Abstract
Fluidized bed gasifiers (FBGs) and gas engines (GEs) could be available as waste-to-energy technology, because most small- and medium-scale municipal solid waste treatment plants have low electricity generation efficiencies. As feedstock composition vary widely based on regional characteristics, clarifying the relationship between gas and tar generation behaviors and feedstock is useful for the design of the GE generation process to predict gas and tar yields and compositions. To understand the synergistic effect of feedstock characteristics in fluidized bed gasification, flash pyrolysis of wood pellet, polyethylene, and polypropylene at 900 °C was conducted. Yields and compositions of gasses and tar from single and co-pyrolysis were investigated. The results reveal that co-pyrolysis increases the gas yield because of oxygenates and moisture present in the wood pellet. Tar yields found to decrease while polycyclic aromatic hydrocarbons (PAHs) are not reduced even during co-pyrolysis. That is because most chain hydrocarbons of tar are converted to CmHn gases and PAHs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It was estimated that in 2017 in Japan, 86% of the 9.03 million tons of plastic from both municipal and industrial waste streams was recycled, of which the highest percentage (58%) was attributable to thermal (energy) recycling, followed by material (mechanical) recycling at 23%, and chemical recycling at 4% [1]. As an example of thermo-chemical recycling technologies for plastics waste stream, Lee et al. [2] conducted circulating fluidized bed gasification to study complex hydrodynamic characteristics by numerical modeling and simulation using computational fluid dynamics (CFD). There is a need to design efficient reactors to improve effective resource utilization with fluidized bed technology. Currently in Japan, only a few small- and medium-scale (i.e., capacity below 100 t/day) municipal solid waste (MSW) treatment plants can produce electricity because such plants have low electricity generation efficiency [3, 4]. The use of fluidized bed gasifiers (FBGs) and gas engine (GEs) could potentially enhance this efficiency. The lower heating value of product gas, the concentrations of tar and char, and impurities are important factors for the stable operation of GE and for the design of gas treatment processes. These factors depend on operational conditions and feedstock characteristics. However, as MSW consists of varying fractions of different components such as paper, food, plastics, wood, and rubber; therefore, it is difficult to estimate the composition of the product gas and by-products, such as tar, char, and impurities, generated via pyrolysis.
Pyrolysis tests conducted in previous studies are summarized in the supplementary materials. The typical pyrolysis temperature range for thermal processes is between 350 and 900 °C [5]. The pyrolysis mechanisms of biomass and plastics have also been studied [5,6,7,8,9,10,11]. Based on the rate of heat transfer to the materials, pyrolysis technologies are generally categorized into slow pyrolysis, fast pyrolysis, and flash (very fast) pyrolysis [12]. Slow pyrolysis involves a low heating rate (approximately 10 °C/s) within a temperature range of 400–500 °C and long residence time (5–30 min). The heating rate and residence time of fast pyrolysis are approximately 100 °C/s at 400–650 °C and 0.5–2 s, respectively, while those for flash pyrolysis are above 500 °C/s at 700–1000 °C and below 0.5 s, respectively [13]. Slow pyrolysis typically forms solid products with low oil production, while fast pyrolysis and flash pyrolysis generate large amounts of gases and oil. Several studies have explored different pyrolysis modes for pure and mixed biomass and plastics, including slow pyrolysis of pure wood biomass [14] and mixed wood biomass with plastics [15,16,17,18], fast pyrolysis [19], and flash pyrolysis for pure wood biomass [20], pure plastics [21, 22] and mixed wood biomass with plastics [23, 24]. Furthermore, several studies have conducted the co-pyrolysis of wood biomass and plastics for a wider temperature range (450–750 °C) and investigated the yield and composition of the target products (gas, tar, and char) to understand the interaction between these two feedstock types [15,16,17,18,19, 23, 24]. However, product type and proportion and the mechanism of product formation for different modes of single and co-pyrolysis require further clarification. Furthermore, knowledge of the yield and composition of gas and tar, and their behaviors and interactions during flash co-pyrolysis remains limited.
