Abstract
Effects of carbon concentration and Cu additive in simulated fly ash (SFA) and real fly ash (RFA) on the formation of polychlorinated dibenzofurans (PCDFs), polychlorinated dibenzo-p-dioxins (PCDDs), chlorobenzenes, and polychlorinated biphenyls which were all regarded as persistent chlorinated aromatics in iron ore sintering were investigated. In the annealing process of SFA with various carbon contents, the yield of chlorinated aromatics and the I-TEQ obtained their maximum at 10 wt% carbon content. Active carbon in SFA acted as the carbon source as well as an adsorbent which led to higher production of PCDD/F in solid phase at 10 wt% carbon content. The increase of carbon content will be beneficial on the formation of 2,3,7,8-Chloro-substituted PCDF compared with 2,3,7,8-Chloro-substituted PCDD. In addition, the CuCl2·2H2O was a much more powerful catalyst in the formation of chlorinated aromatic compounds compared with elementary Cu, since it served as both a catalyst and a chlorine donor. However, the RFA behaved similarly with SFA with elementary Cu in the formation of chlorinated aromatic compounds. The effect of carbon content and copper additives on formation of 2,3,7,8-chloro-substituted congeners displayed similar characteristics with the tetra- to octa-PCDD/F isomers and even the total PCDD/Fs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dioxins are the typical and toxic persistent aromatic compounds, composed of polychlorinated dibenzofurans (PCDFs), polychlorinated dibenzo-p-dioxins (PCDDs), and coplanar polychlorinated biphenyles (PCBs) [1]. Most dioxins are produced in the thermal treatment process, such as combustion, incineration, and metallurgical processes [2–4].
Dioxins are first discovered in the fly ash and flue gas in municipal solid waste incineration (MSWI) by Olie and Hutzinger [5]. Then, dioxin formation mechanism and pathway in the MSWI were investigated widely, and the emission control principles and technologies were also carried out in MSWI in the developed countries. As a consequence, iron ore sintering process became the most important sources of dioxins [6]. In 1995, in China, the produced dioxins in the iron ore sintering process accounted about 10 % of the total emissions [7], becoming the most dominated source of dioxins.
The mechanisms of dioxin formation can be summarized in three main pathways [6, 8]: (1) the homogeneous gas-phase reactions at high temperature of more than 500 °C, the heterogeneous gas–solid reactions at low temperature between 200 and 500 °C, including, (2) the precursor pathway which means that dioxins are mainly generated from precursors, such as chlorophenols, chlorobenzenes (CBzs), and diphenyl ethers [9], and (3) the de novo synthesis which means that dioxins are generated at 300–350 °C in the presence of carbon source, chloride source, and metal catalysts (Cu, Fe, etc.) [10]. The dioxin formation mechanism in the iron ore sintering is quite different from that of MSWI which might be caused by the different combustion technologies and raw materials between the two thermal treatment process. The sintering bed is divided vertically into four layers of sintering layer, combustion layer, dry layer, and moist layer. Dioxins are mainly generated by de novo synthesis from the dry layer [10, 11]. The dry zone of the sintering bed possesses the suitable raw materials and conditions for the de novo dioxin formation.
Carbon source is an essential factor in de novo dioxin formation. The type, size, concentration, and structure of the carbon source will all influence dioxin formation [12–14]. Coke had a similar structure with active carbon and had abundant specific surface area. It could be decomposed to carbon residue which could promote the formation of PCDD/Fs. Kawaguchi et al. [15] studied the effect of coke particle size on the formation of PCDD/Fs. The result indicated that the yield of PCDD/Fs increased almost ten times with the particle size of coke decreasing from 3 to 0.2–0.5 mm. Iino et al. [16, 17] treated the simulated fly ash (PAHs 0.1 %, CuCl2 5 %) at 400 °C in atmosphere of 10 % O2. PAHs which served as the carbon source reacted to generate 1,2,8,9-TCDF by the ring cleavage reaction and de novo synthesis. Ryan and Altwicker [18] tested the PCDD/Fs formation in the carbon mode system in the presence of five different carbon sources of activated carbon, graphic powder, two carbon blacks, and Spherocarb. The results showed that the effect of different carbon source in the PCDD/Fs formation was quite different which might be attributed to the morphology of the carbon. Activated carbon exhibited the most activity in the PCDD/Fs formation among all the carbon tested. Stieglitz et al. [19] investigated the effect of carbon concentration on the formation of PCDD/Fs, and discovered that the production of PCDD/Fs in the annealing process of fly ash at 300 °C showed a linear increase with carbon concentration increasing to 4 wt% and then showed no further increase. In the annealing of fly ash, just a tiny fraction of carbon was released as PCDD/Fs. Most carbon tended to react with oxygen and was released as oxocarbon [20].
