Abstract
This paper aims to make an overview on the current status and new tendency for recycling cathodic active materials from spent lithium-ion batteries. Firstly, it introduces several kinds of pretreatment technologies, followed by the summary of all kinds of single recycling processes mainly focusing on organic acid leaching and synergistic extraction. Then, several examples of typical combined processes and industrial recycling processes are presented in detail. Meanwhile, the advantages, disadvantages and prospect of each single process, combined process, as well as industrial recycling processes, are discussed. Finally, based on a novel acidic organic solvent, the authors briefly introduce an environmental friendly process to directly recycle and resynthesize cathodic active material LiNi1/3Co1/3Mn1/3O2 from spent lithium-ion batteries. The preliminary experimental results demonstrated the advantages of low reaction temperature, high separation efficiency and organic solvent cycling and preventing secondary pollution to the environment. This process may be used for large-scale recycling of spent lithium-ion batteries after further study.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lithium-ion batteries (LIBs) offer several advantages over other rechargeable batteries (e.g., nickel–cadmium and nickel metal–hydride rechargeable batteries), including high power and energy density, high potential, long storage life, low self-discharge efficiency and a wide operating temperature range [1, 2]. Thus, they are currently extensively used as electrochemical power sources in cellular phones, laptop computers, video cameras and other modern-life appliances [3, 4], and are expanding their market share in the area of rechargeable batteries. For example, according to the machinery statistics released by the Japanese Ministry of Economy, Trade and Industry (METI), the market share of LIBs in the secondary batteries increased from 28 % in 2001 to 67 % in 2010 [5]. Meanwhile, China, with the total production capacity of 2 966 million units and an increasing rate of 18.22 % in 2011, has become the largest production, consumption and exportation country of LIBs throughout the world [6]. Moreover, it is anticipated that the potential application of LIBs in the fields of electric vehicles and renewable energy storage will further expand their consumption in the near future [7].
With the wide application of LIBs in portable electronic equipment, it is obvious that a great number of spent LIBs will be produced due to the average service lifetime of 1–3 years [8]. In China, for instance, according to the operating report of the communication industry released by the Ministry of Industry and Information Technology (MIIT) in February 2012, the users of mobile telephones have exceeded one billion [9]. Assuming that one user is equipped with two units of LIBs, about 60 thousand tons spent LIBs will be generated in the next few years. Typically, lithium-ion rechargeable batteries are composed of crusts, electrodes (usually copper foil for anode and aluminium foil for cathode), electrolyte, and polymers [8, 10]. Most lithium-ion batteries use a kind of material like Li x MO2 in the cathode and graphite in the anode. The active materials used in the cathode include LiCoO2, LiNiO2, LiMn2O4, and so on. The LIBs contain toxic and flammable electrolyte, an organic liquid with dissolved substances like LiClO4, LiBF4 and LiPF6 [11–14]. Heavy metals, organic chemicals and plastics are also found in the spent LIBs with the proportion of 5–20 % cobalt, 5–10 % nickel, 5–7 % lithium, 15 % organic chemicals and 7 % plastics, and the composition varies slightly with different manufacturers [15]. The concentrations of some metals in spent LIBs are in relatively high levels, sometimes even higher than those in natural ores [16]. If the spent LIBs are disposed properly, these metals can be recovered and reused to manufacture LIBs in order to alleviate the pressure of natural resources shortage. Otherwise, these wastes will pose a threat to the environment and human health.
From the viewpoints of environmental preservation and valuable resources recovery, the recycling of spent LIBs is highly desirable. Actually, it could be very meaningful to recover battery materials to recycle in the manufacturing process of new batteries, which could promote the technology upgrade and sustainable development of the battery industry. Therefore, researchers all over the world have laid more emphasis on the study of technologies for recycling spent LIBs. The current status of the recycling processes and technologies of spent LIBs has been reviewed in several studies [11, 17–19]. Since more and more cathode materials are to be used in lithium-ion batteries, it is necessary to have a comprehensive understanding of the current status and new development on the recycling of spent LIBs and develop some new recycling technologies accordingly.
This paper mainly introduces the current status of recycling technologies of spent LIBs, including pretreatment technologies, several kinds of single processes, some typical examples of combined processes and industrial recycling processes. In addition, based on a novel acidic organic solvent, a novel process was proposed to directly recycle and resynthesize new LiNi1/3Co1/3Mn1/3O2 cathodic active material from spent LIBs.
Pretreatment technologies
Unlike other batteries, lithium-ion batteries often blow up during the recycling process due to radical oxidation, which is caused by the mechanical shock of lithium metal produced from battery overcharge from exposure to the air. [20]. Therefore, a preliminary treatment of spent LIBs before recycling is necessary. Meanwhile, this process can also reduce scrap volume, separate battery components and enrich valuable metals.
Li et al. [21] presented a pretreatment process combining crushing with ultrasonic washing, in which ultrasonic washing was used as an alternative process to improve the recovery efficiency of cobalt and reduce energy consumption and environmental pollution. After being crushed with a 12 mm aperture screen, the undersize products were put into an ultrasonic washing container to separate electrode materials from their support substrates. In addition, Li et al. [22, 23] used ultrasonic energy together with N-methylpyrrolidone (NMP) to separate cathodic active material and aluminium foil. As a kind of polar organic solvent, NMP was usually employed to separate the electrode materials from their support substrates, in which polar organic substrates (for instance, polyvinylidene fluoride, PVDF) were usually used as binders [8, 22–25]. The mechanism of this method is “Like Dissolves Like Theory” which means that any substance that has the same polarity (either polar or non-polar) can easily dissolve into each other. However, if the non-polar organic binders (for example, polytetrafluoroethylene, PTFE) were used in the LIBs, NMP can hardly separate the electrode material from aluminium foil because of the highly non-polar property of PTFE.