In our previous fluidized bed gasification experiment [25], the composition of generated gas differed not only with change in equivalence ratio and temperature but also with changes in feedstock characteristics. Gas yield and composition are important factors as they significantly affect the amount of electricity produced through GE. On the other hand, the amount of tar generated from refuse paper and plastic fuel (RPF) and wood pellet were similar. However, it was thought that their tar compositions differed as the chemical characteristics of the RPF and wood pellet used were different. In addition to the amount of tar generated, tar composition is also a significant factor for GE operation; for instance, heavy tar results in a higher dew point, which causes serious problems. It is necessary to properly select the solvent of the gas scrubber depending on the composition of tar. As there is an interaction between gas and tar generation behavior, which will also be affected by feedstock, clarifying such behavior is important for process design.
Gasification is a partial oxidation process that is usually applied for syngas while pyrolysis is oxidant free process. Flash pyrolysis with momentary residence time (below 0.5 s) generates gas, tar and char. Flash pyrolysis process is essential to know the devolatilization characteristics and to maximize the evolved volatile gases in high heating rate with shorter duration. The process was previously studied in several works [26,27,28] in different reaction furnaces to be probably reproduced the reaction conditions closer to those encountered as one of the processes in practical fluidized bed gasification. Fluidized bed gasification could be attributed a co-relationship between (1) flash pyrolysis and (2) pyrolysis gas partial combustion as it is generally known that the gasification reaction in FBG is completed immediately. To collect and organize data on the differences among feedstocks by fluidized bed gasification, this study first examined differences in flash pyrolysis behavior. Through flash co-pyrolysis of wood pellet, as representative of biomass, and polyethylene (PE) and polypropylene (PP), as representative of plastics, both synergistic and non-synergistic effects among difference of feedstock characteristics were observed.
Materials and methods
Feedstock
Wood pellet, PE, and PP were used as feedstock because the wood biomass and plastic fractions remaining in MSW after the removal of food waste are the main sources for RPF. RPF is produced from plastics, paper, and other materials such as wood and fabric from industrial wastes and well-separated MSW from both municipalities and the private sector [29, 30]. In addition, PE and PP account for 33.8% and 30.1% (total: 63.9%) of the waste plastic stream in Japan, respectively [31]. Although PE and PP have the same chemical composition, their structures are different.
The wood pellet was produced from a blend of white spruce, red pine, various cedar species and other woods without bark. Wood pellet was pulverized and screened to obtain particle sizes between 0.6 and 2.0 mm. Commercial grade resins of PE and PP with 3 mm were used. The characteristics of the wood pellet, PE, and PP are listed in Table 1. The mixing ratios between wood pellet, PE, and PP were varied in different tests as listed in Table 2.
Experimental procedure
Test conditions
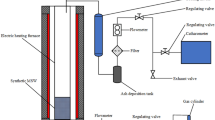
Flash pyrolysis experiments have been conducted using an electric heater at the gasification temperature under an inert atmosphere. A schematic diagram of the flash pyrolysis process and the associated experimental apparatus is shown in Fig. 1. The heating temperature of the electric furnace was fixed at 900 °C to achieve complete volatilization of the sample based on JIS M 8812:2004. After this set temperature was attained, 1.5 g of the sample to be studied was placed in a quartz sample boat, which was inserted into the center of the furnace, and heated for 120 min. N2 was used as the carrier gas at a flow rate of 50 ml/min, regulated using a mass flow controller (Model 3660, Kojima Instruments Inc., Kyoto, Japan). Each experimental test was done one time.
Gas sampling and analysis
Gas samples were collected using 1 L sampling bags every 15 min for 120 min (1, 15, 30, 45, 60, 75, 90, 105, and 120 min). These samples were collected after initiation of heating so that all the gas generated in the furnace could be captured. The gas samples were analyzed for H2, O2, N2, CH4, CO, CO2, and hydrocarbons (CmHn: C2H4, C2H6,C3H6,C3H8, i-C4H10, n-C4H10) using a gas chromatograph-thermal conductivity detector (GC-TCD, 490 Micro GC; Agilent Technologies Japan, Ltd.) with Molsieve 5A (Ar carrier) and PoraPLOT Q (He carrier) columns. Furthermore, the gas volume in each gas bag was measured with a Pyrex syringe. Product yields were calculated using Eq. 1:
where Py is the product yield, Pw is the product weight, and Fth is the fuel throughput.
