Abstract
Recycling of materials is becoming progressively more significant for society due to the depletion of natural resources. Jewelry wastes are considered as secondary sources of raw material and contain a very high proportion of precious metals. Scraps and wastes from jewelry activities need to be pre-treated before refining to reduce costs and maximize the recovery of precious metals. In this paper, the recovery of gold (Au) and silver (Ag) from floor sweeping jewelry wastes (FSJW) of jewelry workshops using flotation followed by leaching process was studied. In flotation experiments, optimum pH value, particle size, type, and amount of reagent were the investigated parameters. Experimental results demonstrated that the flotation method was very successful in diminishing much of the waste fraction. A froth product, with more than 280 g/t Au and 2800 g/t Ag, was produced from a feed containing 174 g/t Au and 1834 g/t Ag with approximately 87% Au and 82% Ag recoveries. To investigate the dissolution behaviors of gold and silver, cyanide leaching tests were carried out directly on the feed and the froth product. 68% Au and 75% Ag in leach that were obtained with direct feed after 48 h of leaching in 12 g/L NaCN concentration at 40 °C and 1/5 solid/liquid ratio (w/w) were enhanced to 79% Au and 84% Ag when the froth sample was subjected to leaching under the same conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the jewelry sector, the most important noble metals are undoubtedly Au and Ag. Gold is a reusable element by its formation and is used in multiple sectors because of its resistance to corrosion, malleability and ductility properties. Silver is widely used in industry due to its high electrical conductivity, thermal conductivity, and the lowest electrical contact resistance. Noble metals are highly demanded in different modern electronics such as information, military, space, telecommunications, etc. [1, 2]. Due to all these specialties, gold and silver are used in various other industrial applications along with jewelry manufacturing [3,4,5].
Today, parallel to population growth, the production of electrical and electronic products is constantly increasing; therefore, electronic waste amounts rise to very high levels [6,7,8]. Precious metals are certainly important for recycling and Au&Ag are the most popular precious metals that are generated from mostly the jewelry and electronics industry [9, 10]. Approximately 80% of the gold and 20% of the silver produced in the market is used in the jewelry industry. Therefore, the dust of these metals generated from workshops is very critical for economic recycling process [10,11,12,13,14]. In industrial applications, wastes generated from workshops are collected and then sent out to final processing [11, 12]. The scrap materials that have higher grades of noble metals are cleaned and then recycled. However, the contaminated scrap and other wastes that contain less amounts of noble metals are collected and then refined to form pure metals. The wastes generated at different stages of the manufacturing process contain noble metals at a much lower rate than the scraps [13,14,15].
Usually, three different types of waste: jewelry polishing waste, floor sweepings waste (FSJW), and handwashing waste, are produced in jewelry workshops [13]. Jewelry polishing waste consists of free gold or silver particles that coat the jewelry equipment. It includes plastics, abrasive paste, and metal dust as a mixture. Floor sweepings waste, one of the most produced jewelry waste, is composed of precious metals with high amounts of dust and material debris. A significant amount of noble metal is lost as a result of rubbish from operator’s hands, faces, and clothes for laboratory cleaning. These wastes are called handwashing residues and are composed of organic wastes, such as soap and coffee powder that cause problems in the recycling process [11, 14,15,16]. Apart from all these wastes, some part of noble metals are discharged into the sewage system by digestion of operators. There is no study in the literature and industrial-scale application, due to the difficulty of recovering metallic values from this organic waste.