Besides the mechanical and physical methods, selective precipitation is also employed to separate the cathodic active material by dissolving aluminium foil with alkali solution [4, 26–28]. This method can efficiently remove more than 97 wt% aluminium. Moreover, the metals in the cathode materials (for instance, nickel, cobalt, manganese and lithium) hardly react with the alkali solution. However, this process makes the following separation steps complicated due to the introduction of aluminium ion into the leachate. In addition, thermal treatment was widely used to eliminate the conducting reagent and organic binder remained in the separated cathodic active materials [10, 22–26, 28, 29]. However, it often had a negative effect on the following chemical leaching process. For instance, Shin et al. [15] found that incineration of lithium cobalt oxide particles at 900 °C for 1 h to remove carbon and organic binder before chemical leaching significantly reduced the leaching efficiency.
Recently, vacuum pyrolysis was employed to separate the cathodic active material from spent LIBs [30, 31]. In these studies, the separated cathode sheets were uncurled manually after batteries dismantling and then placed in a vacuum pyrolysis system in which the temperature was kept at 600 °C for 30 min with the heating rate of 10 °C min−1 and the pressure was kept lower than 1.0 kPa. During the pyrolysis process, the organic materials (including electrolyte and binder) were evaporated or decomposed to low molecular weight products, so most of the cathodic active materials could not bind aluminium foil firmly and could be separated easily. Moreover, the recovered aluminium foil was intact because of no reactions in vacuum. It was also demonstrated that the quantity of active cathodic material peeled from aluminium foil was obviously different due to different kinds of adhesive reagents used and manufacturing methods, but the phenomenon of peeling was an inevitable result [30].
Recycling processes
The existing processes for recycling spent LIBs are pyrometallurgy [32], hydrometallurgy [16] and biometallurgy [33–35]. Pyrometallurgical processes are often associated with high gases emission and they require stringent gases filtration standards. The biometallurgical processes have been gradually replacing the hydrometallurgical processes because of their higher efficiency, lower cost and fewer industrial requirements [33], but the treatment period is longer and the bacteria required are difficult to incubate.
Hydrometallurgy is a kind of well-established process for the separation and recovery of metal ions, with such benefits as complete recovery of metals with high purity, low energy consumption [36], the minimization of wastewater and no gases emission [37]. It mainly consists of acid leaching [4, 8, 10, 15, 16, 21–31, 38–45], chemical precipitation [4, 8, 16, 21, 26, 28, 30, 38–45], chemical replacement [42, 43], solvent extraction [4, 16, 26, 28, 38, 41, 44, 46–52], hydrothermal reaction method [53–59], crystallization [27] and electrochemical methods [46]. In this paper, we only focus on two kinds of new processes developed in recent years.
Organic acid leaching process
The cathodic active material, which has been separated by a series of pretreatment steps, is leached by an acidic solution in order to transfer the metals of interest to the solution. The leaching of LiCoO2 from spent LIBs is usually carried out by using inorganic acids such as H2SO4 [4, 15, 16, 26–28, 31, 40, 44, 45], HCl [8, 21, 38, 41–43] and HNO3 [10, 22, 29, 39] as leachants, followed by the treatment of the acid leachate and final wastes. Metals were leached according to the following sequence in sulfuric media: aluminium > lithium > cobalt > copper [39]. During the leaching process, H2O2 [10, 15, 16, 22, 24–29, 31, 40, 42–45] is usually added to convert all cobalt or manganese to their divalent states, which are easily leached out by acid solution. A large amount of literature focusing on acid leaching has been published during the last decade in which the inorganic acid leaching reactions and mechanisms were well presented. When a strong acid solution is used as leachant, more than 99 % lithium and cobalt can be recovered. However, Cl2, SO3 and NO x are released during the leaching process and the acid obtained after leaching is a threat to the environment and human health.
Compared with inorganic acids, some organic acids have some favorable properties, including easy degradation, recyclable and avoiding secondary pollution to the environment. Most importantly, they also have excellent leaching efficiency. Thus, organic acid leaching processes are increasingly attracting the attention of researchers throughout the world.