Tar sampling and analysis
The sampling of tars was carried out in line with the pyrolysis experiment. A tar extraction module was fixed to the gas discharge side, of the pyrolysis system, before the gas sampling bags. The temperature at this point was maintained at 300 °C with a mantle heater to prevent tar condensation along the path of product gas. The product gas was allowed to pass through two impingers filled with 2-propanol in the tar extraction module to extract the tar present in the gas stream. Both impingers filled with the tar extraction solvent (2-propanol) were submerged in an ice cooling bath. The tars adhered to the reactor tube inside the furnace were washed with acetone and collected in the impingers. The tar extraction solvent was suction-filtered using a membrane filter (0.45 μm) to remove dust. The filtrate was concentrated in a rotary evaporator at 55 °C and 20 kPa (abs) to enable determination of tar yield. Tar components in the solvent were identified using a gas chromatograph-mass spectrometer (GC–MS; GC: 7820A/MS: 5977B, column: L 30 m × I. D. 0.25 mm, df: 0.25 μm, DB-5; Agilent Technologies Japan, Ltd.). Measurement of tar weight in both qualitative and quantitative analysis was carried out based on the guidelines provided in the sampling and analysis of tar and particles in biomass producer gases version 3.3 [32].
Results and discussion
Mass balance
Table 3 showed gas yield and composition and tar yield of each test. Approximately 60–84% of the input (1.5 g) was measured as gas, tar, and residue fractions. It was thought that there were (1) undetected tar which failed to be captured in two impingers and, therefore, partitioned into gas bags, and/or (2) volatilized tar fractions with lower boiling point such as benzene and toluene during concentrating the filtrate in a rotary evaporator at 55 °C and 20 kPa.
Gas yield and composition
Gas yield and composition and tar yield were different for pure and mixed feedstocks as listed in Table 3. In the flash pyrolysis experiments with pure feedstock (RUN1-3), CO was produced from wood pellet, while CmHn was produced from plastics. Flash pyrolysis of PE (RUN2) tended to generate higher levels of C2H4, while that of PP (RUN3) released higher amounts of CH4 and C3H6. The total gas yields were higher for mixed feedstock (69.6–73.5% in RUN4-6) than for pure feedstock (41.7–51.0% in RUN1-3).
The relationship between plastic content and gas yield is illustrated in Fig. 2. As shown in Fig. 2a and b, CO and CO2 yields increased with a decrease in plastic (PE and PP) content. This is derived from the cracking of oxygen containing function groups such as carboxyl and carbonyl [33]. The effect of water–gas reactions (C + H2O → CO + H2, C + 2H2O → CO2 + 2H2) caused by the oxygenates and moisture present in the wood pellet also contributed to it [16, 20]. CO and CO2 yields decreased from 31.6 to 9.4 wt.%, respectively, at 0% plastic content (pure wood pellet) to 24.1–24.6 wt.% and 6.3–6.6 wt.%, respectively, at 50% plastic content. As plastics do not contain oxygen, CO and CO2 yield at 100% plastic content were almost zero.
CH4 is generated by the cracking of methyl and methoxy and methylene groups [34]. As shown in Fig. 2c–e, the yields of CH4 and C3Hn from PP were higher than those from PE; however, the C2Hn yield from PE was higher than that from PP. This difference in CmHn yield may be attributable to the different structures of PP, which is a polymer of propylene, and PE, which is an ethylene polymer. The study findings confirm that feedstock structure affects syngas composition, even if the feedstocks have the same chemical composition. Furthermore, the methyl group present in PP may be converted into CH4 and C3Hn through cracking. For fluidized bed gasification process design, not only do chemical composition and heating value of feedstocks need to be accounted for, but also the chemical structure needs to be considered.
The yield of H2 from wood pellet was higher than that from plastics (Fig. 2f). This is likely because of the water–gas reaction caused by the fixed carbon and moisture present in wood pellet. However, the H2 yield was the highest (1.3–1.5 wt.%) at a plastic content of 50%. This may be because the moisture/steam and oxygen in wood pellet promotes H2 release from the aromatic hydrocarbons present in plastics. Hydrogen donor radicals promoted the H2 yield in the co-pyrolysis experiments owing to the higher H/C ratio of plastics [16].
