Abstract
Individuals with bilateral vestibular hypofunction (BVH) often report symptoms of oscillopsia (the perception that the world is bouncing or unstable) during walking. Efference copy/proprioception contributes to locomotion gaze stability in animals, sometimes inhibiting the vestibulo-ocular reflex (VOR). Gaze stability requires both adequate eye velocity and appropriate timing of eye movements. It is unknown whether eye velocity (VOR gain), timing (phase), or both are impaired for individuals with BVH during walking. Identifying the specific mechanism of impaired gaze stability can better inform rehabilitation options. Gaze stability was measured for eight individuals with severe BVH and eight healthy age- and gender-matched controls while performing a gaze fixation task during treadmill walking. Frequency response functions (FRF) were calculated from pitch eye and head velocity. A one-way ANOVA was conducted to determine group differences for each frequency bin of the FRF. Pearson correlation coefficients were calculated to determine the relationship between the real and imaginary parts of the FRF and the Oscillopsia Visual Analog Scale (oVAS) scores. Individuals with BVH demonstrated significantly lower gains than healthy controls above 0.5 Hz, but their phase was ideally compensatory for frequencies below 3 Hz. Higher oVAS scores were correlated with lower gain. Individuals with BVH demonstrated ideal timing for vertical eye movements while walking despite slower than ideal eye velocity when compared to healthy controls. Rehabilitation interventions focusing on enhancing VOR gain during walking should be developed to take advantage of the intact timing reported here. Specifically, training VOR gain while walking may reduce oscillopsia severity and improve quality of life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During locomotion, the ability to fixate gaze on objects and use optic flow for heading is essential (Pozzo et al. 1991; Patla and Vickers 2003). It has been suggested that the primary purpose of the vestibulo-ocular reflex (VOR) is to stabilize gaze during locomotion, when frequencies of head movement far exceed the compensatory capabilities of pursuit or optokinetic systems (Grossman et al. 1989; Leigh and Brandt 1993). Functionally, gaze stability can be thought of as eye movements at the correct speed (VOR gain) that occur at the correct time (compensatory phase). Inadequate gaze stability occurs when either the eye velocity is insufficient to compensate for head velocity, or when eye movement timing is delayed. Gaze instability during walking has been attributed to loss of function of the VOR (Crawford 1964; Leigh and Brandt 1993; Fetter 2007; Badaracco et al. 2010). After VOR failure, stationary environmental objects appear to “jump” during walking (Crawford 1964). However, complaints of oscillopsia are not consistent across all individuals with bilateral vestibular hypofunction (BVH) (Bhansali et al. 1993) and oscillopsiamay be more related to angular VOR capabilities than otolith (Brantberg and Löfqvist 2007).
Current physiological vestibular function tests do not characterize walking gaze stability or the daily life impact of oscillopsia experienced by individuals with BVH. Oscillopsia has been studied using scales of symptom severity (Oscillopsia Visual Analog Scale) (Herdman et al. 2007; Badaracco et al. 2010) and symptom frequency (Guinand et al. 2012). However, oscillopsia severity and frequency do not consistently relate to physiological (i.e., VOR) or perceptual assessments of vestibular function (McGath et al. 1989; Bhansali et al. 1993; Schubert et al. 2002; Badaracco et al. 2010; Guinand et al. 2012). Oscillopsia complaints were not consistently associated with caloric abnormalities (McGath et al. 1989) or rotational VOR gain (McGath et al. 1989; Bhansali et al. 1993). Some studies have reported no relationship between oscillopsia and dynamic visual acuity (DVA) (McGath et al. 1989; Bhansali et al. 1993; Guinand et al. 2012) while others reported better DVA scores with worse oscillopsia (Schubert et al. 2002; Badaracco et al. 2010). This disconnect between diagnostic testing and daily activities likely reflects a focus on isolated physiology necessary for diagnostics vs. more integrative systems during daily activities. Moreover, comparing oscillopsia that primarily occurs in the vertical plane during walking with active or passive gaze stability tests in the yaw plane may also contribute to the disconnect (Schubert et al. 2002; Herdman et al. 2007; Badaracco et al. 2010). Testing methods that are more relevant to daily life activities are needed to describe the relationship between vestibular pathology, oscillopsia, and the individual outside of the laboratory testing environment.
