Abstract
The objective of this large population-based cross-sectional study was to evaluate the association between smoking, passive smoking, alcohol consumption, and hearing loss. The study sample was a subset of the UK Biobank Resource, 164,770 adults aged between 40 and 69 years who completed a speech-in-noise hearing test (the Digit Triplet Test). Hearing loss was defined as speech recognition in noise in the better ear poorer than 2 standard deviations below the mean with reference to young normally hearing listeners. In multiple logistic regression controlling for potential confounders, current smokers were more likely to have a hearing loss than non-smokers (odds ratio (OR) 1.15, 95 % confidence interval (CI) 1.09–1.21). Among non-smokers, those who reported passive exposure to tobacco smoke were more likely to have a hearing loss (OR 1.28, 95 %CI 1.21–1.35). For both smoking and passive smoking, there was evidence of a dose-response effect. Those who consume alcohol were less likely to have a hearing loss than lifetime teetotalers. The association was similar across three levels of consumption by volume of alcohol (lightest 25 %, OR 0.61, 95 %CI 0.57–0.65; middle 50 % OR 0.62, 95 %CI 0.58–0.66; heaviest 25 % OR 0.65, 95 %CI 0.61–0.70). The results suggest that lifestyle factors may moderate the risk of hearing loss. Alcohol consumption was associated with a protective effect. Quitting or reducing smoking and avoiding passive exposure to tobacco smoke may also help prevent or moderate age-related hearing loss.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Age-related hearing impairment is highly prevalent, with 36.7 % of UK adults aged between 61 and 70 years having hearing loss (mean hearing threshold level of >25 dB hearing level (HL) over 500 to 4000 Hz in the better ear; Davis 1989). Hearing loss has been viewed as an inevitable consequence of aging (Gates and Mills 2005). Encouragingly, there is some evidence that this may not be the case; some older individuals have normal hearing (Cruickshanks et al. 1998b), and in younger generations, the prevalence of hearing loss is lower than in older generations (Zhan et al. 2009; Hoffman et al. 2012). Further, hearing loss is associated with various modifiable risk factors, including noise exposure (Agrawal et al. 2008), cardiovascular disease (Gates et al. 1993; Helzner et al. 2005), exercise (Hull and Kerschen 2010) and diabetes (Horikawa et al. 2013). Smoking and alcohol consumption (reviewed below) may represent additional modifiable risks, presenting opportunities to delay the onset and/or moderate the severity of hearing loss.
Smoking may impact upon the auditory system via direct ototoxic effects of nicotine or other ototoxic substances found in cigarette smoke (Maffei and Mianil 1962) or vascular effects, such as increased blood viscosity and reduced available oxygen causing cochlear hypoxia (Lowe et al. 1980; Browning et al. 1986).
Several studies report an association between hearing loss and smoking (Siegelaub et al. 1974; Barone et al. 1987; Rosenhall et al. 1993; Cocchiarella et al. 1995; Cruickshanks et al. 1998a; Noorhassim and Rampal 1998; Nakanishi et al. 2000; Itoh et al. 2001; Sharabi et al. 2002; Mizoue et al. 2003; Palmer et al. 2004; Burr et al. 2005; Helzner et al. 2005; Nomura et al. 2005; Uchida et al. 2005; Pouryaghoub et al. 2007; Fransen et al. 2008; Gopinath et al. 2010), but the evidence is not entirely consistent (Gates et al. 1993; Brant et al. 1996). A 2005 meta-analysis concluded that there are moderate-to-large associations between smoking and hearing loss (Nomura et al. 2005). Nomura and colleagues’ meta-analysis reported an overall risk ratio of 1.33 (95 % confidence interval (CI) 1.24–1.44) over five cross-sectional studies, 1.97 (1.44, 2.70) over four cohort studies, and 2.89 (2.26, 3.70) in one case-control study. Passive smoking may also be associated with hearing loss; Cruickshanks and colleagues (1998a) reported that non-smokers who lived with a smoker were more likely to have hearing loss than those who did not live with a household member who smokes.