There are some previous studies to support the results observed in this study. Hwang et al., Nedjalkow et al., and Matsunaga et al. [20,21,22] conducted flash pyrolysis of pure wood biomass and plastics at relatively high temperatures (700–900 °C). Hwang et al. [20] used wood chip, Refuse derived fuel, and RPF (70% paper and 30% plastic) as feedstock and observed that H2 and CH4 yields increased with increase in temperature. They suggest that the increase in the H2 yield was due to dry reforming reactions (CH4 + CO2 → 2CO + 2H2) and that the increase in the CH4 yield was because the decomposition of hydrocarbons released more CH4 than was consumed during the dry reforming reactions. At 900 °C, the CH4 yield from RPF, which contains plastic and wood, was higher than that from wood chip. While the CO and CO2 yields from woody biomass chip were higher than those from RPF due to oxidation and water–gas reactions; as discussed, the CmHn yield of wood chip was lower than that of RPF. This suggests that the oxygen and/or moisture present in the wood fraction reacts with chain hydrocarbons in the tar to form light hydrocarbon gases, as described in “Tar yield and composition”.
Kumagai et al. [18] conducted co-pyrolysis of beech wood and PE when heating from room temperature to 650 °C at a rate of 10 °C/min (slow pyrolysis) and reported an increased gas yield with increasing beech wood content. However, Fujita et al. [17] using the same feedstocks in flash pyrolysis and obtained different results. They observed that gas yield via co-pyrolysis was higher than that via pyrolysis of either beech wood or PE at a constant temperature (650 °C, flash pyrolysis). Fujita et al. [17] suggested that this difference may be attributable to the stabilization of radicals in beech wood due to hydrogen abstraction from PE pyrolysates in the vapor phase under rising temperature conditions [17]. In contrast, at a constant temperature [17], melted PE prevented the aggregation of levoglucosan from beech wood and increased levoglucosan yield, which in turn increased gas yield. In our study, co-pyrolysis at 900 °C improved the gas yield; similar results might be obtained even at medium temperatures (above 600 °C), but might not be obtained at lower temperatures (450–525 °C), at which fixed carbon in the feedstock cannot be decomposed by steam from the moisture in wood pellet [20, 23].
Overall, flash pyrolysis in this study confirmed that gas yield increases by mixing wood pellet and plastic materials because of oxygenates and moisture contents in wood pellet. Previous studies have shown a similar trend.
Tar yield and composition
The higher plastic content displayed higher tar yield as shown in Fig. 3; tar yields at plastic contents of 0%, 50%, and 100% were 4.3 wt.%, 5.5–6.5 wt.%, and 8.7–13.0 wt.%, respectively. Plastics are polymers (PE is a polymer of ethylene and PP is a polymer of propylene), which are hydrolyzed to hydrocarbons, including tar; therefore, plastics tend to have higher tar yields than do wood pellet. Some previous studies reported similar tar yield trends. Increases in tar yield with increasing plastic fraction was observed during co-pyrolysis of PE-wood and PE-paper mixtures at heating rates of 0.1 °C/s and 1 °C/s [15].
Tar composition determined via GC–MS analysis is shown in Table S2 of the supplementary materials. The peak areas of the total iron chromatograph (TIC) are proportional to the respective concentrations; higher peak area values mean a higher concentration of that component in the tar. Tar composition was different for the different feedstocks. However, benzene, toluene, naphthalene, styrene, acenaphthylene, and phenanthrene were always common tar components. Figure 4 shows the TIC of the tar from wood and PP during certain cases of retention time. The results from pure and mixed PE (Fig. S1 of the supplementary materials) show similar tendencies.
When pure PP was used as feedstock, several chain hydrocarbons, which are likely generated via thermal decomposition of plastic, were detected, as shown in Fig. 4a. Unlike PE, PP has a branched structure and contains CH3 and, therefore, branched-chain hydrocarbons were detected in tar generated from PP (see also Fig. S1). According to Murata and Makino [6], the by-products of PP thermal decomposition retain this branched structure, in which CH3 groups, derived from the monomers of PP (C3H6), are regularly arranged. Similarly, branched tars with regularly arranged CH3 groups were observed in this study. As shown in Fig. 4b, the pure PP and mixed PP with wood pellet released higher levels of heavy aromatics such as acenaphthylene, acenaphthylene, and pyrene as compared with pure wood pellet.
Polycyclic aromatic hydrocarbons (PAHs) are one of the major components of tar. As shown in Table S2, the same PAHs (naphthalene, methylnaphthalene, acenaphthylene, and phenanthrene) reported by Zhou et al. [14] were detected in the tar produced from PE in this study. Similarly, wood pellet in this study yielded similar PAHs as that reported by Zhou et al. [14] for cellulose.