The ability to measure eye movements and characterize the capabilities of the gaze stabilization system during more functional tasks, such as locomotion, would add to current clinical assessments. Head rotation and translation during walking includes frequency content up to 5 Hz (King et al. 1992). It has been suggested that vestibular assessments should be more unpredictable and include higher frequency head motion (Grossman and Leigh 1990; King et al. 1992). The challenge in assessing gaze stability during natural head movements is that the resultant eye movements could be generated from multiple sources including the following: (1) residual vestibular function (Schubert et al. 2002; Brantberg and Löfqvist 2007; Agrawal et al. 2012); (2) locomotion-related efference copy from spinal central pattern generators (Solomon and Cohen 1992; Combes et al. 2008; Sadeghi et al. 2012; Chagnaud et al. 2015); (3) central pre-programming via saccades or cerebellar gain adaptation (Herdman et al. 2001; Della Santina et al. 2002; Schubert and Zee 2010; Sadeghi et al. 2012); (4) proprioceptive input from the neck and legs (McCall et al. 2013; Arshian et al. 2014); and (5) visual following systems (Chim et al. 2013). Gaze stability might be augmented during walking for individuals with BVH by multiple different mechanisms that would not be captured by traditional passive diagnostic testing. Thus, measuring overall gaze stability during walking and suggesting that the results are only or even primarily a measure of vestibular function in a more “natural” context is probably misleading.
Since gaze instability is a major complaint during walking, it seems only natural to explore gaze stability during walking. Treadmill walking provides a window for assessing gaze stability using a more natural input stimulus (Grossman and Leigh 1990). Here we compared the gain and phase relationship of pitch rotation eye and head velocity during walking on a treadmill in a group of individuals with BVH and a group of age-matched healthy individuals. We also investigated the relationship between gaze stability and oscillopsia severity. We hypothesized that individuals with BVH would have lower VOR gain and a less compensatory eye: head timing relationship in pitch.
Methods
Subjects
Interested participants were screened by phone to ensure eligibility to participate: (1) the ability to walk unsupported on a treadmill for at least 6 min, (2) have a diagnosis of bilateral vestibular loss. Nine individuals with BVH were recruited to participate in the study from a convenience sample of individuals interested in research. Nine age- (±5 years) and gender-matched individuals were recruited to serve as a control group. One control subject was replaced due to abnormally low head impulse gains and one individual with BVH withdrew during the experiment resulting in matched samples with eight subjects per group. Hearing was not evaluated. Demographic information on the eight individuals with BVH who completed the experiment is provided in Table 1. The average (SD) age for the healthy controls was 55.1 (13.2).
All enrolled participants provided informed consent prior to participating in any aspect of the experiment. This experiment was approved by the Institutional Review Boards at the University of Maryland and Johns Hopkins School of Medicine.
Experimental Setup
Apparatus
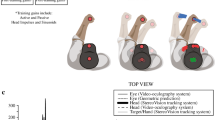
The experiment was conducted on a treadmill (Woodway USA, Inc) in the motion analysis laboratory at Kennedy Krieger Institute. Head and body kinematics were recorded at 120 Hz using two banks of three Optotrak camera systems (Northern Digital, Inc). Eye and head velocities were recorded with an EyeSeeCam (Interacoustics, Eden Prairie, MN) video oculography system at 220 Hz. The EyeSeeCam consists of an integrated 6 degrees-of-freedom inertial sensor to record head movements and an infrared video camera to record 2-D eye movements (Bartl et al. 2009; Schneider et al. 2009).
Experimental Protocol
Oscillopsia Questionnaires
Each subject completed several questionnaires including the following: (1) an OscillopsiaVisual Analog Scale (oVAS) (Herdman et al. 2007); and (2) the Oscillopsia Severity Questionnaire (OSQ) (Guinand et al. 2012). The oVAS has previously been validated as a measure of oscillopsia severity and consists of a 10-cm line anchored by phrases “None at All” and “Worst Possible.” Subjects were instructed to draw a mark across the 10-cm line to indicate the severity of oscillopsia during walking. The distance in centimeter from the end anchored with “None at All” is the oVAS score. The OSQ has been used to quantify oscillopsia and consists of nine symptom-related questions such as “Is it difficult for you to recognize known faces when you are walking?” The instructions are to indicate how often the subject experiences the symptoms with options and score values as follows: never (1), seldom (2), sometimes (3), often (4), and always (5). The OSQ score was the average of the nine responses. Scores of 3 or more have been interpreted as severe (Guinand et al. 2012).