The chemical composition of such wastes is very different when compared to the run of mine ore. The most common ways to process jewelry wastes are chemical methods such as leaching and smelting. It is well known that hydrometallurgical processes require less capital. If the metal concentration of the subjected ore is low it may become more efficient than pyrometallurgy. The cupellation process is generally preferred in small- to large-scale refineries. Floor sweepings, tissues, and other materials that contain a low amount of noble metals are incinerated under the controlled conditions. In this way, escaping fine particles with flue gas is prevented. To dissolve gold-containing metals in lead, a certain amount of lead is added to the waste material inside the furnace at around 1000–1100 °C. Then, slag-forming materials are added to generate the metal and slag phase. As a result of these processes, while the precious metals remain in the molten phase, the wastes are discharged with the slag. The refining process can be carried out using cyanide. Zinc or aluminum powder is added to precipitate the precious metals from the pregnant solution. After the filtration and drying process, the precipitated metals are mixed with scraps [11]. One of the major disadvantages of the pyrometallurgical process is that it generates hazardous gas and waste production that can cause high pollution in the atmosphere. Besides, due to the outdated production techniques and relatively low temperature, the dispersed metal particles remain in slag and cause the loss of metallic values [17].
In terms of metal recovery, the jewelry wastes are important resources and, therefore, various researchers have made their studies on this subject [12, 18,19,20,21,22,23,24,25]. Chmielewsky and co-workers have applied a dual process containing roasting and aqua regia leaching. The gold in pregnant solution was then recovered by diethyl malonate as a selective solvent extraction technique [26]. Potgieter et al. used nitric acid to selectively dissolve silver. The unaffected waste matrix containing gold and platinum was then subjected to aqua regia leaching and the precious metals were then precipitated selectively [24]. Hydrometallurgical techniques are generally subjected to run of mine ore or concentrates to recover precious metals with high purity and recovery rates [27,28,29]. Although the main drawback of this process is the usage of a high amount of water and/or chemicals, the cyanidation process is still the most preferred method for recovering precious metals such as gold and silver [30, 31]. The negative effects of the chemical processes can be reduced by producing a pre-concentrate before the final treatment. Therefore, the efficiency of each stage can be improved [3, 25, 31,32,33]. The coarse gold fraction is easily recovered by gravity concentrators and higher recovery rates can be achieved when the liberated gold is coarser than 50 μm [3]. However, the recovery of gold particles reduces considerably as the particle size diminishes. As a common physicochemical separation technique, flotation is generally applied to ores and increases the content of valuable materials. The fine gold fraction can be easily floated using appropriate collectors (e.g. xanthate). If the gold concentrate grade is high, alternative methods, such as smelting, other than cyanide leaching may be feasible [25]. The concentrate containing valuable fraction is then finalized by chemical/metallurgical. The production of ores that were not economically viable was made possible in parallel with the developments in flotation technology [34,35,36].
Compared to primary resources jewelry sweeping wastes are generated in small quantities; however, they contain a very high amount of Au&Ag contents. In this study, the recovery of noble metals such as Au and Ag from FSJW sample was carried out by direct leach and flotation followed by leaching. Collectors were used to improve the hydrophobicity of metallic fractions and the effects of pH, reagent dosage, and particle size were investigated. The chemical contents of the representative sample and products were examined by inductively coupled plasma atomic absorption spectroscopy (ICP-AAS) after aqua regia digestion. The x-ray powder diffraction (XRD) method was used to characterize the waste sample. Finally, the cyanide leaching method was subjected to compare the Au&Ag dissolution rate with and without flotation.
Materials and methods
Characterization of floor sweeping jewelry waste
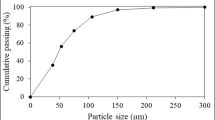
About 200 kg FSJW sample was representatively collected from jewelry workshops at Kuyumcukent (in Turkish, Jewelry-city), Istanbul over a period of 1 month (Fig. 1). Prior to the beneficiation studies, sampling and dividing processes were applied and a quarter of the sample was representatively separated and then taken to the drying process (around 105 °C). Visual studies demonstrated that the representative sample comprises combustible (paper, wood, plastic, etc.) and incombustible (pots, sand particles, molds, etc.) fractions. To avoid any problem of combustible materials during the enrichment process, the sample was fed to a steel container with top-covered sieve (2 mm opening) and incinerated at atmospheric conditions for approximately 12 h. Therefore, the fine particles escaping with ashes were captured. Particle size distributions of FSJW sample were obtained by sieve analysis and classified into eight fractions. Cupellation method was applied to find out Au and Ag contents in feed and different size groups. Ag analysis was finished with gravimetric method and Au with AAS/ICP and gravimetric method. The analysis accuracy was over 99%. The detection limits of Au&Ag were in the range of 0.005–10 ppm and 0.5–100 ppm, respectively.