For example, Li et al. [24] presented a hydrometallurgical process for the recovery of cobalt from spent LIBs which consisted of the following steps: (1) manual dismantling to separate the steel scraps, plastic and electrode material containing cobalt, (2) anode/cathode manual separation and treatment with NMP to recover copper and aluminium and (3) leaching with citric acid and hydrogen peroxide to transfer metals from the cathode material to the aqueous solution. The results showed that nearly 100 % lithium and more than 90 % cobalt were leached by using 1.25 M citric acid solution containing 1.0 vol.% hydrogen peroxide, leaching at 90 °C for 30 min and the solid/liquid (S/L) ratio of 20 g L−1. The leaching reactions of waste LiCoO2 with a C6H8O7·(H2O) solution in the presence of H2O2 may be represented as follows:
Meanwhile, Li et al. [25] reported an environmental friendly leaching process to recover cobalt and lithium from the cathode materials of spent LIBs. In the process, the easily degradable dl-malic acid (C4H6O5) was used as leachant. It was found that the concentration of dl-malic acid greatly affected the leaching efficiency of both cobalt and lithium. An increase in temperature and reaction time also enhanced the leaching efficiency of cobalt and lithium. The introduction of a reducing reagent such as H2O2 was found to be essential in accelerating the dissolution process. Under the optimal leaching conditions of 1.5 M dl-malic acid solution with 2.0 vol.% H2O2, leaching at 90 °C for 40 min and the S/L ratio of 20 g L−1, nearly 100 wt% lithium and more than 90 wt% cobalt were leached from the spent LIBs. The leaching reactions of waste LiCoO2 with dl-malic acid solution in the presence of H2O2 could be represented as follows:
Also, by combining vacuum pyrolysis, oxalate leaching and chemical precipitation, Sun and Qiu [30] developed an environmentally compatible process to recover cobalt and lithium from spent LIBs. In the process, oxalate was introduced as leachant and meanwhile as a precipitant which can leach and precipitate cobalt from LiCoO2 and CoO directly as CoC2O4·2H2O under the optimal experimental conditions of 1.0 M oxalate solution at 80 °C for 120 min with the S/L ratio of 50 g L−1. The reaction efficiency of LiCoO2 could reach more than 98 %; cobalt and lithium could also be separated during the hydrometallurgical process. The LiCoO2 leaching reactions with oxalate solution in the presence of H2O2 could be represented as follows:
Recently, Li et al. [23] put forward a novel process to recover cobalt and lithium from the cathode materials (containing LiCoO2 and aluminium foil) by the combination of ultrasonic washing, calcination and organic acid leaching. In the process, ascorbic acid was selected as both leachant and reducing reagent to improve the cobalt recovery efficiency. It was found that the efficiencies of cobalt and lithium could reach 94.8 and 98.5 %, respectively, under the optimal experimental conditions of 1.25 M ascorbic acid solution, leaching at 70 °C for 20 min and the S/L ratio of 25 g L−1. The LiCoO2 leaching reaction by ascorbic acid solution could be represented as follows:
Synergistic extraction process
After the electrode materials are leached by inorganic/organic acids, the obtained leachate usually contains metal ions of Al(III), Fe(III), Co (II), Ni (II), Mn(II), Cu (II), Li(I), etc. [4, 7, 15, 16, 26, 28, 38, 39, 44, 47]. Before solvent extraction and separation procedures, Al(III) and Fe(III) can be removed by selective precipitation [16, 26, 44]. Therefore, the studies of extraction and separation of nickel, cobalt, manganese, copper and lithium are significant. Such extractants as Acorga M5640 [4, 7, 28, 49], di-(2-ethylhexyl) phosphoric acid (D2EHPA) [49], bis-(2,4,4-tri-methyl-pentyl) phosphinic acid (Cyanex 272) [4, 16, 28, 44, 47, 48], trioctylamine (TOA) [7], diethylhexyl phosphoric acid (DEHPA) or 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester (PC-88A) [7, 26, 38, 47, 48] are usually used to separate the metals in some hydrometallurgical processes, in which nickel, cobalt, manganese, lithium and copper are usually recovered from spent LIBs.
Compared with a single extractant, mixtures of some extractants can produce the synergistic effects and increase the selectivities of metal extraction/separation in solvent extraction processes [49–52]. For instance, Wang et al. [47] investigated the extraction and separation of Co (II), Cu (II) and Mn(II) from sodium sulfate media by Cyanex 272, PC-88A and their mixture in n-heptane systemically. It was found that although the extractability of the mixed and single systems followed the order of PC-88A > Cyanex 272 + PC-88A > Cyanex 272, the mixed system had an evident synergistic effect on the extraction of manganese, cobalt and copper, especially at higher pHe and lower concentration of Na2SO4. The maximum synergistic enhancement coefficient, R max, was obtained at the mole fraction of X Cyanex 272 around 0.5. The sequence of synergistic enhancement effect was manganese > cobalt > copper. Extraction reactions were different between the single and mixed systems. Taking Mn2+ as an example, the extracted species in the single system was MnA2∙2HA or MnL2∙2HL, whereas the extracted species in the mixed system was MnAL∙HL. For the extraction of cobalt, copper and manganese, considering either extractability or selectivity, the mixed system was superior to the single extractant Cyanex 272.
Similarly, aiming at recycling cathode materials from spent LIBs, Zhao et al. [48] presented a process for the separation of Co (II), Mn(II) and Li(I) from sulfate medium by using Cyanex272, PC-88A and their mixture in n-heptane. They also found that the mixed Cyanex 272 and PC-88A system showed a distinct synergistic effect on the extraction of Co (II) and Mn(II). In addition, by adding EDTA into these systems, the separation factors between manganese and cobalt were greatly improved, especially for the mixed system, the separation factor enlarged around two orders of magnitude greater than those without EDTA.
Resynthesis of cathode material from spent lithium-ion batteries
Nowadays, LiCoO2 is used as cathodic active material in almost all commercialized LIBs because of its excellent performances. However, it still has several disadvantages, including high cost, rare cobalt resources, toxicity, etc. Therefore, recycling spent LIBs is significant both for environmental protection and resource reutilization. Meanwhile, it also has several advantages such as providing an alternative cobalt resource, eliminating environmental pollution and promoting the sustainable development of the battery industry. At present, most research work are focused on the recycling of cobalt, lithium and other valuable metals from spent LIBs, the resynthesis of cathode material directly from the spent LIBs is not emphasized, though some processes have been presented.
Contestabile et al. [8] presented a laboratory-scale lithium-ion battery recycling process, in which high temperature solid state reaction was employed to resynthesize LiCoO2. Firstly, the recovered cobalt hydroxide was calcined at 450 °C for 3 h to obtain Co3O4, and then Co3O4 was stoichiometrically mixed with Li2CO3. Secondly, the mixture was calcined in a muffle furnace at 400 °C for 5 h. Finally, the mixture was annealed at 700 °C for about 20 h after homogenizing in a mortar to synthesize the desired LiCoO2. It was found that the resynthesized LiCoO2 had good working capabilities, and could undergo various cycles with good capacity retention. Nan et al. [4] also used high temperature solid state reaction to resynthesize LiCoO2. In their process, the recovered lithium carbonate and cobalt oxalate from spent LIBs were used as precursors to prepare LiCoO2 electrode material. The mixture, in which the molar ratio of cobalt and lithium was adjusted to 1:1 after being ground and mixed equally, was calcined firstly at 600 °C for 6 h, and then ground again and pressed into tablets. The LiCoO2 active material was synthesized after the tablets were calcined at 800 °C for 10 h in the tube type stove. It was tested that the synthesized LiCoO2 had a specific capacity of 136 mAh g−1 at 0.2 C charge–discharge current.