Vestibular Function Testing
A diagnosis of BVH was made based on weak (<10 °/s combined per ear) or absent caloric responses and/or bilaterally pathologic head impulse test (Agrawal et al. 2013). Absence of reversal of nystagmus on sitting from supine after ice water calorics was used to verify the completeness of loss of low frequency lateral canal function. Video head impulse tests using the EyeSeeCam (Interacoustics, Eden Prairie, MN) characterized the VOR response for each semicircular canal (Schneider et al. 2009; Agrawal et al. 2014; Mantokoudis et al. 2014). To minimize artifacts, the strap was tightened maximally and the examiner avoided touching the strap during the testing. Each subject received 5–10 head impulses in each canal plane with peak velocities between 150 and 200 °/s. Methods for cervical and ocular vestibular evoked myogenic potential (VEMPS) testing have been described previously (Li et al. 2015). Air-conducted sound stimuli 500 Hz, 125 dB SPL tone bursts with positive polarity were presented at a rate of 5 Hz monaurally to test the cervical VEMP. The cervical VEMP waveform consists of a positive peak (p13) followed by a negative peak (n23). Cervical VEMPs were classified as absent when no discernable p13 were present. Bone-conducted midline head taps delivered at Fz at the hairline were presented while subjects were instructed to maintain 20 ° of upward gaze. The ocular VEMP waveform consists of a negative peak (n10) followed by a positive peak (p16). Ocular VEMPS were classified as absent when no discernable n10 peak was present. VEMP and caloric testing was only done to verify completeness of BVH in the patient group.
Walking Gaze Stability Measurements
The EyeSeeCam was also used to measure head and eye motion during the walking fixation tasks (Schneider et al. 2009). The EyeSeeCam system has previously been used to measure eye and head movements during human walking (Einhäuser et al. 2009; Dowiasch et al. 2015). All subjects walked on the treadmill at 0.55 m/s (2 km/h). This speed was selected as individuals with BVH (1) have gaze instability while walking at 2 km/h (Guinand et al. 2012) and (2) were able to walk on the treadmill at 2 km/h without holding handrails in pilot experiments. A fixation point (“+” displayed in the center of a screen at a logMAR size of 1.00) was used for the three fixation walking trials (2 min each). The monitor was centered approximately 5 ° below the subject’s standing eye height at a distance of 2.2 m. LogMAR is defined as log10 x where x is the minimum angle resolved in arcmin, with 1 arcmin equal to 1/60 ° (Ferris et al. 1982). All subjects were instructed to “stare at the center of the plus sign” and to minimize the frequency and duration of blinks during walking trials. A 1–2-min standing rest was provided between each walking trial to minimize fatigue.
An overhead harness, which only provided support if upright equilibrium was lost, was worn by all subjects. Light touch stabilizes walking center of mass sway (Dickstein and Laufer 2004); therefore, subjects were instructed to walk without “holding on” to the rails unless a loss of balance occurred. One individual with BVH touched the hand rail for stability in one trial and that trial was terminated and repeated.
Data Analysis
Spectral Analysis
Blinks and saccades were identified using a Kalman filter which compared actual eye velocity to predicted eye velocity (McGibbon et al. 2001). Identified blinks and saccades were removed and missing data were then filled using a cubic spline interpolation (McGibbon et al. 2001). Fourier transforms of vertical eye rotation velocity and head pitch velocity were calculated. One-sided power spectral densities (PSDs) and cross spectral densities (CSDs) using Welch’s method (Bendat and Piersol 2000) with a 20-s Hanning window and one half overlap were then calculated with these transforms. The PSDs and CSDs were averaged across trials for each subject. For each subject, the PSDs and CSDs were binned on a linear logarithmic scale up to 5 Hz, resulting in 12 frequency bins. The frequencies included in each of the 10 bins are as follows: 0.05, 0.1, 0.15–0.2, 0.25–0.3, 0.35–0.45, 0.5–0.6, 0.65–0.9, 0.95–1.3, 1.35–1.8, 1.85–2.55, 2.6–3.55, and 3.6–5 Hz. The frequencies are averaged in each bin for plotting purposes resulting in the following 12 mean frequencies: 0.05, 0.1, 0.175, 0.275, 0.4, 0.55, 0.775, 1.125, 1.575, 2.2, 3.075, and 4.3 Hz.