Flotation
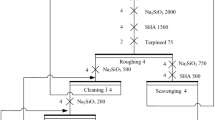
The flotation technique is frequently used in mineral processing and is very beneficial for recovering metallic particles at fine fraction. For this purpose, the froth flotation method was preferred and adapted to FSJW for capturing Au&Ag particles and decrease the amount of material to be fed to the leaching process. 300 g of the sample was mixed with 1500 mL of tap water in a batch-type mechanical flotation cell (Agitator-type, Denver, USA) with an impeller speed of 1500 rpm. Aerofloat 242 and Aerophine 3418A from Cytec Solvay Group (New Jersey, USA) were chosen as flotation collectors. H2SO4 was used to adjust the pH levels. Aero 242 has a frother ability, so no frother was added during flotation studies. Following the aforementioned stages and conditions, the effects of pH, particle size of feed, and reagent dosage were investigated. At the end of the experiments two products, froth and tailing were produced. Materials in the froth and tailing products were identified by microscopic studies. The flotation experiments were repeated two times to assure the validity of the results. The statistical error was calculated as ± 3%.
Leaching
Mining is one of many industrial activities where cyanide is used. Since 1887, low concentration solutions of cyanide were used to obtain gold and silver. Ores including gold particles are dissolved in dilute cyanide solution and, then gold is recovered from the gold-loaded solution at the subsequent stage [36, 37]. Parameters affecting the dissolution of gold in cyanide solutions are cyanide concentration, oxygen concentration, solution temperature, pH value of the solution, size of gold surface area, mixing speed, leaching time, and foreign ions in the solution [38]. The main advantages of cyanide leaching can be listed as widespread use in the industry, high dissolving yield, effective in activated carbon adsorption and well-known leach chemistry and mechanism. Although cyanide leaching is the most widely used method for recovery of gold and silver, the main drawback of this technique is toxicity and slow melting of precious metals.
In leaching experiments, cyanide solution was utilized to recover Au&Ag directly from FSJW sample and the froth product and to compare the efficiency of the two processes. The experiments were conducted by mixing 50 g of the sample and 200 mL of a solution containing NaCN. The IKA RW20 model propeller agitator was used and the slurry in 600 mL beakers was mixed with a speed of 500 rpm. The pH of slurry was monitored using a pH meter per hour. As the natural pH of the material is around 11, no pH adjuster was used during leaching. Oxygen was introduced into the solution by bubbling air into the slurry at a rate of 6 L/min volumetric flow. A vacuum filter was used for solid–liquid separation. In order to determine free cyanide concentration in the pregnant solution, the titration process was performed using a standard silver nitrate (0.02 mol/L) solution and rhodamine as an indicator. The dried filtered cake was assayed for Au and Ag and the dissolution efficiency of the process was calculated.
Results and discussion
Characterization of floor sweeping jewelry waste
In the characterization studies, primarily, the particle size distributions of representative FSJW sample were examined, and then the metal contents at different size groups were identified (Table 1). d50 was found around 400 μm and Au&Ag particles were distributed mostly below 100 µm. It was also determined that the + 6 mm fraction was composed of mainly glass particles, iron scraps, copper wires, and other contaminants that may affect the success of the following beneficiation processes. Therefore, this fraction was removed with 10 g/t Au and 26 g/t Ag and Au&Ag contents of the material to be fed to flotation and leaching were increased to 174 g/t and 1834 g/t from the feed assaying 143 g/t and 1490 g/t, respectively. The − 6 + 1 mm fraction that was unsuitable for flotation enrichment was reduced under 1 mm by roller crusher and then combined with − 1 mm before the beneficiation works.