This study confirmed that the tar yield decreases by mixing wood pellet with plastic fractions. Tar compositions revealed that most chain hydrocarbons are not observed when wood pellet and plastic mixtures are used as the feedstock, which implies that PAHs concentrations are relatively increased.
Mixture effects and behavior
This study considered the effects and interactive behaviors of mixing wood pellet and plastic fractions. Synergistic and non-synergistic effects among the different compositions (feedstocks) were observed.
Firstly, the two synergistic effects by co-pyrolysis were (1) increasing the gas yield, and (2) decreasing the tar yield. The gas yields were higher for mixed feedstock (RUN4-6) than for pure feedstock (RUN1-3) (Table 3; Fig. 2). By mixing wood pellet and plastic fractions, the cracking of oxygen containing function groups such as carboxyl and carbonyl [33] and water–gas reactions (C + H2O → CO + H2, C + 2H2O → CO2 + 2H2) [16, 20], which resulted in increasing CO and CO2 yields.
Furthermore, decreased tar was converted into gases. GC–MS analysis in this study (Fig. 4a) showed that thermal decomposition of plastics alone produces abundant tar as chain hydrocarbons, while in co-pyrolysis, there is less tar of chain hydrocarbons. This could be because O2 and/or steam released from the wood pellet reacted with chain hydrocarbons in tar to form light hydrocarbon gases such as C2Hn and C3Hn (Fig. 2). Therefore, it contributed to increasing the gas yield and reducing the tar yield. As illustrated in Fig. 3, tar yield was not proportional to plastic content; tar yield with mixed wood pellet and PE (RUN4) was slightly lower than at the proportional level with either pure wood pellet or pure PE. However, as shown in Fig. 2, gaseous CO and CmHn yields with mixed wood pellet and plastics were higher than those with either pure wood pellet or plastics. Hwang et al. [20] suggested that this behavior was due to oxygen and/or moisture in the wood fraction reacting with chain hydrocarbons in the tar to form light hydrocarbon gases. It was said that potassium in biomass facilitate the cracking reaction [35]. Jin et al. [36] also reported that the alkali and alkaline earth metals in biomass ash act as catalysts for secondary co-pyrolysis reactions, such as cracking and dehydrogenation to increase gases. According to Zhang et al. [10], as the thermal stability of biomass is lower than that of plastics during the co-pyrolysis process, free radicals from the degradation of biomass (with lignin being the major source) could promote the decomposition of plastic-derived macromolecules.
On the other hand, in terms of non-synergistic effects, this study revealed that PAHs are not reduced even if wood pellet is mixed, which means that the PP or PE fraction was halved (Fig. 4 and Fig. S1). It was reported that co-pyrolysis of cellulose and LDPE generate aromatics through Diels–Alder reactions followed by dehydration reaction [10]. Diels–Alder reactions are cycloaddition in cyclic transition state during harsh reaction conditions and formed by the concerted process for thermodynamically favoring compact cyclic nature such as BTX (benzene, toluene, xylene) and PAHs [10, 14]. It was also assumed that cracking of PP led to the removal of hydrogen radicals, after which carbon atoms would bond to form aromatic structures [10]. It has been suggested that dimerization of propargyl radicals (C3H3) derived from CH2 and C2H2 leads to the formation of benzene rings [37, 38]. In mixed wood pellet and PP (RUN5), as chain hydrocarbons in tar were converted to not only gases but also PAHs, tar yield seemed to the proportional trend (Fig. 4). Thus, the decrease in chain hydrocarbons of tars seemingly means that PAHs concentration is relatively increased. Moreover, it should be noted that increasing concentrations of PAHs derived from plastics increases the dew point of the tar, which significantly impacts GE operation and, therefore, these PAHs should be removed or reformed.
The synergetic effects observed in this study supported the results in our previous gasification test in FBG. Win et al. [25] showed that RPF tends to produce higher hydrocarbon gases than pure wood pellet. Previous gasification tests [25] also show similar tar yields among different feedstocks, which might be because the tar derived from plastic fractions in RPF was decomposed to hydrocarbons and PAHs owing to the existence of steam and oxygen.