Gain and phase were computed to characterize the magnitude and timing of the vertical eye velocity response to head pitch velocity at each of the 12 frequency bins of the frequency response functions (FRF). Gain is computed as the absolute value of the FRF, \( {\overset{-}{H}}_{xy}(f) \), and phase is the argument of the FRF, \( {\overset{-}{H}}_{xy}(f) \) in degrees. The FRF averaged across subjects was defined as \( {\overset{-}{H}}_{xy}(f)={\overset{-}{P}}_{xy}(f)/{\overset{-}{P}}_{yy}(f) \) where \( {\overset{-}{P}}_{xy}(f) \) is the mean CSD between head pitch (x) and eye velocities (y) and \( {\overset{-}{P}}_{yy}(f) \) is the PSD of head pitch velocity (Kiemel et al. 2008).
As a first pass analysis due to the multiple frequency bins, FRFs were averaged across repetitions for each subject and then a one-way ANOVA was used to determine whether the complex valued response for each frequency bin was different from zero. To control for family-wise error (FWE) due to multiple comparisons, alpha levels were corrected using the Holm-Bonferoni method (Holm 1979). All p values are sorted into increasing order. For each comparison (i) the corresponding p value is compared to an adjusted alpha value based on α/(n-i + 1) where n is the total number of comparisons. This Holm-Bonferoni method adjusts the alpha value for each comparison based on the number of comparisons not yet compared (Holm 1979; Aickin and Gensler 1996). Only frequency bins with responses significantly different from zero after correcting for multiple tests were used for subsequent between group comparisons. To compare gain and phase responses between groups (BVH vs. Healthy), the difference between the complex valued FRFs was compared to zero. Both gain and phase are transforms of the same complex valued FRF and as such separate statistical testing is not appropriate. Ninety-five percent confidence intervals were computed based on the complex valued FRFs for gain and phase for each frequency bin. Differences in gain and/or phase between groups were identified (1) when differences between complex valued FRFs were significantly different from zero after correcting for FWE, and (2) when the confidence intervals did not overlap. Statistical analysis on FRFs was conducted using custom software in MATLAB (MathWorks, Inc) with α = 0.05.
Clinical Analysis
A one-way ANOVA was performed to identify significant group differences in video head impulse testing, and in all oscillopsia measures. To determine the relationship between gaze stability and oscillopsia, Pearson correlation coefficients were computed between oVAS scores and both the real and imaginary parts of the FRFs. The oVAS was correlated with the complex valued FRFs rather than gain and phase because gain and phase are both calculated from the same FRF value and thus cannot be analyzed separately. The real and imaginary parts of the FRF can be analyzed separately and can be related back to gain (real part) and phase (imaginary part). Sample size estimates indicated that a sample of 5–12 would be sufficient to identify group differences with power = 0.8. Analysis of clinical variables and correlation of oVAS with real and imaginary FRFs was performed using SPSS version 22. Significance was tested at α = 0.05 for all statistical tests.
Results
Group Comparisons
One control subject was replaced due to abnormally low VOR gains (data not shown) and one individual with BVH withdrew during the experiment because of discomfort from wearing the EyeSeeCam and time constraints (data not shown). None of the individuals with BVH had responses to ocular or cervical vestibular evoked myogenic potentials, see Table 1. Three individuals with BVH had asymmetric responses to bithermal water caloric testing and did not receive ice water caloric testing. One individual with BVH refused ice water caloric testing due to time constraints. Canal-specific VOR gains are presented in Table 2. Individuals with BVH had significantly lower VOR gains for all semicircular canals (ps < 0.001), as shown in Table 2 for each subject and Figure 1 for group average gains. Individuals with BVH reported more severe oscillopsia (p < 0.001) and more frequent oscillopsia (p < 0.001) compared to healthy controls, as shown in Table 3.
Beeswarm plots showing canal-specific VOR gain for all individuals with BVH (red) and healthy controls (blue). The average is represented as an “X” (black for healthy and green for BVH), with error bars representing the standard deviation. Significant group differences indicated by asterisk. R—right, L—left, H—horizontal, A—anterior, P—posterior.
Frequency Response Functions
Eye velocity responses to head velocity for all frequency bins were significantly different from zero; therefore, group and condition analyses were conducted on all frequency bins. Individuals with BVH had significantly lower gains while walking compared to healthy individuals for frequency bins: 0.275, 0.55, 0.775, 1.125, 1.575, 2.2, 3.075, and 4.3 Hz. (ps < 0.004), as shown in Figure 2. Despite lower gains, individuals with BVH demonstrated a stable and compensatory phase response that did not differ from healthy controls below 3 Hz. At 3 Hz, the individuals with BVH displayed a significant phase lag compared to healthy controls (p < 0.004).