The representative sample was comminuted under 50 µm using disc mill and then the characterization studies were completed using XRD analysis. According to XRD, the FSJW sample is composed of mainly calcium and magnesium compounds (71.1% calcite, 3.1% anhydrite, 6.1% magnesite, 14.1% magnesium hydroxide sulfate hydrate, 5.6% mono hydro calcite). These contaminants increase the pH value of the slurry to high levels (around 11). The precious metals such as Au&Ag could not be detected in XRD analysis since their contents are at ppm-level.
In a previous study of the author, more detailed characterizations by SEM and EDS analysis had been provided on a particle in the heavy product as a result of physical enrichment processes [25]. 76.8% Au and 23.2% Nb contents were observed on the layer of particle. Niobium is added to gold in a certain amount to increase its resistance against bending. Therefore, the Nb content of the selected particle was found to be high.
Recovery of Au&Ag by flotation
The optimum beneficiation of Au&Ag from the jewelry waste was studied by froth flotation technique. At primary studies, a wet ball mill was used to grind the material. As a result of milling operation, intense gas output was detected and then the material was flocculated in the following few hours. Therefore, the previously crushed − 1 mm material was ground in dry conditions using a ball mill to make it suitable for flotation. During the experimental studies, the effects of pH, particle size, and collector dosage were investigated.
The effects of the pH level (6.5, 8.5, and 10.8) on flotation were studied using a sample ground below 212 µm. The slurry was highly basic because of the excessive amount of calcite- and magnesite-containing materials. H2SO4 was used as a pH modifier to decrease the pH level. Unfortunately, the pH value could not be further reduced below 6.5 because of the reaction between carbonates and sulfuric acid. The flotation experiments were conducted under the following conditions: mixing speed of 1500 rpm, airflow rate of 3 L/min, 400 g/t of Aerophine 3418A, 400 g/t of Aero 242, condition time of 3 min, flotation time of 3 min. The results are presented in Fig. 2.
The pH value for gold flotation is dependent on the collector type when it is associated with other minerals such as chalcopyrite, pyrite or molybdenite. In a study by Klimpel and Isherwood, the collector S-701 performed the highest gold recovery at around 4.5 pH while F-100 exhibited its best when pH value was greater than 12 [39]. However, the outcome can vary depending on the nature of the gold and mineral matrix. In addition, a passivated mineral surface due to the formation of oxides and/or hydroxides adversely affects the flotation behavior of particles. As can be seen from Fig. 2 pH has a significant role in selectivity and recovery for Au&Ag particles. When pH decreases to 8.5, a concentrate containing 302 g/t Au and 2805 g/t Ag was obtained. However, the recovery rates were maximized (79.1% Au and 74.9% Ag) when the pH level was decreased to around 6.5 and a tailing containing 76 g/t Au and 963 g/t Ag was discharged. Therefore, pH 6.5 was selected as optimum for the subsequent experiments.