The influence of metal catalysts on dioxin formation in iron ore sintering process has received increasing attention, since there exist different kinds of transition metals, such as Fe, Ni, Cu, Cr, and Ti in the iron ore sintering process which might influence the dioxin formation. Xhrouet [21] demonstrated that the oxidation temperature of carbon to CO2 decreased from >700 to 350–450 °C which might be attributed to the presence of copper in the fly ash. Takaoka et al. [22] investigated the effect of copper additives on the formation of chlorinated aromatic compounds in the real MSWI fly ash. They found that CuCl2·3Cu(OH)2 was the most reactive in the formation of chlorinated aromatics. Fujimori et al. [23] ranked the catalytic capacity of different metal compounds in forming chlorinated aromatics as CuCl2·2H2O > Cu2(OH)3Cl > FeCl3·6H2O > FeCl2·4H2O > CuO > Fe2O3 > PbCl2 > Blank > ZnCl2 > PbO > ZnO. Vogg et al. [24] investigated the PCDD/Fs formation by adding different metal chlorides. They demonstrated that the formation of PCDD/F remained at blank level (<0.5 ng/g) with FeCl2, CaCl2, and CdCl2, and still at quite low levels using MgCl2, ZnCl2, SnCl2, MnCl2, NiCl2, and HgCl2. However, the production of PCDD/Fs reached 25–60 ng/g with PbCl2, and relative higher amounts of 600 ng/g PCDD and 4300 ng/g PCDF with CuCl2. Matsumura et al. [25] also claimed that CuCl2 was a most active metal catalyst, with much higher PCDD/Fs productions than CuO, Cu, and CuSO4. CuCl2 was a most attractive metal catalyst in dioxin formation in iron ore sintering process, since, as well as a metal catalyst, CuCl2 could also provide chlorine source which would promote the Deacon reaction in iron ore sintering process [19, 26, 27].
Most researchers focused on the formation of chlorinated aromatics in the real fly ash in iron ore sintering or MSWI. We prepared the simulated fly ash according to the actual composition of RFA and compared the formation characteristics of chlorinated aromatics in the annealing of SFA and RFA to demonstrate the probable formation mechanism of chlorinated aromatics easily and visually. This study investigated the influence of carbon concentration and Cu catalysts on the formation of chlorinated aromatics in the annealing process of fly ash.
Materials and methods
The experiment design is as follows:
-
1.
Real fly ash (RFA) collected from an iron and steel plant was Soxhlet extracted by toluene for about 20 h for 3 times to remove the chlorinated aromatic compounds, and then, it was heated at 300 °C for 30 min.
-
2.
SFA samples were prepared by mixing Fe2O3 (35 wt% Fe), KCl (7 wt% Cl), and SiO2 matrix (other) with different amounts of active carbon (100–150 mesh, 2, 4, and 10 wt%), and then, they were heated at 300 °C for 30 min.
-
3.
SFA samples were prepared by mixing Fe2O3 (35 wt% Fe), active carbon (4 wt% C), KCl (7 wt% Cl), and SiO2 matrix (other) with different kinds of Cu compounds of Cu or CuCl2·2H2O (1.0 wt% Cu, 100–150 mesh), and then, they were heated at 300 °C for 30 min.
All fly ash samples were mixed and grinded to fine power with a particle size of less than 100 meshes. All experiments were repeated (n = 3–5). The RSD was in the range of 4.6–19.6 % and the internal standard recoveries were in the range of 88–126 %.