Pitch plane gaze stability measured with the EyeSeeCam during treadmill walking. Blue circles represent the healthy controls and red squares represent individuals with BVH. Error bars represent 95 % confidence intervals. Asterisk indicates significant group differences after Holm-Bonferoni adjustment for multiple comparisons.
There were significant correlations between oVAS scores and both the real (which corresponds to gain) and imaginary (which corresponds to phase) parts of the walking FRF, as shown in Table 3. The majority of significant correlations during the walking condition were between the oVAS and the real part of the FRF (frequency bins 0.175, 0.275, 0.55, 0.775, 2.2, 3.075, and 4.3 Hz), which corresponds to gain. Only one frequency bin (3.075 Hz) was significantly correlated with the imaginary part of the FRF, which corresponds to phase (Table 4).
Discussion
In this experiment, gaze stability during treadmill walking was compared between a group of individuals with severe BVH and a group of age-matched healthy individuals. Overall, individuals with BVH reported more severe oscillopsia and had lower VOR gains on clinical head impulse testing. During walking, individuals with BVH displayed lower gains as frequency increased compared to healthy controls. The lower eye/head velocity gain during walking for individuals with BVH was consistent with low VOR gains measured with video head impulses. This suggests that the gain component of the FRF may be directly attributable to vestibular function. In contrast, individuals with BVH demonstrated ideally compensatory phase for frequencies below 3 Hz. When walking, individuals with BVH had lower velocity eye movements, but they were appropriately timed. These results suggest that the timing for eye movements in response to head motion during walking was not dependent on a fully functioning vestibular system. Animal investigations have identified proprioception and spinal efference copy signals as mechanisms that contribute to control of eye movements during locomotion (Solomon and Cohen 1992; Combes et al. 2008; Shanidze et al. 2010; Sadeghi et al. 2012; McCall et al. 2013). The compensatory phase we observed at frequencies below 3 Hz for individuals with BVH while walking suggests that timing information is available for feed forward control of gaze stability. This is consistent with prior research describing efference copy enhanced gaze stability for self-generated head movements (Herdman et al. 2001; Della Santina et al. 2002; Schubert et al. 2006).
Whether the timing information came from an efference copy/central pattern generator or somatosensory contributions to the central vestibular system, the overall effect during walking appears to be enhanced gaze stability. Despite slower eye velocity, timing was ideally compensatory, such that close to the middle of the arc of head rotation, the eyes would also be near the middle of their movement arc allowing stable vision. The specific mechanism contributing this timing information cannot be elucidated based on this experiment. The ideal timing suggests that the pursuit system is not likely responsible since onset latencies of the smooth pursuit system of ∼100 ms are much longer than the typical VOR latency of 7–13 ms or other anticipatory behaviors (Lencer and Trillenberg 2008). This suggests that the pursuit system may not be responsible for the compensatory timing of eye movements during walking. Additional mechanistic studies are needed to determine what if any role the pursuit system contributes to ongoing steady state walking gaze stability. Additionally, future studies should investigate how much stable image time is needed for functional gaze stability during walking. Additionally, future studies should elucidate the mechanisms which drive the timing of eye movements during walking.
Individuals with BVH reported that oscillopsia symptoms were more severe and more frequent compared to the matched controls in this experiment. Previous attempts to relate subjective reports of oscillopsia to measures of vestibular function have been inconsistent (McGath et al. 1989; Bhansali et al. 1993; Grunfeld et al. 2000; Schubert et al. 2002; Herdman et al. 2007; Badaracco et al. 2010; Guinand et al. 2012). In this experiment, the real part of the gaze stability FRF was positively correlated with oVAS scores during walking. This corresponds to higher oVAS scores (greater oscillopsia severity) being associated with lower gain during walking. Practically speaking, gaze instability could be caused by inadequate gain, non-compensatory phase, or both (Wist et al. 1983). In this experiment, individuals with BVH demonstrated inadequate gain but compensatory phase during walking below 3 Hz. Interestingly, the only frequency bin with a significant correlation between the imaginary part of the FRF (phase) and oVAS scores was 3.075 Hz. This was also the only frequency where individuals with BVH had a significant phase delay relative to the healthy controls. Subjective ratings of oscillopsia severity were primarily associated with aspects of impaired gaze instability for these individuals with BVH. Our results differ from a recent report in which gaze stability was measured by DVA (Guinand et al. 2012). This discrepancy may be due to differences in how gaze stability or oscillopsia was measured. In the present experiment, gaze stability was the relationship between eye and head velocity, while in the other experiment DVA was the measure of gaze stability (Guinand et al. 2012). While DVA scores are related to VOR gain (Schubert et al. 2006), DVA scores only account for 45 % of the variance in VOR gain abnormalities for individuals with vestibular disease. Additionally, Guinand et al. (2012) reported oscillopsia frequency rather than oscillopsia severity. Currently, there is not an activity participation scale characterizing the impact of oscillopsia symptoms on daily life activities. A better understanding of the relationship between gaze stability and activity participation could lead to more specific intervention strategies that improve functional behavior and quality of life. Individuals with BVH often also present with gait and balance impairments that may contribute to changes in activity independent of oscillopsia. Future work is needed to determine whether oscillopsia independently contributes to activity participation.