Hydrothermal reaction [53–59] was also employed to resynthesize cathodic active material from spent LIBs. For example, Kim et al. [53] reported a novel process to resynthesize LiCoO2 from spent lithium-ion batteries in a single synthetic step by using hydrothermal method in a concentrated LiOH solution at 200 °C without any scraping procedures. The renovated LiCoO2 phase crystallized in the rhombohedral system with the space group R-3m and exhibited the first discharge capacity of 144.0 mAh g−1 and the discharge capacity retention of 92.2 % after 40 cycles. This method is expected to serve as an effective route for the direct resynthesis of cathodic active material from spent LIBs. The reaction mechanism is fully based on the “dissolution–precipitation” mechanism [56–59].
Besides the methods mentioned above, some other methods, such as the amorphous citrate precursor process (ACP) [29] and electrochemical technology [22], were also used to resynthesize LiCoO2 cathode material from spent LIBs. Moreover, the resynthesized LiCoO2 cathode material exhibited good electrochemical performances and cycling properties.
Examples of typical combined recycling processes
It is obvious that a single kind of recycling process such as dismantling, physical dissolution, thermal treatment, acid/alkali leaching, solvent extraction, chemical precipitation and electrochemical processes cannot complete the recycling tasks of spent LIBs. Therefore, several kinds of recycling processes should be combined together in order to recycle and resynthesize cathode material from spent LIBs. In the following part, the authors would like to introduce some examples of typical combined recycling processes developed in recent years.
Combined process of crushing, ultrasonic washing, acid leaching and chemical precipitation
Li et al. [21] proposed a new process of recovering cobalt from spent LIBs by a combination of crushing, ultrasonic washing, acid leaching and chemical precipitation, in which ultrasonic washing was used as an alternative process to improve the recovery efficiency of cobalt and reduce energy consumption and pollution. This process consisted of the following steps. Firstly, the spent LIBs were crushed with a 12 mm aperture screen, and the underflow products were put into an ultrasonic washing container with the ultrasonic frequency 40 Hz and electric power 100 W under 55 °C for 10 min, along with agitation to separate electrode materials from their support substrates. Then, the washed materials were filtered through a screen with a 2 mm aperture to get underflow products. Ninety-two percent of cobalt was transferred to the recovered electrodes where cobalt accounted for 28 % of the mass, and impurities including aluminium, iron, and copper accounted for 2 %. The valuable materials left in the products with diameter 2–12 mm, including copper, aluminium, and iron, were presented as thin sheets and could be easily separated. Finally, the recovered electrodes were leached with 4.0 M HCl at 80 °C for 2 h, along with concurrent agitation. About 97 % of lithium and 99 % of cobalt in the recovered electrodes could be leached out. The impurities could be removed at pH 4.5–6.0 with little loss of cobalt by the chemical precipitation method.
Compared with selective precipitation and physical dissolution methods, this combined process employed mechanical treatment as a pretreatment method which was an environmental friendly method if controlled properly. On the other hand, ultrasonic washing significantly alleviated both energy consumption and environmental pollution. Furthermore, the separation efficiency was enhanced by the “cavitation effect” resulting from ultrasonic waves. However, the cathodic active materials could hardly be separated from the aluminium substrate completely only by mechanical methods. Accordingly, the recovery efficiency would decrease.
Combined process of dismantling, crushing, physical dissolution, thermal treatment and organic acid leaching
Li et al. [24] presented a combined process of dismantling, crushing, physical dissolution, thermal treatment and organic acid leaching. Firstly, the spent LIBs were dismantled manually to remove both the plastic and steel cases that cover the batteries. Once dismantled, the anode and cathode were manually uncurled and separated, followed by immersion with N-methylpyrrolidone (NMP) at 100 °C for 1 h. The cathodic active materials were effectively separated from their support substrates and the recovery of both copper and aluminium in their metallic form was achieved. Secondly, after being dried at 60 °C for 24 h, the separated cathodic active materials were calcined at 700 °C for 5 h to remove the carbon and residual PVDF. Finally, the obtained cathodic active materials were leached with citric acid and hydrogen peroxide to transfer metals to the solution after being ground for 2 h. It was found that nearly 100 % lithium and more than 90 % cobalt were leached from the spent LIBs under the optimal reaction conditions of 1.25 M citric acid containing 1.0 vol.% hydrogen peroxide, leaching at 90 °C for 30 min with the S/L ratio of 20 g L−1.
In this method, NMP was employed to separate cathodic active material and aluminium foil from the spent LIBs. As a kind of polar solvent, NMP can effectively dissolve the organic binder such as PVDF. Consequently, the cohesive force between cathodic active material and aluminium substrate decreases dramatically. In addition, NMP can be recycled which will definitely decrease the operating cost, and citric acid is easily degradable compared with inorganic acids such as sulfuric acid. However, the dissolving ability of NMP depends on the properties of the organic binder and the manufacturing process of LIBs. If the non-polar organic binder (for instance, PTFE) was used in the electrode material, NMP can hardly dissolve it and separate the electrode material from aluminium substrate.