Characterization of real fly ash
Real fly ash used in this research was collected from the electrostatic precipitator (EP) of an iron and steel factory in China. The EP dust contains the essential materials de novo dioxin formation and is recycled in iron ore sintering [8, 21]. The RFA was analyzed by X-ray diffraction (LabX XRD-6000, Shimadzu Co., Japan) and X-ray fluorescence (EDX-1050, Sense instrument Co. Ltd, China) to determine the components and ratios of RFA. Moreover, the carbon concentration in RFA was analyzed by the elemental analyzer (Costech ECS 4010, Italy). The XRD scanning range of RFA was 10°–90° with a scanning speed of 5°/min. The XRD and XRF results were shown in Fig. 1 and Table 1, respectively. It can be concluded that the RFA mainly contains Fe2O3, KCl, CaSO4, and SiO2, and the concentrations of different elements are as follows: Fe 34.13 wt%, O 29.12 wt%, Ca 9.94 wt%, Cl 7.07 wt%, K 4.37 wt%, C 3.92 wt%, S 2.59 wt%, and Si 2.36 wt%. As we know, Cu compounds were effective catalysts in the de novo formation of chlorinated aromatics. However, the results showed that the Cu might exist as the probable form of CuFe2O4.
Experimental system
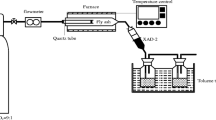
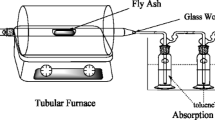
Figure 2 shows the schematic diagram for the annealing of fly ash. Five gram of fly ash was heated at 300 °C for 30 min in a tube furnace, (OTF-1200X, Hefei Kejing Material Technology Co., Ltd., China). 100 mL/min of mixed air (21 % O2 and 79 % N2, 99.999 vol.%, Beijing Haipu Gas Co., Ltd., China) was used as the carrier gas. The produced chlorinated aromatic compounds were absorbed by 200 mL toluene (J.T. Baker Chemical Co., USA). The pretreatment procedure for CBzs and PCB analysis was reported in the authors’ previous paper [28]. Moreover, the pretreatment of samples for PCDD/F analysis followed the US EPA Method 1613B (1997) similarly with Liu’s publication [29]. Quantitative analysis of the 17 2,3,7,8-chloro-substituted congeners and all tetra- to octa-chlorinated PCDD/F isomers was carried out.
Experiment setup for thermal treatment of fly ash [29]
Analytical methods
The produced CBzs and PCBs were analyzed according to the US Environmental Protection Agency (USEPA) Method 8280B by a gas chromatograph coupled with a mass spectrometer (GCMS-QP2010, Shimadzu Co., Ltd., Japan). The produced PCDD/Fs were analyzed according to the USEPA Method 1613B (1997) by a Hewlett-Packard Model 6890 gas chromatograph coupled with an AutoSpec Ultima mass spectrometer (Waters, USA) (HRGC-HRMS). A DB-5 capillary column (60 m, 0.25 mm, 0.20 µm, Agilent J&W) was used for sample separation of PCDD/Fs, CBzs, and PCBs.
The normalized distribution patterns were calculated by the formula as follows:
where N(XCDD) and N(XCDF) represent the normalized distribution ratio of each PCDD and PCDF homologue, XCDD and XCDF represent the amounts of different PCDD and PCDF homologues; and \(\sum {\text{PCDD}}\) and \(\sum {\text{PCDF}}\) represent the total amounts of PCDDs and PCDFs.
Results and discussion
Effect of carbon content
Carbon is a basic and essential element for the formation of chlorinated aromatics. The effect of carbon content on the total amount of PCDDs (sum of all tetra- to octa-chlorinated congeners), PCDFs (sum of all tetra- to octa-chlorinated congeners), dioxin-like PCBs (12 kinds of dl-PCBs), CBzs (sum of all di- to hexa-chlorinated congeners), and PCBs (sum of all di- to deca-chlorinated congeners) was shown in Fig. 3. The yield of PCDD, PCDF, dl-PCBs, and CBzs all displayed increasing trend from 449 pg/g-fly ash, 260 pg/g-fly ash, 4920 pg/g-fly ash, and 558 ng/g-fly ash to much higher values of 4779 pg/g-fly ash, 3302 pg/g-fly ash, 7540 pg/g-fly ash, and 846 ng/g-fly ash with the carbon content increasing from 2 wt% to 10 wt%. The yield of PCDD/Fs, dl-PCBs, CBzs, and PCBs all reached the maximum at a 10 wt% carbon concentration which was 1.2–13-fold the production of chlorinated aromatics at 2 wt% carbon content.
