed chickpea germination.
Germination Index of chickpea seeds [recorded in 0.5–5 days after sowing (DAS)] (a) and average dry weight of chickpea plants 4 weeks after germination (b). “control” represents the control treatment, “PFW30” and “PPG30” represent the composts containing 30% initial raw materials (dry weight) of PFW and PPG, respectively, and “PFW15 + PPG15” represents the compost containing 15% PFW and 15% PPG. Asterisk significantly different from the control at p ≤ 0.05
The effect of PFW and PPG enriched animal manure on plant growth was also evaluated using chickpeas seeds. Addition of PFW and PPW to compost mixtures significantly increased growth of chickpea plants compared to control. Chickpea plant dry weight increased by 170% in PFW15 + PPG15, followed by 140% increase in PFW30 and 100% increase in PPG30 (Fig. 7b), indicating that addition of PFW and PPG enhances plant growth and development especially when both PFW and PPG are added (Fig. 7b).
Conclusion
The addition of PFW and PPG to compost mixture improved compost quality, they enhanced organic matter degradation, increased fertilizing elements (P2O5, K2O, MgO, CaO) and decreased C/N ratio relative to Control formulation. In addition, supplementing compost mixtures with PFW and PPG had no observable negative effect on seed germination, and significantly enhanced plant growth and development, especially when both PFW and PPG were added to the comport. These results indicate that PFW and PPG can be used in compost formulations to improve plant growth while at the same time reducing environmental and health risks associated with their production.
References
Sciubba L, Cavani L, Grigatti M, Ciavatta C, Marzadori C (2015) Relationships between stability, maturity, water-extractable organic matter of municipal sewage sludge composts and soil functionality. Environ Sci Pollut Res Int 22(17):13393–13403
Himanen M, Hänninen K (2009) Effect of commercial mineral-based additives on composting and compost quality. Waste Manag 29(8):2265–2273
Jasinski SM (2011) Minerals yearbook, phosphate rock (1994-2013). US Geological Survey [Online]. http://minerals.usgs.gov/minerals/pubs/commodity/phosphate_rock/. Accessed 5 Feb 2019
Hu TG, Zeng D, Huang H, Yu X, Jiang F, Huang G (2007) Use of potassium dihydrogen phosphate and sawdust as adsorbents of ammoniacal nitrogen in aerobic composting process. J Hazard Mater 141(3):736–744
Li Y, Luo W, Li G, Wang K, Gong X (2018) Performance of phosphogypsum and calcium magnesium phosphate fertilizer for nitrogen conservation in pig manure composting. Bioresour Technol 250:53–59
Kammoun M, Ghorbel I, Charfeddine S, Kamoun L, Gargouri-Bouzid R, Nouri-Ellouz Q (2017) The positive effect of phosphogypsum-supplemented composts on potato plant growth in the field and tuber yield. J Environ Manag 200:475–483
Gabhane J, William SP, Bidyadhar R, Bhilawe P, Anand D, Vaidya AN, Wate SR (2012) Additives aided composting of green waste: effects on organic matter degradation, compost maturity, and quality of the finished compost. Bioresour Technol 114:382–388
Yang F, Li G, Shi H, Wang Y (2015) Effects of phosphogypsum and superphosphate on compost maturity and gaseous emissions during kitchen waste composting. Waste Manag 36:70–76
Elfadil S, Bouchdoug M, Jaouad A (2016) Physico-chemical characterization of phosphate flotation waste and its potential as a composting amendment. Imperial J Interdiscip Res 2(5):71–77
Puspitaloka H, Mimoto H, Tran QNM, Koyama M, Nakasaki K (2019) Effect of aeration method on organic matter degradation and ammonia emission of composting in a laboratory-scale reactor. J Mater Cycles Waste Manag. https://doi.org/10.1007/s10163-019-00956-9
Ragauskas AJ, Beckham GT, Biddy MJ, Chandra R, Chen F, Davis MF, Davison BH, Dixon RA, Gilna P, Keller M, Langan P, Naskar AK, Saddler JN, Tschaplinski TJ, Tuskan GA, Wyman CE (2014) Lignin valorization: improving lignin processing in the biorefinery. Science 344(6185):1246843