The results from this experiment demonstrated that gaze stability may be better during walking for individuals with BVH in ways not predicted by passive testing. Individuals with BVH may benefit from gaze stabilization activities performed while walking in order to take advantage of the walking-specific phase enhancement in gaze stability. Recent advances in vestibular rehabilitation demonstrate that incremental VOR gain adaptation can directly increase VOR gain (Schubert et al. 2008; Fadaee and Migliaccio 2016). It is not clear whether this enhancement can be leveraged to facilitate better gaze stability during walking for individuals with BVH, and future studies are needed.
Limitations
The individuals with BVH in this study may have residual vestibular function, particularly in the vertical semicircular canals, which may have contributed to the results. Some of the healthy individuals had hyperfunctional vertical semicircular canal VOR gains which may reflect testing artifact such as goggle slippage (Mantokoudis et al. 2015). Goggle slippage artifact is less likely during the walking portion of the experiment since head velocities were significantly lower. However, this population may be more representative of individuals with bilateral vestibular disease who are referred to rehabilitation (Ward et al. 2013; Lucieer et al. 2016). The results of this study may not be generalizable to individuals with surgically induced bilateral vestibular loss.
Conclusion
Intrinsic knowledge of timing during walking possibly via a locomotion efference copy contributes to gaze stability during walking for individuals with BVH. Oscillopsia scores were related to the specific component of gaze stability that was impaired, namely gain but not phase. Rehabilitation interventions focusing on enhancing VOR gain during walking should be developed to take advantage of the intact timing reported here. Specifically targeting the impaired component of walking gaze stability (VOR gain) may reduce complaints of oscillopsia severity and improve quality of life.
References
Agrawal Y, Zuniga MG, Davalos-Bichara M et al (2012) Decline in semicircular canal and otolith function with age. Otol Neurotol 33:832–839
Agrawal Y, Bremova T, Kremmyda O, Strupp M (2013) Semicircular canal, saccular and utricular function in patients with bilateral vestibulopathy: analysis based on etiology. J Neurol 260:876–883
Agrawal Y, Schubert MC, Migliaccio AA et al (2014) Evaluation of quantitative head impulse testing using search coils versus video-oculography in older individuals. Otol Neurotol 35:283–288
Aickin M, Gensler H (1996) Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health 86:726–728
Arshian MS, Hobson CE, Catanzaro MF et al (2014) Vestibular nucleus neurons respond to hindlimb movement in the decerebrate cat. J Neurophysiol 111:2423–2432. doi:10.1152/jn.00855.2013
Badaracco C, Labini FS, Meli A, Tufarelli D (2010) Oscillopsia in labyrinthine defective patients: comparison of objective and subjective measures. Am J Otolaryngol-Head Neck Med Surg 31:399–403. doi:10.1016/j.amjoto.2009.06.002
Bartl K, Lehnen N, Kohlbecher S, Schneider E (2009) Head impulse testing using video-oculography. Ann N Y Acad Sci 1164:331–333
Bendat J, Piersol A (2000) Random data: analysis and measurement procedures, 3rd edn. Wiley, New York
Bhansali SA, Stockwell CW, Bojrab DI (1993) Oscilopsia in patients with loss of vestibular function. Otolaryngol-Head Neck Surg 109:120–125
Brantberg K, Löfqvist L (2007) Preserved vestibular evoked myogenic potentials (VEMP) in some patients with walking-induced oscillopsia due to bilateral vestibulopathy. J Vestib Res 17:33–38
Chagnaud BP, Banchi R, Simmers J, Straka H (2015) Spinal corollary discharge modulates motion sensing during vertebrate locomotion. Nat Commun 6:7982. doi:10.1038/ncomms8982