In this research, we just focused on the isomer patterns of PCDD/Fs. The detailed homologue distribution patterns of CBzs and PCBs were published in the author’s another paper [28]. The isomer patterns of PCDD/Fs in the heating process of SFA with different carbon concentrations were shown in Fig. 4. It was obviously observed in Fig. 4a that the yields of almost all PCDD/F isomers showed increasing trends with increasing carbon contents in the SFA from 2 to 10 wt%. Figure 4b shows the calculated percentage (Eqs. 1 and 2) of the PCDD/Fs homologues. It can be concluded that with a lower carbon content of 2–4 wt%, the higher chlorinated aromatics, such as OCDD/F and HpCDD/F, seemed to occupy more proportions in the total PCDD/Fs. OCDD and OCDF were the most dominant homologues, the amounts of which accounted for 76–79 and 48–65 % in the total PCDDs and PCDFs. However, with carbon content increasing further to 10 wt%, the distribution patterns moved to the lower chlorinated homologues. The proportions of OCDD/F and HpCDD/F decreased sharply, and instead, the proportions of lower chlorinated aromatics as TCDD/F and PeCDD/F increased to much higher values. As a consequence, the TCDD/F changed to be the most dominated homologues of PCDD/Fs. The increase of the carbon concentration in the fly ash would increase the adsorption ability of fly ash. Consequently, the solid-phase reaction dominated in the formation of chlorinated aromatics at higher carbon concentrations which might lead to the different reaction mechanism at lower carbon contents and higher carbon contents [30].
Figure 5 showed the homologue distribution of PCDD/Fs in gas and solid phases in the annealing process of SFA with different carbon concentrations. As can be seen from the depicted data, the chlorinated aromatics mainly occurred in the gas phase at the lower carbon contents of 2–4 wt%. At the high carbon content of 10 wt%, the main fraction of PCDD/Fs was found in the solid phase. Eventually, each PCDD/F homologue in the solid phase occupied more than 50 % of its total amount. It can be attributed to the specific porous structure and abundant adsorption superficial area of active carbon in SFA. The active carbon can serve as a carbon source as well as an adsorbent [14]. Therefore, the chlorinated aromatics mainly generate in the solid phase at a higher carbon content of 10 wt%.
Effect of copper additive
As most researchers’ discoveries, copper, and its compounds are principal catalysts in the formation of chlorinated aromatics [31]. In this research, the elementary Cu and CuCl2·2H2O were selected to be the typical copper additives in the SFA to investigate the effect of Cu catalysts on the formation of chlorinated aromatics. As shown in Fig. 6, the addition of Cu and CuCl2·2H2O in SFA resulted in the productions of 2.3 × 103–5.7 × 104 pg PCDDs/g-fly ash, 1.9 × 103–2.6 × 104 pg PCDFs/g-fly ash, 2.2 × 104–3.0 × 105 pg dl-PCBs/g-fly ash, 2.8 × 104–1.0 × 106 ng CBzs/g-fly ash, and 142–5.3 × 103 ng PCBs/g-fly ash, 3.5–89-, 3.5–48-, 3.5–48-, 47–1716-, and 1.9–71-fold that of SFA without Cu compounds, respectively. Moreover, CuCl2·2H2O exhibited a much powerful catalytic effect and obtained abundant chlorinated aromatic productions than elementary Cu, consistent with the results of the previous research [32]. CuCl2·2H2O served as both a metal catalyst and a chlorine donor. Chlorine promotes chlorination of the carbonaceous structures to form CBzs, PCBs, and PCDD/F. On the other hand, for SFA with elementary Cu, Cu just functioned as catalysts, and KCl was the only chlorine source [33].
In addition, Fig. 6 also displayed the production of chlorinated aromatics in the RFA. The amounts of chlorinated aromatics in the thermal treatment process of RFA were generally higher than that of SFA without any Cu additives except for dl-PCBs which can be attributed to the presence of copper compounds in the RFA; however, they were much lower than that of SFA with CuCl2·2H2O. It can be concluded that the Cu compounds in RFA had much worse catalytic activity that CuCl2·2H2O which corresponded with the XRD result of RFA. In fact, the amounts of chlorinated aromatics in RFA were nearly at the same level with that in the SFA with elementary Cu. In addition, almost all the chlorinated aromatics in RFA obtained a bit lower productions than in SFA with Cu except the PCDF.