Combined process of dismantling, vacuum pyrolysis, organic acid leaching and chemical precipitation
Sun and Qiu [30] put forward an environmentally compatible process based on vacuum pyrolysis, oxalate leaching and chemical precipitation to recover cobalt and lithium from spent LIBs. In their process, the spent cathode materials were separated and uncurled manually after dismantling of batteries, and then placed in a vacuum pyrolysis system in which the separated cathode materials were calcined at 600 °C for 30 min and the system pressure was kept below 1.0 kPa. After lithium cobalt oxide was separated, oxalate was introduced as leachant meanwhile as precipitant which leached and precipitated cobalt from LiCoO2 and CoO directly as CoC2O4·2H2O with 1.0 M oxalate solution at 80 °C for 120 min and with the S/L ratio of 50 g L−1. The reaction efficiency of more than 98 % of LiCoO2 could be achieved and cobalt and lithium could also be separated efficiently during the hydrometallurgical process.
In this process, oxalate was employed as both leachant and precipitant which could shorten the process. Furthermore, oxalate is easily decomposed compared with inorganic acids. However, it is difficult to separate the cathodic active material from aluminium substrate completely only by vacuum pyrolysis, which will decrease the recovery efficiency in the following steps.
Typical industrial recycling processes
The economy of the spent LIBs recycling depends on the values of the metals separated from the spent LIBs as well as the recycling cost. Currently, some valuable metals are often used in LIBs. For example, cobalt, which is less available and, thus, more costly than other transition metals such as manganese, iron and nickel, is considered as a strategic metal in many countries. Indeed, data from the London Metal Exchange show that the price of cobalt is about twice as expensive as nickel and 15 times more expensive than copper. Also, with the popularity and familiarity of electric and hybrid vehicles increasing, the demand for nickel in the global automotive industry has increased remarkably. As a result, the cash price of nickel gradually recovered and returned to above $19 000 per ton in March 2010 [60]. It does not make any economic sense to recover lithium from LIBs in the existing electronic devices as they contain only a small fraction of lithium carbonate which is much less expensive compared to cobalt or nickel. It is obvious that the future development of electric vehicles will induce a large demand of lithium, while this could be partly provided from the spent LIBs. Considering the high cost and toxicity of cobalt, some less expensive metals, such as manganese and phosphorus, are used in the manufacturing of LIBs. In this case, the attraction of recycling spent LIBs would diminish, thus, it would be mainly for ecological benefits and for meeting the environmental regulations in the long-term. Furthermore, to get more economical profit, the recycling processes should be simple, flexible, and require less treatment procedures and equipment.
At present, the existing industrial recycling processes can basically be divided into pyrometallurgical and hydrometallurgical processes. Some recycling processes combine both pyrometallurgical and hydrometallurgical steps and often have integrated pretreatment steps like pyrolysis or mechanical processing. Typical examples of industrial recycling process are given as follows.
The company Batrec mainly employs a mechanical process to recycle Lithium-ion battery cells. Firstly, the batteries are crushed in a CO2 gas atmosphere. Thereby, the volatile organic electrolyte evaporates and is collected as non-usable condensate. A subsequent material separation is done and the different material fractions are sold and represent feedstock materials in other processes [18, 61, 62].
At first, the hydrometallurgical Toxco process was developed to recycle spent lithium primary batteries safely. Today’s facility processes lithium-ion battery scrap as well. The scrap is stored in earth covered concrete storage bunkers. Residual electrical energy is removed from larger and more reactive batteries. Then they are safely shredded and the materials are separated. Metals recovered from the spent batteries are collected and sold. The lithium components are separated and converted to lithium carbonate for resale. If cobalt is also contained in the batteries, it is also recovered to prepare LiCoO2 as new cathodic active material [18, 61, 63].
Some of the existing industrial recycling processes were not developed for recycling spent LIBs originally, such as the pyrometallurgical Inmetco process and the Xstrata process. The former process was developed for recycling spent NiCd, NiMH as well as Li-ion batteries. Thereby, only a small amount of Li-ion battery scrap is charged into an electric arc furnace as secondary feedstock besides iron containing material. The main aim is the recovery of cobalt, nickel and iron for the production of an iron-based alloy. Ignoble metals are slagged and organic materials as well as carbon are burned and used as reducing agents [18, 61]. In the Inmetco process, most of the battery components including the lithium are lost. Similar to the Inmetco process, the Xstrata process includes pyrometallurgical and hydrometallurgical process steps, in which only the cobalt, nickel and copper contents of spent Li-ion batteries are of interest, and all other battery materials are slagged or used as an energy source and reducing agents [64–67].
The Umicore VAL’EAS™ process represents a dedicated combined pyrometallurgical and hydrometallurgical process for recycling spent Lithium-ion and NiMH batteries. The battery scrap is directly melted down in a purpose-built shaft furnace without any pretreatment. Umicore is also a producer of cathode material for Lithium-ion batteries, i.e., the recycling process aims at a closed-loop recovery of cobalt and nickel in the form of LiCoO2 and Ni(OH)2. Ignoble metals, such as aluminium and lithium, are lost whereas copper, iron and manganese are recovered in an aqueous solution. Organic materials as well as carbon are burned and used as reducing agents [68–72].
The disadvantages of all pyrometallurgical recycling processes include high energy consumption, rigorous requirements for the treatment equipment as well as the lost of lithium. Therefore, a combination of pyrometallurgical and hydrometallurgical process steps is necessary as is the case for the Umicore process. However, the Umicore process does not aim at the recycling of lithium, which is the main disadvantage of this process. Furthermore, ignoble metals are slagged and organic materials as well as carbon are lost. With the increased potential application of lithium-ion batteries in (hybrid) electric vehicles, and, hence, the dramatically growing future demand for lithium, some alternative competing recycling processes need to be developed to recycle as many battery components as possible. Moreover, to adapt the evolution of cathode materials in the future, these recycling processes should be simple, flexible, low cost as well as environmental friendly.