Chim D, Lasker DM, Migliaccio AA (2013) Visual contribution to the high-frequency human angular vestibulo-ocular reflex. Exp Brain Res 230:127–135. doi:10.1007/s00221-013-3635-9
Combes D, Le Ray D, Lambert FM et al (2008) An intrinsic feed-forward mechanism for vertebrate gaze stabilization. Curr Biol 18:R241–R243. doi:10.1016/j.cub.2008.02.018
Crawford J (1964) Living without a balancing mechanism. Br J Ophthalmol 48:357–360
Della Santina CC, Cremer PD, Carey JP, Minor LB (2002) Comparison of head thrust test with head autorotation test reveals that the vestibulo-ocular reflex is enhanced during voluntary head movements. Arch Otolaryngol Head Neck Surg 128:1044–1054
Dickstein R, Laufer Y (2004) Light touch and center of mass stability during treadmill locomotion. Gait Posture 20:41–47
Dowiasch S, Marx S, Einhäuser W, Bremmer F (2015) Effects of aging on eye movements in the real world. Front Hum Neurosci 9:46. doi:10.3389/fnhum.2015.00046
Einhäuser W, Moeller GU, Schumann F et al (2009) Eye-head coordination during free exploration in human and cat. Ann N Y Acad Sci 1164:353–366. doi:10.1111/j.1749-6632.2008.03709.x
Fadaee SB, Migliaccio AA (2016) The effect of retinal image error update rate on human vestibulo-ocular reflex gain adaptation. Exp Brain Res 234:1085–1094. doi:10.1007/s00221-015-4535-y
Ferris FL, Kassoff A, Bresnick GH, Bailey I (1982) New visual acuity charts for clinical research. Am J Ophthalmol 94:91–96
Fetter M (2007) Vestibulo-ocular reflex. Dev Ophthalmol 40:35–51
Grossman GE, Leigh RJ (1990) Instability of gaze during walking in patients with deficient vestibular function. Ann Neurol 27:528–532
Grossman GE, Leigh RJ, Bruce EN et al (1989) Performance of the human vestibuloocular reflex during locomotion. J Neurophysiol 62:264–272
Grunfeld EA, Morland AB, Bronstein AM et al (2000) Adaptation to oscillopsia a psychophysical and questionnaire investigation. Brain 123:277–290
Guinand N, Pijnenburg M, Janssen M, Kingma H (2012) Visual acuity while walking and oscillopsia severity in healthy subjects and patients with unilateral and bilateral vestibular function loss. Arch Otolaryngol Head Neck Surg 138:301–306
Herdman SJ, Schubert MC, Tusa RJ (2001) Role of central preprogramming in dynamic visual acuity with vestibular loss. Arch Otolaryngol Head Neck Surg 127:1205–1210
Herdman SJ, Hall CD, Schubert MC et al (2007) Recovery of dynamic visual acuity in bilateral vestibular hypofunction. Arch Otolaryngol Head Neck Surg 133:383–389. doi:10.1001/archotol.133.4.383
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70
Kiemel T, Elahi AJ, Jeka JJ (2008) Identification of the plant for upright stance in humans: multiple movement patterns from a single neural strategy. J Neurophysiol 100:3394–3406. doi:10.1152/jn.01272.2007
King OS, Seidman SH, Leigh RJ (1992) Control of head stability and gaze during locomotion in normal subjects and patients with deficient vestibular function. In: Berthoz A, Graf W, Vidal P-P (eds) The head-neck sensory motor system. Oxford University Press, New York, pp 568–570
Leigh RJ, Brandt T (1993) A reevaluation of the vestibulo-ocular reflex: new ideas of its purpose, properties, neural substrate, and disorders. Neurology 43:1288–1295
Lencer R, Trillenberg P (2008) Neurophysiology and neuroanatomy of smooth pursuit in humans. Brain Cogn 68:219–228. doi:10.1016/j.bandc.2008.08.013
Li C, Layman AJ, Carey JP, Agrawal Y (2015) Epidemiology of vestibular evoked myogenic potentials: data from the Baltimore Longitudinal Study of Aging. Clin Neurophysiol 126:2207–2215
Lucieer F, Vonk P, Guinand N et al (2016) Bilateral vestibular hypofunction: insights in etiologies, clinical subtypes, and diagnostics. Front Neurol 7:26. doi:10.3389/fneur.2016.00026
Mantokoudis G, Schubert MC, Tehrani ASS et al (2014) Early adaptation and compensation of clinical vestibular responses after unilateral vestibular deafferentation surgery. Otol Neurotol 35:148–154