Figure 7 showed the isomer patterns of PCDD/Fs in the thermal treatment process of SFA with different copper additives and RFA. It can be concluded from Fig. 7a that the amounts of almost all PCDD/F homologues catalyzed by different copper additives followed the order: CuCl2·2H2O ≫ Cu > Blank which was similar to the total PCDD/Fs. It can be observed from the normalized distribution pattern of PCDD/Fs (Fig. 7b) that higher chlorinated PCDD/Fs (OCDD/F and HpCDD/F) were more dominated homologues. OCDD and OCDF were the most dominant PCDD/F homologues. The proportions of OCDD and OCDF ranged at 86–94 and 63–76 % for SFA with and without copper additives as well as for RFA. In addition, although the yield of higher chlorinated PCDD/F homologues and total PCDD/Fs in RFA was at the similar level with that in the heating process of SFA with Cu, however, the productions of lower chlorinated PCDD/Fs (TCDD/F and PeCDD/F) in RFA were much higher than that in the SFA with Cu.
The distribution ratio of PCDD/F homologue in the gas and solid phases was shown in Fig. 8. Almost all PCDD/F homologues were detected in the gas phase in the annealing process of SFA with various Cu additives and RFA except for the TCDD/F and PeCDD/F in the SFA without Cu additives. As a consequence, the distribution pattern in gas and solid phase had a little relationship with copper additives and mainly related to the carbon in the fly ash.
Formation of 2,3,7,8-chlorinated aromatics in iron ore sintering
The 2,3,7,8-chlorinated PCDD/Fs attract most attention in the previous research, since they exhibit higher toxicity and threat human health and environment. The concentrations of all 17 toxic 2,3,7,8-chloro-substituted PCDD/F congeners, the ratios of 2,3,7,8-chlorinated PCDD/2,3,7,8-chlorinated PCDF, and the I-TEQ values for all experiments are listed in Table 2. It can be concluded that, similarly with total amounts and homologue amounts of PCDD/F, the yield of most 2,3,7,8-chlorinated PCDD/Fs increased generally with carbon content increasing from 2 to 10 wt% and obtains the maximum productions at a 10 wt% carbon content. In addition, the I-TEQ also increased from 1.1 to 17 with carbon content increasing from 2 to 10 wt%. Among all 2,3,7,8-chlorinated PCDD/F homologues, OCDD/F and HpCDD/F were also the most important homologues. The total amount of OCDD/F and HpCDD/F occupied more than 80 % of the total 2,3,7,8-chlorinated PCDD/Fs. With carbon content increasing from 2 to 4–10 wt%, the ratio of PCDD to PCDF decreased from 1.9 sharply to 1.1–1.2. Consequently, the increase of carbon content will enhance the formation of 2,3,7,8-chlorinated PCDFs.
Moreover, in the thermal treatment process of SFA with various copper additives, CuCl2·2H2O had the most efficient catalytic activity on the amounts of 2,3,7,8-chlorinated PCDD/Fs and the I-TEQ. OCDD/F and HpCDD/F also came to be the most dominated homologues. Among all the 2,3,7,8-chloro-substituted congeners, 2,3,7,8-TCDD/F, which was regarded as the most toxic compounds, obtained even the least production. The catalytic activity on the ratio of PCDD to PCDF was as follows: CuCl2·2H2O > Cu > Blank. It meant that the catalytic effect of copper additives on the formation of PCDD was superior to that of PCDF.
Summary of findings
This study aimed to identify the formation mechanism of chlorinated aromatics in fly ash from iron ore sintering. The findings can be summarized as follows:
-
1.
The total amounts and homologue amounts of PCDD, PCDF, dl-PCBs, and CBzs increased from 449 pg/g-fly ash, 260 pg/g-fly ash, 4920 pg/g-fly ash, and 558 ng/g-fly ash to much higher values of 4779 pg/g-fly ash, 3302 pg/g-fly ash, 7540 pg/g-fly ash, and 846 ng/g-fly ash with carbon concentration increasing from 2 to 10 wt%, and obtained the maximum productions at a 10 wt% carbon content. The chlorinated aromatics were mainly found in the solid phase at 10 wt% carbon content as a consequence of the porous structure and abundant adsorption surface area of active carbon.