Moderate alcohol consumption—typically defined as consumption of one to two drinks per day—is associated with protective effect against cardiovascular disease (Baum-Baicker 1985; Moore and Pearson 1986; Rimm et al. 1991; Ronksley et al. 2011), possibly via increasing levels of high-density lipoprotein (HDL) cholesterol and reduced coagulation (Pearson 1996). In contrast, high levels of alcohol consumption are associated with increased risk of cardiovascular disease (Criqui 1987). High levels of alcohol consumption do not result in increased HDL but are associated with increased levels of low-density lipoprotein, increased blood clotting, histological changes in the myocardium, and reduced threshold for ventricular fibrillation, all linked to adverse cardiovascular outcomes (McKee and Britton 1998).
Since cardiovascular disease may be associated with hearing loss (Johnsson 1973; Rubinstein et al. 1977; Makishima 1978; Susmano and Rosenbush 1988; Gates et al. 1993; Brant et al. 1996), an effect of alcohol consumption on hearing may be via a cardiovascular causal pathway. The small amount of research in this area appears partly to bear this out; heavy drinking was associated with increased risk of hearing loss (Rosenhall et al. 1993; Popelka et al. 1998) or no increased risk versus non-drinkers (Itoh et al. 2001). Moderate alcohol consumption was associated with a protective effect on hearing (Popelka et al. 1998; Itoh et al. 2001; Helzner et al. 2005; Fransen et al. 2008; Gopinath et al. 2010). Findings are not consistent, however, as some studies have not detected any significant association between moderate or heavy alcohol consumption and hearing (Brant et al. 1996; Curhan et al. 2011).
In summary, smoking and passive smoking may be associated with hearing loss. There is some evidence for a protective effect of alcohol consumption against hearing loss. High levels of alcohol consumption are associated with reduced benefit compared to moderate levels of consumption or with an increased risk of hearing loss. The aim of the present study was to test associations between smoking, passive smoking, alcohol consumption, and hearing loss, independent of age, sex, socioeconomic status, ethnicity, and other known risks for hearing loss (including cardiovascular factors, diabetes, ototoxic medications, and noise exposure (Cruickshanks et al. 2010)). The expectation was that smoking and passive smoking would be associated with greater risk of hearing loss. Moderate alcohol consumption would be associated with reduced risk, while higher levels of alcohol consumption would be associated with less benefit.
METHODS
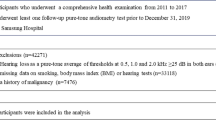
This research was conducted using the UK Biobank (Collins 2012), which contains data from over 500,000 people. The very large sample size was designed to facilitate research into the environmental and genetic causes of disease in middle and older ages. Additional measures were added to the UK Biobank protocol throughout the duration of data collection, and so, the present study was focused on a subsample of 164,770 participants who completed a hearing test (the Digit Triplet Test, described below). Participants were aged between 40 to 69 years at the time of testing. UK Biobank recruitment took place between March 2007 and July 2010 via the UK National Health Service and aimed to be as representative and inclusive as possible of the general UK population. Recruitment was via postal invitation with a telephone follow-up, and the overall response rate was 5.47 %. Table 1 shows the sex, ethnicity, and Townsend deprivation index score (Norman 2010) for the subset of the UK Biobank sample included in the present study versus the corresponding section of the UK population aged 40 to 69 years. The Townsend deprivation scheme is a proxy measure of socioeconomic status that is widely used in health studies. It comprises four input variables on unemployment, non-car ownership, non-home ownership, and household overcrowding based on area of residence, each of which is expressed as a z-score relative to the national level which is then summed to give a single deprivation score. Lower Townsend scores represent areas associated with less-deprived (i.e., more affluent) socioeconomic status.
The study sample contains a slightly higher proportion of female subjects and people living in more affluent areas than in the general population. The proportion of White ethnicity is similar to that in the general population. Participants attended a UK Biobank assessment and provided written informed consent. They completed a “whole-body” assessment of 90-min duration that included a computerized questionnaire on lifestyle and medical history as well as physical measures, including hearing testing, body mass index (BMI) assessment, and pulse wave arterial stiffness assessment. Detailed information about the assessment procedure and the additional data collected (not reported in the present study) may be found elsewhere (http://www.ukbiobank.ac.uk/).