Raut M, Prince William S, Bhattryya J, Chakrabarti T, Devotta S (2008) Microbial dynamics and enzyme activities during rapid composting of municipal solid waste—a compost maturity analysis perspective. Bioresour Technol 99(14):6512–6519
Liang C, Das KC, McClendon RW (2003) The influence of temperature and moisture contents regimes on the aerobic microbial activity of a biosolids composting blend. Bioresour Technol 86(2):131–137
Seng B, Kaneko H, Hirayama K, Katayama-Hirayama K (2012) Development of water movement model as a module of moisture content simulation in static pile composting. Environ Technol 33(13–15):1685–1694
Dubelman S, Fischer J, Zapata F, Huizinga K, Jiang C, Uffman J, Levine S, Carson D (2014) Environmental fate of double-stranded RNA in agricultural soils. PLoS ONE 9(3):e93155
Sundberg C, Yu D, Franke Whittle I, Kauppi S, Smårs S, Insam H, Jönsson H (2013) Effects of pH and microbial composition on odour in food waste composting. Waste Manag 33(1):204–211
Sundberg C, Smars S, Jonsson H (2004) Low pH as an inhibiting factor in the transition from mesophilic to thermophilic phase in composting. Bioresour Technol 95(2):145–150
Parkinson R, Gibbs P, Burchett S, Misselbrook T (2004) Effect of turning regime and seasonal weather conditions on nitrogen and phosphorus losses during aerobic composing of cattle manure. Bioresour Technol 91:171–178
Janczak D, Krystyna M, Wojciech C, Rafaela C, Andrzej L, Dach J (2017) Biochar to reduce ammonia emissions in gaseous and liquid phase during composting of poultry manure with wheat straw. Waste Manag 66:36–45
López M, Soliva M, Martínez-Farré FX, Bonmatí A, Huerta-Pujol O (2010) An assessment of the characteristics of yard trimmings and recirculated yard trimmings used in biowaste composting. Bioresour Technol 101(4):1399–1405
Magrí A, Teira-Esmatges MR (2015) Assessment of a composting process for the treatment of beef cattle manure. J Environ Sci Health Part B 50(6):430–438
Iglesisas-Jimenez E, Perez-Garcia V (1991) Composting of domestic refuse and sewage sludge. I: evolution of temperature, pH, C/N ratio and cation exchange capacity. Resour Conserv Recycl 6(1):45–60
Chefetz B, Hatcher P, Hadar Y, Chen Y (1996) Chemical and biological characterization of organic matter during composting or municipal solid waste. J Environ Qual 25(4):776
Makan A, Mountadar (2012) Effect of C/N ratio on the in-vessel composting under air pressure of organic fraction of municipal solid waste in Morocco. J Mater Cycles Waste Manag 14:241. https://doi.org/10.1007/s10163-012-0062-0
Song G, Hayes MH, Novotny EH, Simpson AJ (2011) Isolation and fractionation of soil humin using alkaline urea and dimethylsulphoxide plus sulphuric acid. Naturwissenschaften 98(1):7–13
Tomati U, Madejon E, Galli E (2000) Evolution of humic acid molecular weight as an index of compost stability. Compost Sci Util 8(2):108–115
Zhao XL, Li BQ, Ni JP, Xie DT (2016) Effect of four crop straws on transformation of organic matter during sewage sludge composting. J Integr Agric 15(1):232–240
Tiquia S, Tam N, Hodgkiss I (1997) Effects of turning frequency on composting of spent pig-manure sawdust litter. Bioresour Technol 62(1–2):37–42
Tiqiua S, Tam N (1998) Elimination of phytotoxicity during co-composting of spent pig-manure sawdust litter and pig sludge. Bioresour Technol 65(1–2):43–49
Alburquerque J, Gonzalvez J, Garcia D, Cegarra J (2006) Measuring detoxification and maturity in compost made from “Alperujo”, the solid by-product of extracting olive oil by the two-phase centrifugation system. Chemosphere 64(3):470–477
Acknowledgements
We thank the Moroccan Cherifian Office of Phosphates (COP), the Moroccan Society of Phosphates, and the Ministry of Higher Education in Morocco for their financial support. We also thank the COP personnel for their discussions and inputs.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Elfadil, S., Hamamouch, N., Jaouad, A. et al. The effect of phosphate flotation wastes and phosphogypsum on cattle manure compost quality and plant growth. J Mater Cycles Waste Manag 22, 996–1005 (2020). https://doi.org/10.1007/s10163-020-00997-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-020-00997-5