The particle size has a great effect on gold recovery due to its high specific density. For effective flotation of gold particles, the range of 20–200 μm is generally preferred. At grain sizes less than 20 μm, the selectivity decreases due to a large amount of floating tailings. After the determination of optimum pH value the experiments were performed at different feeding sizes (73, 100, 212, and 500 µm). The flotation conditions at the previous tests were kept constant. As seen in Fig. 3, a froth containing 478 g/t Au and 4876 g/t Ag with 53.8% and 52.1% recoveries was produced when the particle size of feed was selected as − 500 µm. However, considerable amounts of gold were observed in the − 500 µm flotation tail compared to smaller size fractions. Au&Ag concentrations of froth products of − 500 µm were greater than other fractions and the recovery of metallic fractions leveled off below 212 µm. To explain this situation, froth and tailing products were observed under the microscope. Some gold and silver particles were found to be stuck to the contaminant fraction in waste, whereas the other grains were in free form and in plated shape. Due to the fact that the surface shapes of the free metallic particles are close to the plane, they easily transport to the foam zone. Even their sizes are relatively coarse as the contents of metallic values in the froth increase. However, the short grinding time caused these particles to be insufficiently separated from the waste matrix to which they were attached, and Au&Ag recovery rates decreased due to the small amount of floated material. In parallel with the increase in milling time, the grain size decreased and a part of these metals took the plate shape while the rest were in a spherical formation due to their ductile and malleable properties (Fig. 4). Because of this reason, especially spherical shaped Au&Ag grains, having high specific weight, could not be efficiently moved to the bubble zone and sunk in the cell. Recovery decreases for small sizes is mainly related to the difficulty of particle/bubble adhesion. They can not acquire enough kinetic energy to produce a stable particle/bubble aggregate. Slime coating of particles might prevent direct contact of collectors and air bubbles and increase the pulp viscosity. To understand and control slime coatings in flotation is quite difficult. Although the contents of the concentrates obtained in sizes other than 212 µm were obtained higher, the recovery rates could not be improved for this reason. According to these findings, the optimum feeding size was accepted as − 212 µm.
In order to investigate the effect of collector dosage on metal recovery, the flotation experiments were conducted with 600 g/t and 800 g/t reagent dosages at various aforementioned stages. The other flotation conditions were kept the same as the previous tests and the results are illustrated in Fig. 5. It is clear that the flotation performance of metallic fractions was improved in terms of content and recovery by increasing the collector’s dosage. The results showed that a froth product containing 283 g/t Au and 2799 g/t Ag could be obtained with 86.6% and 81.5% recoveries at 600 g/t collector addition. 800 g/t reagent dosage could not improve the metal contents of the froth product. Only a slight increase in the recovery rates was observed due to a parallel increase in the amount of froth product. 600 g/t collector dosage was chosen as optimum because it gave a high gold grade and recovery. Consequently, the precious metal recovery was successfully improved adding more reagent; however, metallic components are still relatively heavy to be influenced by bubbles and remained in the cell during flotation.
Effects of flotation on cyanide leaching of Au&Ag
The recovery of precious metals from FSJW sample was investigated using cyanide leaching. At the first stage of the experiments, the jewelry waste ground below 212 µm was subjected to direct leaching and the parameters (leaching temperature, NaCN concentration, and time) affecting the dissolution efficiency were optimized. Then, the values of the parameters were adapted to the froth product to make a comparison.
The temperature has a direct effect in leaching reactions in two ways: it changes the reaction rate and improves the overall efficiency of leaching by increasing or decreasing the solubility of compounds. The effect of temperature was investigated by adapting leaching at 20, 40, and 60 °C temperatures under the following conditions: 1/5 of S/L ratio, 4 h of leaching time, and 2 g/L of NaCN concentration.
According to the results presented in Fig. 6, about 31% of the gold is extracted at 20 °C increased to 35% at elevated pulp temperature (40 °C). Similar trends were also observed for Ag dissolution rates. Although not very high, leaching temperature has a positive effect on gold and silver dissolution yields. The increase in temperature above 40 °C did not result in better dissolution results both for Au&Ag. At around 60 °C, the viscosity of the leach pulp increased dramatically and accordingly a decrease in dissolution yield was observed. The increased decomposition or consumption of cyanide due to temperature rise may result in loss of precious metal recovery. Another factor affecting the dissolution efficiency of metals in cyanide solution could be the decrease in gas dissolution rate (oxygen) at high temperatures. Considering the fact that the dissolution efficiency difference due to leaching temperature would increase more in the next parameters to be investigated, 40 °C of leaching temperature was kept constant and the tests were performed at higher NaCN concentrations.