-
2.
The addition of Cu compounds accelerated the formation of chlorinated aromatic compounds. The catalytic activity of Cu additives was as follows: CuCl2·2H2O ≫ Cu > blank. CuCl2·2H2O was the most potential catalyst in the formation of chlorinated aromatics, since it served as both a metal catalyst and a chlorine donor.
-
3.
The effect of carbon content and copper additives on the formation of 2,3,7,8-chlorinated PCDD/Fs was quite similar with that on the total amounts and fingerprints of PCDD/Fs. Consequently, the efficient control of carbon contents and the deactivation of the metal catalysts in iron ore sintering process will be helpful in the minimization of persistent chlorinated aromatics.
References
Fernandez-Gonzalez R, Yebra-Pimentel I, Martinez-Carballo E, Simal-Gandara J (2015) A critical review about human exposure to polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs) and polychlorinated biphenyls (PCBs) through foods. Crit Rev Food Sci Nutr 55:1590–1617
Liu G, Liu W, Cai Z, Zheng M (2013) Concentrations, profiles, and emission factors of unintentionally produced persistent organic pollutants in fly ash from coking processes. J Hazard Mater 261:421–426
Sun Y, Liu X, Kainuma M, Wang W, Takaoka M, Takeda N (2015) Dechlorination of polychlorinated biphenyls by iron and its oxides. Chemosphere 137:78–86
Sun Y, Takaoka M, Takeda N, Wang W, Zeng X, Zhu T (2012) Decomposition of 2,2′,4,4′,5,5′-hexachlorobiphenyl with iron supported on an activated carbon from an ion-exchange resin. Chemosphere 88:895–902
Olie K, Vermeulen P, Hutzinger O (1977) Chlorodibenzo-p-dioxins and chlorodibenzofurans are trace components of fly ash and flue gas of some municipal incinerators in the Netherlands. Chemosphere 6:455–459
Karasek F (1995) An overview of dioxin formation in combustion processes. Organohalogen Compd 23:315–319
Jia H, Song C, Dai Z, Gao B (2008) Formation mechanism and control of dioxins in the process of sintering. Sintering Pellet 33:25–30 (in Chinese)
Buekens A, Stieglitz L, Hell K, Huang H, Segers P (2001) Dioxins from thermal and metallurgical processes: recent studies for the iron and steel industry. Chemosphere 42:729–735
Kasai E, Hosotani Y, Kawaguchi T, Nushiro K, Aono T (2001) Effect of additives on the dioxins emissions in the iron ore sintering process. ISIJ Int 41:93–97
Harjanto S, Kasai E, Terui T, Nakamura T (2002) Behavior of dioxin during thermal remediation in the zone combustion process. Chemosphere 47:687–693
Nakano M, Hosotani Y, Kasai E (2005) Observation of behavior of dioxins and some relating elements in iron ore sintering bed by quenching pot test. ISIJ Int 45:609–617
Grandesso E, Gullett B, Touati A, Tabor D (2011) Effect of moisture, charge size, and chlorine concentration on PCDD/F emissions from simulated open burning of forest biomass. Environ Sci Technol 45:3887–3894
Cains PW, McCausland LJ, Fernandes AR, Dyke P (1997) Polychlorinated dibenzo-p-dioxins and dibenzofurans formation in incineration: effects of fly ash and carbon source. Environ Sci Technol 31:776–785
Stanmore BR (2004) The formation of dioxins in combustion systems. Combust Flame 136:398–427
Kawaguchi T, Matsumura M (2002) Method of sinter pot test evaluation for dioxins formation on iron ore sintering. Tetsu to Hagane 88:16–22
Iino F, Imagawa T, Takeuchiet M, Sadakata M, Weber R (1999) Formation rates of polychlorinated dibenzofurans and dibenzo-p-dioxins from polycyclic aromatic hydrocarbons, activated carbon and phenol. Chemosphere 39:2749–2756
Iino F, Imagawa T, Takeuchiet M, Sadakata M (1999) De novo synthesis mechanism of polychlorinated dibenzofurans from polycyclic aromatic hydrocarbons and the characteristic isomers of polychlorinated naphthalenes. Environ Sci Technol 33:1038–1043