Conclusions
Clarifying the relationship between gas and tar generation behaviors and feedstock is useful for process design of fluidized bed gasification and to predict gas and tar yields and compositions, as feedstock compositions vary widely based on regional characteristics. To collect and organize data on the differences of gas and tar generation behavior among feedstocks, flash pyrolysis tests for pure and mixed wood pellet, PE, and PP at 900 °C were conducted. As the synergetic effects of co-pyrolysis, tar yields were decreased because it was converted into light hydrocarbon gases. On the other hand, tar composition analysis via GC–MS revealed that co-pyrolysis did not reduce PAHs because chain hydrocarbons were converted into not only gasses but also PAHs.
Abbreviations
- FBG:
-
Fluidized bed gasifier
- GE:
-
Gas engine
- MSW:
-
Municipal solid waste
- PAHs:
-
Polycyclic aromatic hydrocarbons
- PE:
-
Polyethylene
- PP:
-
Polypropylene
- TIC:
-
Total ion chromatograph
- RPF:
-
Refuse paper and plastic fuel
References
PWMI (2019) An introduction to plastic recycling. Plast Waste Manag Inst (in Japanese). https://www.pwmi.or.jp/pdf/panf1.pdf. Accessed 8 Nov 2019
Lee JE, Choi HS, Seo YC (2014) Study of hydrodynamic characteristics in a circulating fluidized bed gasifier for plastic waste by computational fluid dynamics modeling and simulation. J Mater Cycles Waste Manag 16:665–676
MoEJ (2017) Japan waste dispoal status and processing situation. Minist Environ Japan (in Japanese). http://www.env.go.jp/recycle/waste_tech/ippan/h27/data/disposal.pdf. Accessed 25 Nov 2018
MoEJ (2007) Technologies to support a sound material-cycle society. Ministry of Environment Japan. https://www.env.go.jp/en/recycle/smcs/arep/2007gs_full.pdf. Accessed 25 May 2017
Al-Salem SM, Antelava A, Constantinou A, Manos G, Dutta A (2017) A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J Environ Manag 197:177–198
Murata K, Makino T (1975) Thermal degradation of polypropylene (in Japanese). Nippon Kagaku Kaishi 1:192–200
Takeshi K, Takashi S, Toshio H (1976) Thermal gasification of polypropylene (in Japanese). Nippon Kagaku Kaishi 976:322–327
Collard F-X, Blin J (2014) A review on pyrolysis of biomass constituents: mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew Sustain Energy Rev 38:594–608
Anuar Sharuddin SD, Abnisa F, Wan Daud WMA, Aroua MK (2016) A review on pyrolysis of plastic wastes. Energy Convers Manag 115:308–326
Zhang X, Lei H, Chen S, Wu J (2016) Catalytic co-pyrolysis of lignocellulosic biomass with polymers: a critical review. Green Chem 18:4145–4169
Wang S, Dai G, Yang H, Luo Z (2017) Lignocellulosic biomass pyrolysis mechanism: a state-of-the-art review. Prog Energy Combust Sci 62:33–86
Scheirs J, Kaminsky W (2006) Feedstock recycling and pyrolysis of waste plastics_converting waste plastics into diesel and other fuels. John Wiley Sons Ltd, NJ
John A. duton e-Education Institute (2018) The Pennsylvania State University Alternative fuels from biomass sources, Chapter 5.1 Biomass Pyrolysis. https://www.e-education.psu.edu/egee439/node/537. Accessed 19 Nov 2018
Zhou H, Wu C, Onwudili JA, Meng A, Zhang Y, Williams PT (2015) Polycyclic aromatic hydrocarbons (PAH) formation from the pyrolysis of different municipal solid waste fractions. Waste Manag 36:136–146
Grieco EM, Baldi G (2012) Pyrolysis of polyethylene mixed with paper and wood: interaction effects on tar, char and gas yields. Waste Manag 32:833–839
Alvarez J, Kumagai S, Wu C, Yoshioka T, Bilbao J, Olazar M, Williams PT (2014) Hydrogen production from biomass and plastic mixtures by pyrolysis-gasification. Int J Hydrog Energy 39:10883–10891
Fujita K, Kumagai S, Kameda T, Yoshioka T (2015) Co-pyrolysis behavior of beech wood and polyethylene mixtures at isothermal temperature. In: Proc 25th Annu Conf Japan Inst Energy, pp 72–73