This waste material, which is based on experimental studies, contains much more precious metal than an economic ore in which classical gold mining is processed. Generally, the cyanide concentration is practiced industrially in the range of 0.15–0.5 g/L. In some cases, the feed material may contain significant amounts of cyanide consumers and/or high content of Au&Ag. The subjected FSJW sample contains approximately 30–50 times more gold and silver contents than the classical gold ore. For this reason, the dissolution process requires higher cyanide concentration (2–10 g/L NaCN) than usual. Due to the low dissolution yields obtained in previous tests, it was decided to carry out experiments at higher cyanide concentrations. The effect of various NaCN concentrations on the amount of Au&Ag extracted from the jewelry waste is presented in Fig. 7.
As clearly seen from Fig. that both Au&Ag recoveries increased dramatically when NaCN concentration was shifted from 0.5 g/L to 12 g/L. When 12 g/L NaCN concentration was exceeded, no further gain in the leaching efficiency would be observed. High concentration of cyanide does not affect the dissolution rate of gold and excessive cyanide might cause the formation of cyanide complexes with impurities in the system. A concentration of 12 g/L NaCN may seem to be very high compared to the concentration used in industrial applications, which is around 0.3 g/L. Since the ore sample used in this study contains 174 g/t Au and 1834 g/t Ag, the use of 12 g/L NaCN concentration would be suitable. In this context, this value was kept constant for further tests.
By choosing the following conditions: 1/5 of S/L ratio, 12 g/L of NaCN concentration, and 40 °C pulp temperature. the effect of leaching time on Au&Ag dissolution efficiencies was studied. 4, 8, 12, 24, and 48 h leaching times were investigated and the results are represented by curves in Fig. 8. The results of the leaching test clearly showed that Au&Ag dissolution efficiencies that were 56% and 63% in the 4-h leaching test increased dramatically to 68% and 75% after 48 h leaching duration. No big change in the rate of recovery occurred when the leaching time was longer than 48 h. In industrial applications, very long leaching times are not preferred due to capacity problems. However, for this kind of material that contains high amounts of precious metals and normally fed in much lower amounts, the leaching times of 48 h and/or longer remain within acceptable limits.
The optimum leaching parameters at 1/5 of S/L ratio were 48 h cyanidation time, − 212 μm particle size, 12 g/L NaCN concentration, 40 °C pulp temperature, and 500 rpm stirring speed. Under these conditions, the NaCN consumption was found as 10.25 g/L. The leaching of the froth product at different leaching times was investigated under the optimum conditions mentioned above. Figure 9 shows the dissolution efficiencies of noble metals in direct leaching and flotation followed by leaching processes.
According to the repeated experimental results, it was seen that the test results slightly improved recovery at around 10% on average. NaCN consumptions had a parallel trend to Au&Ag recoveries. It increased to 11.35 g/L after flotation followed by leaching. The Au extraction efficiency before flotation was 68% that increased to 79% after flotation at 48 h leaching time. In parallel, 84% of Ag in froth product was extracted at the end of cyanide leaching. By producing a pre-concentrate before the final hydrometallurgical treatment the amount of unwanted fraction that was previously reported above was minimized and their negative effects on precious metal leaching were reduced. Reagents’ usage during flotation did not cause an adverse effect on Au&Ag extraction at cyanide leaching. According to these results, flotation could be a promising pre-processing technique to recover precious metals from FSJW.
Conclusions
This study investigated the recovery of noble metals from FSJW using flotation followed by cyanide leaching. The findings of this study could be summarized as follows:
- 1.
As alone flotation separation is not suitable for the final treatment of Au&Ag particles from FSJW. If the flotation conditions are investigated in more detail (bubble size, solid in pulp ratio, mixing speed, etc.) it may be possible to produce a pre-concentrate with Au content about 1000 g/t.
- 2.
Leaching reagent concentration, temperature, and time improved the recovery kinetics and the optimum recovery rate of the two noble metals. Au dissolution rates increased to 79% from 68% after flotation and the same trends were observed for Ag as well.
- 3.