Ryan SP, Altwicker ER (2000) The formation of polychlorinated dibenzo-p-dioxins/dibenzofurans from carbon model mixtures containing ferrous chloride. Chemosphere 40:1009–1014
Stieglitz L, Zwick G, Beck J, Roth W, Vogg H (1989) On the de-novo synthesis of PCDD/PCDF on fly ash of municipal waste incinerators. Chemosphere 18:1219–1226
Hell K, Stieglitz L, Altwicker ER, Addink R, Will R (2001) Reactions of 2, 4, 6-trichlorophenol on model fly ash: oxidation to CO and CO2, condensation to PCDD/F and conversion into related compounds. Chemosphere 42:697–702
Xhrouet C, Pirard C, De Pauw E (2001) De novo synthesis of polychlorinated dibenzo-p-dioxins and dibenzofurans on fly ash from a sintering process. Environ Sci Technol 35:1616–1623
Takaoka M, Yamamoto T, Shiono A, Takeda N, Oshita K, Matsumoto T, Tanaka T (2005) The effect of copper speciation on the formation of chlorinated aromatics on real municipal solid waste incinerator fly ash. Chemosphere 59:1497–1505
Fujimori T, Takaoka M, Takeda N (2009) Influence of Cu, Fe, Pb, and Zn chlorides and oxides on formation of chlorinated aromatic compounds in MSWI fly ash. Environ Sci Technol 43:8053–8059
Vogg H, Metzger M, Stieglitz L (1987) Recent findings on the formation and decomposition of PCDD/PCDF in municipal solid waste incineration. Waste Manag Res 5:285–294
Matsumura M, Kawaguchi T, Kasai E (2006) Effects of promoting and suppressing materials on dioxin emissions in iron ore sintering process. Proc Asia Steel Int Conf 2006:416–421
Suzuki K, Kasai E, Aono T, Yamazaki H, Kawamoto K (2004) De novo formation characteristics of dioxins in the dry zone of an iron ore sintering bed. Chemosphere 54:97–104
Li X, Zhang J, Yan J, Chen T, Lu S, Cen K (2006) Effect of water on catalyzed de novo formation of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans. J Hazard Mater 137:57–61
Sun Y, Liu L, Fu X, Zhu T, Buekens A, Yang X, Wang Q (2016) Mechanism of unintentionally produced persistent organic pollutant formation in iron ore sintering. J Hazard Mater 306:41–49
Liu H, Zhang Q, Cai Z, Li A, Wang Y, Jiang G (2006) Separation of polybrominated diphenyl ethers, polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins and dibenzo-furans in environmental samples using silica gel and florisil fractionation chromatography. Anal Chim Acta 557:314–320
Milligan MS, Altwicker E (1993) The relationship between de novo synthesis of polychlorinated dibenzo-p-dioxins and dibenzofurans and low-temperature carbon gasification in fly ash. Environ Sci Technol 27:1595–1601
Oberg T, Bergback B, Oberg E (2007) Different catalytic effects by copper and chromium on the formation and degradation of chlorinated aromatic compounds in fly ash. Environ Sci Technol 41:3741–3746
Takaoka M, Fujimori T, Shiono A, Yamamoto T, Takeda N, Oshita K, Uruga T, Sun YF, Tanaka T (2010) Formation of chlorinated aromatics in model fly ashes using various copper compounds. Chemosphere 80:144–149
Fujimori T, Takaoka M (2009) Direct chlorination of carbon by copper chloride in a thermal process. Environ Sci Technol 43:2241–2246
Acknowledgments
This research was financially supported by the National Natural Science Foundation of China (Project Nos. 21277010, 2141101075). We also gratefully acknowledge the funding for this research provided by the Beijing Municipal Science and Technology Committee (Project No. Z151100001515001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Liu, L., Sun, Y. et al. Formation of persistent chlorinated aromatic compounds in simulated and real fly ash from iron ore sintering. J Mater Cycles Waste Manag 19, 1437–1445 (2017). https://doi.org/10.1007/s10163-016-0537-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-016-0537-5