Mantokoudis G, Saber Tehrani AS, Kattah JC et al (2015) Quantifying the vestibulo-ocular reflex with video-oculography: nature and frequency of artifacts. Audiol Neurotol 20:39–50. doi:10.1159/000362780
McCall AA, Moy JD, Puterbaugh SR et al (2013) Responses of vestibular nucleus neurons to inputs from the hindlimb are enhanced following a bilateral labyrinthectomy. J Appl Physiol 114:742–751
McGath JH, Barber HO, Stoyanoff S (1989) Bilateral vestibular loss and oscillopsia. J Otolaryngol 18:218–221
McGibbon CA, Palmer T, Goldvasser D, Krebs DE (2001) Kalman filter detection of blinks in video-oculography: applications for VVOR measurement during locomotion. J Neurosci Methods 106:171–178
Patla AE, Vickers JN (2003) How far ahead do we look when required to step on specific locations in the travel path during locomotion? Exp Brain Res 148:133–138. doi:10.1007/s00221-002-1246-y
Pozzo T, Berthoz A, Lefort L, Vitte E (1991) Head stabilization during various locomotor tasks in humans. II. Patients with bilateral peripheral vestibular deficits. Exp Brain Res 85:208–217
Sadeghi SG, Minor LB, Cullen KE (2012) Neural correlates of sensory substitution in vestibular pathways following complete vestibular loss. J Neurosci 32:14685–14695
Schneider E, Villgrattner T, Vockeroth J et al (2009) EyeSeeCam: an eye movement-driven head camera for the examination of natural visual exploration. Ann N Y Acad Sci 1164:461–467. doi:10.1111/j.1749-6632.2009.03858.x
Schubert MC, Zee DS (2010) Saccade and vestibular ocular motor adaptation. Restor Neurol Neurosci 28:9–18. doi:10.3233/RNN-2010-0523.Saccade
Schubert MC, Herdman SJ, Tusa RJ (2002) Vertical dynamic visual acuity in normal subjects and patients with vestibular hypofunction. Otol Neurotol 23:372–377
Schubert MC, Migliaccio AA, Della Santina CC (2006) Modification of compensatory saccades after aVOR gain recovery. J Vestib Res 16:285–291
Schubert MC, Della Santina CC, Shelhamer M (2008) Incremental angular vestibulo-ocular reflex adaptation to active head rotation. Exp Brain Res 191:435–446. doi:10.1007/s00221-008-1537-z
Shanidze N, Kim AH, Loewenstein S et al (2010) Eye-head coordination in the guinea pig II. Responses to self-generated (voluntary) head movements. Exp Brain Res 205:445–453. doi:10.1007/s00221-010-2375-3
Solomon D, Cohen B (1992) Stabilization of gaze during circular locomotion in darkness. II. Contribution of velocity storage to compensatory eye and head nystagmus in the running monkey. J Neurophysiol 67:1158–1170
Ward BK, Agrawal Y, Hoffman HJ et al (2013) Prevalence and impact of bilateral vestibular hypofunction: results from the 2008 US National Health Interview Survey. JAMA Otolaryngol Head Neck Surg 139:803–810
Wist ER, Brandt T, Krafczyk S (1983) Oscillopsia and retinal slip evidence supporting a clinical test. Brain 106:153–168
Acknowledgements
Thanks to Amy Bastian, PT, PhD, for sharing lab and equipment space for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Source of Funding
This work was supported in part by PODS Scholarships from the Foundation for Physical Therapy, Inc. (E Anson, PI); a Wylie Dissertation Fellowship from the University of Maryland Graduate School (E Anson, PI); the University of Maryland’s Department of Kinesiology Graduate Student Research Initiative Fund (E Anson, PI); and National Institutes of Health grant R21 AG041714-01A1 (J Jeka, PI). For the remaining authors, none were declared.
Additional information
A version of this manuscript has been published in electronic form as a dissertation chapter in fulfillment of doctoral degree requirements under copyright by E Anson.
Rights and permissions
About this article
Cite this article
Anson, E.R., Kiemel, T., Carey, J.P. et al. Eye Movements Are Correctly Timed During Walking Despite Bilateral Vestibular Hypofunction. JARO 18, 591–600 (2017). https://doi.org/10.1007/s10162-017-0626-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-017-0626-8