It should be very beneficial if the coarser metallic fractions are recovered prior to the flotation process. Herewith, coarser Au&Ag particles that could not be efficiently frothed are concentrated by gravity or centrifugal separation techniques and the resulting metal loss could be reduced.
- 4.
In spite of some adversities such as metal losses in flotation tailings and slow leaching with NaCN, the findings of this study present an optional route for recycling of Au&Ag metals from jewelry wastes.
- 5.
This study was carried out to extract Au&Ag metallic fractions from floor sweeping jewellery wastes in a laboratory scale. The final refining of Au&Ag metals needs to be investigated further.
References
Ebin B, Petranikova M, Ekberg C (2018) Physical separation, mechanical enrichment and recycling-oriented characterization of spent NiMH batteries. J Mater Cycles Waste Manag 20:2018–2027
Mejame MPP, Kim YM, Lee DS, Lim SR (2018) Effect of technology development on potential environmental impacts from heavy metals in waste smartphones. J Mater Cycles Waste Manag 20:100–109
Burat F, Ozer M (2018) Physical separation route for printed circuit boards (PCBs). Physicochem Probl Mi 54:554–566
Prassad MS, Mensah Biner R, Pizarro RS (1991) Modern trends in gold processing—overview. Miner Eng 4:1257–1277
Loewen R (1989) Refining jeweler’s wastes. Proceedings, Santa Fe Symposium on Jewelry Manufacturing Technology 331
Yano J, Sakai S (2015) Waste prevention indicators and their implications from life cycle perspective: a review. J Mater Cycles Waste Manag 18:38–56
Yano J, Hirai Y, Okamoto K, Sakai S (2013) Dynamic flow analysis of current and future end-of-life vehicles generation and lead content in automobile shredder residue. J Mater Cycles Waste Manag 16:52–61
Arya S, Gupta A, Bhardwaj A (2018) Electronic waste management approaches—a pilot study in Northern Indian States. Int J Waste Resour 8:1–5
Sakai S, Yano J, Hirai Y, Asari M, Yanagawa R, Matsuda T, Yoshida H, Yamada T, Kajiwara N, Suzuki G, Kunisue T, Takahashi S, Tomoda K, Wuttke J, Mählitz P, Rotter VS, Grosso M, Astrup TF, Cleary J, Oh GJ, Liu L, Li J, Ma HW, Chi NG, Moore S (2017) Special feature: review, waste prevention for sustainable resource and waste management. J Mater Cycles Waste Manag 19:1295–1313
Canda L, Heput T, Ardelean E (2016) Methods for recovering precious metal from industrial waste. Mater Sci Eng, IOP Conf. Series, p 106
Corti CW (2002) Recovery and refining of gold jewellery scraps and wastes. The Santa Fe Symposium on Jewellery Manufacturing Technology 1–20. London
Delfini M, Manni A, Massacci P (2000) Gold recovery from jewellery waste. Miner Eng 13:663–666
Ferrini M, Manni A, Massacci P (1998a) Characterization and sampling of jewellery waste in Italy. In Proc. Second Biennial International Conference on chemical measurement and monitoring of the environment. Enviro Analysis 98, Ottawa, 529–534
Ferrini M, Manni A, Massacci P (1998b) Chemical analyses by ICP-AES of Jewellery waste in Italy. In Proc. second Biennial International Conference on chemical measurement and monitoring of the environment. Enviro Analysis 98, Ottawa, 501–506
Hoke CM (1982) Refining precious metal wastes: gold-silver-platinum metals: a handbook for the jeweler, Dentist and Small Refiner Metallurgical Publishing Company
Manni A, Saviano G, Massacci P (2001) Technical note: Characterization of gold particles in recoverable waste matrix. Miner Eng 14:1679–1684
Bellemans I, De Wilde E, Moelans N, Verbeken K (2018) Metal losses in pyrometallurgical operations—a review. Adv Colloid Interface Sci 255:47–63