Development of a novel process for recycling spent lithium-ion batteries
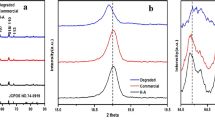
Recently, we invented a novel process in which an acidic organic solvent was employed to separate LiNi1/3Co1/3Mn1/3O2 cathode material and aluminium foil from spent lithium-ion batteries. The separation effect, purity of the recovered aluminium foil, mass distribution of the main metal species in leachate and recovered cathodic active material are illustrated in Fig. 1. The spent cathode materials containing LiNi1/3Co1/3Mn1/3O2 were cut into small pieces in order to promote the dissolving and leaching process. Then they were put into the solution with the acidic organic solvent concentration of 15 vol.% at 40 °C for 180 min and with the S/L ratio of 125 g L−1 along with agitation. It was found that nickel, cobalt and manganese mostly remained in the recovered cathodic active materials, and the impurities (aluminium, iron, copper, etc.) were less than 2 wt%. Furthermore, the molar ratio of nickel, cobalt and manganese in the recovered cathodic active materials was appropriately equal to 1:1:1. Therefore, after removing impurities, conducting reagent, residual binder and mixing stoichiometrically with Li2CO3, the LiNi1/3Co1/3Mn1/3O2 cathode material could be resynthesized by a high temperature solid state reaction method. The nickel, cobalt, manganese and lithium remaining in the leachate could also be used to prepare the precursor of cathode material by a co-precipitation method.
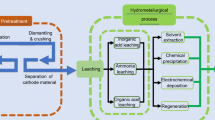
Compared with inorganic acids and alkalis, the acidic organic solvent could be reused in the subsequent separating and leaching steps. The preliminary experimental results demonstrated the advantages of low reaction temperature, high separation efficiency and organic solvent cycling and preventing secondary pollution to the environment. Based on this novel acidic organic solvent, an environmental friendly process to directly recycle and resynthesize LiNi1/3Co1/3Mn1/3O2 cathode material from spent LIBs may be developed, which was presented in Fig. 2.
At present, many manufacturers are trying to use less cobalt or quit using cobalt due to its toxicity and risks in supply. Therefore, many other transition metals, such as nickel, manganese and iron are substituting cobalt in lithium-ion batteries. However, the existing cobalt recycling processes could also be employed to recycle these batteries after a bit of modification. In the future, it is clearly that more and more power lithium-ion batteries with high capacity and excellent rate capability are needed to meet the requirements of electric vehicles (EVs) and hybrid electric vehicles (HEVs). The components and manufacturing technologies of these batteries are quite different from the present LIBs. To improve the capacity and rate capability, the water-based binders (for instance, PTFE) are more likely to be used in power lithium-ion batteries. On the other hand, the compress process would be adopted to prepare the cathodes of these batteries. Consequently, it is more difficult to recycle these batteries due to the highly non-polar property of PTFE and thus the greater cohesive force between the cathodic active material and aluminium. By the novel process we presented in this paper, the difficulty of recycling these spent batteries may be effectively solved.
Summary
At present, most work has focused on the recycling of valuable metals from spent lithium-ion batteries because of their attractive profits. Among these studies, LiCoO2 batteries are taken into consideration by most of the existing recycling processes. The studies on the recovery or disposal of other materials such as graphite, electrolyte in spent LIBs are very few. Therefore, the studies on the recycling of all components of spent LIBs need to be emphasized in the near future.
Hydrometallurgical processes are still the main methods employed to recycle or dispose of the spent LIBs. The potential application of other cathode materials, such as LiNi x Co1−x O2, LiNi x Mn1−x O2 and LiNi x Co y Mn1−x−y O2, will partly change the composition elements and manufacturing technologies of LIBs. This requires the recycling processes to be more flexible in order to recycle different kinds of cathode materials. Accordingly, the new recycling processes need to be further studied. In the future, the direct resynthesis processes from spent LIBs need to be developed in which the cathode materials are renovated through less pretreatment procedures and simple steps. The comprehensive recycling technologies are also needed to be developed to solve the problems of environmental pollution and secondary resource recovery due to the generation of spent lithium-ion batteries.
References
Etacheri V, Marom R, Elazari R, Salitra G, Aurbach D (2011) Challenges in the development of advanced Li-ion batteries: a review. Energy Environ Sci 4:3243–3262
Bruno Scrosati B, Hassoun J, Sun YK (2011) Lithium-ion batteries. A look into the future. Energy Environ Sci 4:3287–3295
Ra DI, Han KS (2006) Used lithium ion rechargeable battery recycling using Etoile-Rebatt technology. J Power Sources 163:284–288
Nan J, Han D, Zuo X (2005) Recovery of metal values from spent lithium-ion batteries with chemical deposition and solvent extraction. J Power Sources 152:278–284
http://www.meti.go.jp/english/statistics/index.html. Accessed 28 Dec 2011
http://www.chinabattery.org/index.php/archives/14608. Accessed 1 Oct 2012