Kumagai S, Fujita K, Kameda T, Yoshioka T (2016) Interactions of beech wood–polyethylene mixtures during co-pyrolysis. J Anal Appl Pyrolysis 122:531–540
Sophonrat N, Sandström L, Johansson AC, Yang W (2017) Co-pyrolysis of mixed plastics and cellulose: an interaction study by Py-GC × GC/MS. Energy Fuels 31:11078–11090
Hwang I-H, Kobayashi J, Kawamoto K (2014) Characterization of products obtained from pyrolysis and steam gasification of wood waste, RDF, and RPF. Waste Manag 34:402–410
Nedjalkov I, Yoshiie R, Nunome Y et al (2016) Products of high temperature gasification of ABS, PS and PE. J Combust Soc Jpn 186(58):402–410
Matsunaga S, Ueki Y, Yoshiie R, Naruse I (2017) Evaluation of pyrolysis behavior of waste plastic (in Japanese). In: The 28th Annual Conference of Japan Society of Material Cycles and Waste Management, pp 301–302
Bhattacharya P, Steele PH, Hassan EIBM, Mitchell B, Ingram L, Pittman CU Jr (2009) Wood/plastic copyrolysis in an auger reactor: chemical and physical analysis of the products. Fuel 88:1251–1260
Ephraim A, Minh DP, Lebonnois D, Peregrina C, Sharrock P, Nzihou A (2018) Co-pyrolysis of wood and plastics: influence of plastic type and content on product yield, gas composition and quality. Fuel 231:110–117
Win MM, Asari M, Hayakawa R, Hosoda H, Yano J, Sakai S (2019) Characteristics of gas from the fluidized bed gasification of refuse paper and plastic fuel (RPF) and wood biomass. Waste Manag 87:173–182
Önal E, Uzun BB, Pütün AE (2014) Bio-oil production via co-pyrolysis of almond shell as biomass and high density polyethylene. Energy Convers Manag 78:704–710
Scott DS, Plskorz J, Radleln D (1985) Liquid products from the continuous flash pyrolysis of biomass. Ind Eng Chem Process Des Dev 24:581–588
Shuangning X, Zhihe L, Baoming L, Weiming Y, Xueyuan B (2006) Devolatilization characteristics of biomass at flash heating rate. Fuel 85:664–670
Kodera Y (2012) Plastics recycling—technology and business in Japan. In: Waste Manag—An Integr Vision, Luis Fernando Marmolejo Rebellon, IntechOpen, pp 1–26
MoEJ (2012) Manufacturing business survey on refused paper and plastics fuel. RPF; Ministry of Environment Japan (in Japanese), pp 1–78
Japan Plastics Recycling Association (2018) Basic knowledge of plastics recycling (in Japanese). http://www.pwmi.or.jp/. Accessed 26 July 2019
Neeft JPA, Knoef HAM, Zielke U, Sjöström K, Hasler P, Simell PA, Dorrington MA, Thomas L, Abatzoglou N, Deutch S, Greil C, Buffinga GJ, Brage C, Suomalainen M (2001) Guideline for sampling and analysis of tar and particles in biomass producer gases. Version 3.3. Energy project ERK6-CT1999-20002 (Tar protocol)
Qu T-T, Guo W-J, Shen L-H, Xiao J, Zhao K (2011) Experimental study of biomass pyrolysis based on three major components: hemicellulose, cellulose, and lignin. Ind Eng Chem Res 50:10424–10433
Ma Z, Chen D, Gu J, Bao B, Zhang Q (2015) Determination of pyrolysis characteristics and kinetics of palm kernel shell using TGA-FTIR and model-free integral methods. Energy Convers Manag 89:251–259
Higman C, van der Burgt M (2008) Gasification, 2nd edn. Gulf Professional Publishing, Elsevier
Jin Q, Wang X, Li S, Mikulčić H, Bešenić T, Deng S, Vujanović M, Tan H, Kumfer BM (2017) Synergistic effects during co-pyrolysis of biomass and plastic: gas, tar, soot, char products and thermogravimetric study. J Energy Inst 92(1):108–117
Stein SE, Walker JA, Suryan MM, Fahr A (1990) A new path to benzene in flames. In: Twenty-Third Symp Combust, pp 85–90
Tonokura K (2014) Formation of PAH and soot particle in combustion. Earozoru Kenkyu 29(1):5–9
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Win, M.M., Asari, M., Hayakawa, R. et al. Gas and tar generation behavior during flash pyrolysis of wood pellet and plastic. J Mater Cycles Waste Manag 22, 547–555 (2020). https://doi.org/10.1007/s10163-019-00949-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-019-00949-8