Loewen R (1995) Small scale refining of jewellery wastes. Ronda R.Cordray Met-Chem. Research, Texas, pp 161–167
Embleton FT (1989) Refining of gold from jewellery scrap. Johnson Matthey Chemicals Ltd., England, pp 315–319
Ammen CW (1997) Recovery and refining of precious metals, 2nd edn. Chapman & Hall, New York
Corti CW (1997) Recovery and recycling in gold jewellery production. Gold Technol 21:11
Corti CW (1997) In-House gold refining: the options. Gold Technol 21:31
Mbaya RKK (2004) Recovery of noble metals from jewellery wastes, Doctorate Thesis, Tshwane University of Technology
Potgieter JH, Potgieter SS, Mbaya RKK, Teodorovic A (2004) Small-scale recovery of noble metals from jewellery wastes. J S Afr Inst Min Metall 104:563–572
Burat F, Baştürkcü H, Özer M (2019) Gold&silver recovery from jewelry waste with combination of physical and physicochemical methods. Waste Manag 89:10–20
Chmielewski AG, Urbanski TS, Migdal W (1997) Separation technologies for metals recovery from industrial wastes. Hydrometallurgy 45:333–344
Ashtari P, Pourghahramani P (2018) Hydrometallurgical recycling of cobalt from zinc plants residue. J Mater Cycles Waste Manag 20:155–166
Wang WY, Yen CH, Lin JL, Xu RB (2019) Recovery of high-purity metallic cobalt from lithium nickel manganese cobalt oxide (NMC)-type Li-ion battery. J Mater Cycles Waste Manag 21:300–307
Saca N, Dimache A, Radu LR, Iancu I (2017) Leaching behavior of some demolition wastes. J Mater Cycles Waste Manag 19:623–630
Habashi F (1997) Precious metals, refractory metals, scattered metals, radioactive metals, rare earth metals. In: Handbook of extractive metallurgy. Wiley, London, pp 1186–1279
Hoffmann JE (1992) Recovering precious metals from electronic scrap. JOM 44:43–48
Jha MK, Lee JC, Kumari A, Choubey PK, Kumar V, Jeong J (2011) Pressure leaching of metals from waste printed circuit boards using sulphuric acid. JOM 63:29–32
Menad N, Björkman B, Allain EG (1998) Combustion of plastics contained in electric and electronic scrap. Resour Conserv Recycl 24:65–85
Mallampati SR, Lee CH, Park MH, Lee BK (2018) Processing plastics from ASR/ESR waste: separation of poly vinyl chloride (PVC) by froth flotation after microwave-assisted surface modification. J Mater Cycles Waste Manag 20:91–99
Wills BA, Napier-Munn T (2006) Wills’ mineral processing technology, 7th edn. Butterworth-Heinemann, Oxford
Jeon S, Ito M, Tabelin CB, Pongsumrankul R, Kitajima N, Hiroyoshi N (2018) Gold recovery from shredder light fraction of e-waste recycling plant by flotation-ammonium thiosulfate leaching. Waste Manag 77:195–202
Logsdon M, Hagelstein K, Terry I (2001) The management of cyanide in gold extraction. ICME 1–40
Yüce E (1997) Gold mining with environmental impacts and trends (in Turkish), TMMOB Chamber of Mining Engineers Istanbul Branch Working Report
Klimpel R, Isherwood S (1993) Some new flotation products for the improved recovery of gold and platinum, Randol Gold Forum, Beaver Creek '93, 105–111
Acknowledgements
The present study is based on the results of the undergraduate theses conducted at the Istanbul Technical University. We would like to thank SAY Ramat Company for providing the sample and financial support of chemical analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Burat, F., Demirağ, A. & Şafak, M.C. Recovery of noble metals from floor sweeping jewelry waste by flotation-cyanide leaching. J Mater Cycles Waste Manag 22, 907–915 (2020). https://doi.org/10.1007/s10163-020-00982-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-020-00982-y