Suzuki T, Nakamura T, Inoue Y, Niinae M, Shibata J (2012) A hydrometallurgical process for the separation of aluminum, cobalt, copper and lithium in acidic sulfate media. Sep Purif Technol 98:396–401
Contestabile M, Panero S, Scrosati B (2001) A laboratory-scale lithium-ion battery recycling process. J Power Sources 92:65–69
http://www.miit.gov.cn/n11293472/n11293832/n11294132/n12858447/14536179.html. Accessed 30 Mar 2012
Lee CK, Rhee KI (2003) Reductive leaching of cathodic active materials from lithium ion battery wastes. Hydrometallurgy 68:5–10
Bernardes AM, Espinosa DCR, Tenório JAS (2004) Recycling of batteries: a review of current processes and technologies. J Power Sources 130:291–298
Wu Q, Lu W, Prakash J (2000) Characterization of a commercial size cylindrical Li-ion cell with a reference electrode. J Power Sources 88:237–242
Iwakura C, Fukumoto Y, Inoue H, Ohashi S, Kobayashi S, Tada H, Abe M (1997) Electrochemical characterization of various metal foils as a current collector of positive electrode for rechargeable lithium batteries. J Power Sources 68:301–303
Chen JM, Yao CY, Sheu SP, Chiou YC, Shih HC (1997) The study of carbon half-cell voltage in lithium-ion secondary batteries. J Power Sources 68:242–244
Shin SM, Kim NH, Sohn JS, Yang DH, Kim YH (2005) Development of a metal recovery process from Li-ion battery wastes. Hydrometallurgy 79:172–181
Dorella G, Mansur MB (2007) A study of the separation of cobalt from spent Li-ion battery residues. J Power Sources 170:210–215
Bernardes AM, Espinosa DCR, Tenório JAS (2003) Collection and recycling of portable batteries: a worldwide overview compared to the Brazilian situation. J Power Sources 124:586–592
Espinosa DCR, Bernardes AM, Tenório JAS (2004) An overview on the current processes for the recycling of batteries. J Power Sources 135:311–319
Xu J, Thomas HR, Francis RW, Lum KR, Wang J, Liang B (2008) A review of processes and technologies for the recycling of lithium-ion secondary batteries. J Power Sources 177:512–527
Contestabile M, Panero S, Scrosati B (1999) A laboratory-scale lithium battery recycling process. J Power Sources 83:75–78
Li J, Shi P, Wang Z, Chen Y, Chang CC (2009) A combined recovery process of metals in spent lithium-ion batteries. Chemosphere 77:1132–1136
Li L, Chen R, Sun F, Wu F, Liu J (2011) Preparation of LiCoO2 films from spent lithium-ion batteries by a combined recycling process. Hydrometallurgy 108:220–225
Li L, Lu J, Ren Y, Zhang XX, Chen RJ, Wu F, Amine K (2012) Ascorbic-acid assisted recovery of cobalt and lithium from spent Li-ion batteries. J Power Sources 218:21–27
Li L, Ge J, Wu F, Chen R, Chen S, Wu B (2010) Recovery of cobalt and lithium from spent lithium ion batteries using organic citric acid as leachant. J Hazard Mater 176:288–293
Li L, Ge J, Chen R, Wu F, Chen S, Zhang X (2010) Environmental friendly leaching reagent for cobalt and lithium recovery from spent lithium-ion batteries. Waste Manag 30:2615–2621
Chen L, Tang X, Zhang Y, Li L, Zeng Z, Zhang Y (2011) Process for the recovery of cobalt oxalate from spent lithium-ion batteries. Hydrometallurgy 108:80–86
Ferreira DA, Prados LMZ, Majuste D, Mansur MB (2009) Hydrometallurgical separation of aluminium, cobalt, copper and lithium from spent Li-ion batteries. J Power Sources 187:238–246
Nan J, Han D, Yang M, Cui M, Hou X (2006) Recovery of metal values from a mixture of spent lithium-ion batteries and nickel-metal hydride batteries. Hydrometallurgy 84:75–80
Lee CK, Rhee KI (2002) Preparation of LiCoO2 from spent lithium-ion batteries. J Power Sources 109:17–21
Sun L, Qiu K (2012) Organic oxalate as leachant and precipitant for the recovery of valuable metals from spent lithium-ion batteries. Waste Manag 32:1575–1582
Sun L, Qiu K (2011) Vacuum pyrolysis and hydrometallurgical process for the recovery of valuable metals from spent lithium-ion batteries. J Hazard Mater 194:378–384
Paulino JF, Busnardo NG, Afonso JC (2008) Recovery of valuable elements from spent Li-batteries. J Hazard Mater 150:843–849
Mishra D, Kim D, Ralph DE, Ahn J, Rhee Y (2008) Bioleaching of metals from spent lithium ion secondary batteries using Acidithiobacillus ferrooxidans. Waste Manag 28:333–338
Zeng G, Deng X, Luo S, Luo X, Zou J (2012) A copper-catalyzed bioleaching process for enhancement of cobalt dissolution from spent lithium-ion batteries. J Hazard Mater 199–200:164–169
Xin B, Zhang D, Zhang X, Xia Y, Wu F, Chen S, Li L (2009) Bioleaching mechanism of Co and Li from spent lithium-ion battery by the mixed culture of acidophilic sulfur-oxidizing and iron-oxidizing bacteria. Bioresource Technol 100:6163–6169
Huang K, Li J, Xu ZM (2009) A novel process for recovering valuable metals from waste nickel-cadmium batteries. Environ Sci Technol 43:8974–8978
Pietrelli L, Bellomo B, Fontana D, Montereali M (2005) Characterization and leaching of NiCd and NiMH spent batteries for the recovery of metals. Waste Manag 25:221–226
Zhang P, Yokoyama T, Itabashi O, Suzuki TM, Inoue K (1998) Hydrometallurgical process for recovery of metal values from spent lithium-ion secondary batteries. Hydrometallurgy 47:259–271
Castillo S, Ansart F, Laberty-Robert C, Portal J (2002) Advances in the recovering of spent lithium battery compounds. J Power Sources 112:247–254
Aktas S, Fray DJ, Burheim O, Fenstad J, Acma E (2006) Recovery of metallic values from spent Li ion secondary batteries. Miner Process Extr M (Trans Inst Min Metall C) 115:95–100
Wang RC, Lin YC, Wu SH (2009) A novel recovery process of metal values from the cathode active materials of the lithium-ion secondary batteries. Hydrometallurgy 99:194–201
Li J, Li X, Hu Q, Wang Z, Zheng J, Wu L, Zhang L (2009) Study of extraction and purification of Ni, Co and Mn from spent battery material. Hydrometallurgy 99:7–12
Li J, Li X, Zhang Y, Hu Q, Wang Z, Zhou Y (2009) Study of spent battery material leaching process. Trans Nonferrous Met Soc China 19:751–755
Kang J, Senanayake G, Sohn J, Shin SM (2010) Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex 272. Hydrometallurgy 100:168–171
Kang J, Sohn J, Chang H, Senanayake G, Shin SM (2010) Preparation of cobalt oxide from concentrated cathode material of spent lithium ion batteries by hydrometallurgical method. Adv Powder Technol 21:175–179
Lupi C, Pasquali M (2003) Electrolytic nickel recovery from lithium-ion batteries. Miner Eng 16:537–542
Wang F, He F, Zhao J, Sui N, Xu L, Liu H (2012) Extraction and separation of cobalt(II), copper(II) and manganese(II) by Cyanex 272, PC-88A and their mixtures. Sep Purif Technol 93:8–14
Zhao JM, Shen XY, Deng FL, Wang FC, Wu Y, Liu HZ (2011) Synergistic extraction and separation of valuable metals from waste cathodic material of lithium ion batteries using Cyanex 272 and PC-88A. Sep Purif Technol 78:345–351
Cerpa A, Alguacil FJ (2004) Separation of cobalt and nickel from acidic sulfate solutions using mixtures of di(2-ethylhexyl)phosphoric acid (DP-8R) and hydroxyoxime (ACORGA M5640). J Chen Technol Biot 79:455–460
Cheng CY (2006) Solvent extraction of nickel and cobalt with synergistic systems consisting of carboxylic acid and aliphatic hydroxyoxime. Hydrometallurgy 84:109–117
du Preez AC, Preston JS (2004) Separation of nickel and cobalt from calcium, magnesium and manganese by solvent extraction with synergistic mixtures of carboxylic acids. J S Afr Inst Min Metall 104:333–338
Cheng CY, Zhang WS, Pranolo Y (2010) Separation of cobalt and zinc from manganese, magnesium, and calcium using a synergistic solvent extraction system consisting of Versatic 10 and LIX 63. Solvent Extr Ion Exch 28:608–624
Kim DS, Sohn JS, Lee CK, Lee JH, Han KS, Lee YI (2004) Simultaneous separation and renovation of lithium cobalt oxide from the cathode of spent lithium ion rechargeable batteries. J Power Sources 132:145–149
Yoshimura M, Han KS, Tsurimoto S (1998) Direct fabrication of thin-film LiNiO2 electrodes in LiOH solution by electrochemical-hydrothermal method. Solid State Ionics 106:39–44
Han KS, Song SW, Fujita H, Yoshimura M (2000) Single-step fabrication of Li1−x Ni1+x O2 and LiCoO2 films by soft solution-processing at 20–200°C. Solid State Ionics 135:273–276
Han KS, Tsurimoto S, Yoshimura M (1999) Fabrication temperature and applied current density effects on the direct fabrication of lithium nickel oxide thin-film electrodes in LiOH solution by the electrochemical-hydrothermal method. Solid State Ionics 121:229–233
Song SW, Han KS, Sasagawa I, Watanabe T, Yoshimura M (2000) Effect of LiOH concentration change on simultaneous preparation of LiCoO2 film and powder by hydrothermal method. Solid State Ionics 135:277–281
Watanabe T, Uono H, Song SW, Han KS, Yoshimura M (2001) Direct fabrication of lithium cobalt oxide films on various substrates in flowing aqueous solutions at 150°C. J Solid State Chem 162:364–370
Han KS, Song SW, Tsurimoto S, Fujita H, Sasagawa I, Choi KH, Kang HK, Yoshimura M (2002) Soft solution processing for direct fabrication of LiMO2 (M=Ni and Co) film. Solid State Ionics 151:11–18
U.S. Geological Survey (2011) Mineral commodity summaries 2011. U.S. Geological Survey, Reston, Virginia
Rentz O, Engels B, Schultmann F (2001) Environmental research plan of the German Federal Ministry for the Environment, nature conservation and nuclear safety. Research Project 299 35 330. French-German Institute for Environmental Research, Universität Karlsruhe (TH)
Bau-, Verkehrs- und Energiedirektion des Kantons Bern, GSA – Amt für Gewässerschutz und Abfallwirtschaft (Ed.) (2003) Altbatterien gehören nicht in den Kehrrichtsack, Abfallsplitter, Waste Information Canton Bern
Pistoia G, Wiaux JP, Wolsky SP (2001) Used battery collection and recycling. Elsevier Science, Amsterdam
Henrion P (2004) ICBR—international congress for battery recycling, Como
Henrion P (2008) EBR—electronics & battery recycling, Toronto
Henrion P (2008) ICBR—international congress for battery recycling, Düsseldorf
Tollinsky N (2008) Xstrata boosts recycling capacity. Sudbury Min Solut J 5:1–36
Chéret D (2004) ICBR—international congress for battery recycling, Como
Chéret D (2006) ICBR—international congress for battery recycling, Interlaken
Meskers CEM, Hagelüken C, Van Damme G (2009) Greeen recycling of EEE: special and precious metal recovery from EEE. In: Stanley M, Howard (eds) Proceedings of sessions and symposia sponsored by the extraction & processing division (EPD) of the minerals, metals & materials society (TMS), San Franscisco, California, pp 1131–1136
Siret C (2008) ICBR—international congress for battery recycling, Düsseldorf
Siret C, Van Damme G (2008) EBR—electronics & battery recycling, Toronto
Acknowledgments
This study was supported by the Chinese Academy of Sciences (No. 2011YDHZ-JSC02), Guangdong Province (No. 2011A032302001) and the China Postdoctoral Science Foundation (No. 2012M510553).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, X., Xie, Y., Lin, X. et al. An overview on the processes and technologies for recycling cathodic active materials from spent lithium-ion batteries. J Mater Cycles Waste Manag 15, 420–430 (2013). https://doi.org/10.1007/s10163-013-0140-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-013-0140-y