Assessments
Hearing—Digit Triplet Test
The Digit Triplet Test (DTT) is a speech-in-noise test developed for reliable large-scale hearing screening (Smits et al. 2004; Vlaming et al. 2011). The DTT correlates strongly with measures of hearing sensitivity (PTA; r = 0.77 (Smits et al. 2004)) and with other speech-in-noise tests (for example, sentences-in-noise (Plomp and Mimpen 1979); r = 0.85 (Smits et al. 2004)). The DTT is therefore a reliable measure of hearing impairment. As listening in noise is a key function of hearing and difficulty hearing in noise is the most common complaint by people with hearing loss, speech recognition testing in noise arguably provides a more ecologically valid measure than detection of tones in a quiet environment (Arlinger et al. 2009). In the version of the DTT used in the UK Biobank, 15 sets of three monosyllabic digits were presented via circumaural headphones (Sennheiser HD-25). Left and right ears were tested separately with the order of testing randomized across participants. The participants first set the volume of stimuli to a comfortable listening level. Digits were then presented in background noise shaped to match the spectrum of the speech stimuli. Noise levels varied contingent on correct identification of the three digits via a touch screen interface, with the signal to noise ratio (SNR) for the 50 % correct recognition threshold estimated adaptively. The recognition threshold was taken as the mean SNR for the last eight triplets. Lower (more negative) scores correspond to better performance. In the present study, hearing loss was based on performance of the better ear (i.e., the ear with the lower recognition threshold). Hearing loss was identified if the better ear recognition threshold was more than two standard deviations poorer with respect to a reference group of participants aged 18 to 29 years with normal hearing (defined as pure-tone audiometric thresholds <25 dB HL between 250 and 8,000 Hz bilaterally)(Dawes et al. 2014), i.e., a threshold greater than or equal to −5.5 dB.
Age, Sex, Ethnicity, and Socioeconomic Status
Data on sex, age at time of assessment, ethnicity (2001 UK Census categories), and the Townsend deprivation score corresponding to area of residence were recorded for each participant. For the regression analyses, Townsend scores were categorized into quartiles from the least to the most deprived sections of the sample. Ethnicity was coded according to “White” or “non-White” ethnic background.
Smoking
Smoking status was based on responses to two questions “Do you smoke tobacco now?” and “In the past, how often have you smoked tobacco?” Current smokers are those who reported currently smoking occasionally or on most or all days. Ex-smokers are those who reported previously smoking occasionally or on most or all days. Non-smokers are those who reported never smoking or who reported just having tried smoking once or twice. Current and ex-smokers were asked “About how many cigarettes do/did you smoke on average each day?”Pack years were calculated according to daily consumption of cigarettes divided by 20 (to index the number of packs per day) and multiplied by the duration of smoking in years. Pack-year category was then assigned based on the bottom 25th percentile (defined as greater than 0 and less than or equal to 10 pack years), the middle range between the 25th percentile and 75th percentile (greater than 10 and less than or equal to 33 pack years), and the top 25th percentile (greater than 33 pack years).
Non-smokers were asked the additional questions “At home, about how many hours per week are you exposed to other people’s tobacco smoke?” and “Outside of your home, about how many hours per week are you exposed to other people’s tobacco smoke?” Participants were identified as being exposed to tobacco smoke if they reported any weekly exposure either at home or outside the home. Exposure was quantified further by summing the weekly hours of exposure in and outside the home and then grouping according to three levels of exposure: no exposure or 1 h or less per week, 2–9 h per week, and 10 or more hours per week.
Alcohol Consumption
Alcohol drinkers and non-drinkers were identified on the basis of responses to the question “About how often do you drink alcohol?” Non-drinkers were identified on the basis of the response “Never,” while drinkers were identified on the basis of the remaining response options (“Special occasions only,” “One to three times a month,” “One or twice a week,” “Three or four times a week,” “Daily or almost daily”). Those who answered “Never” were asked the additional question “Did you previously drink alcohol?” (yes/no). If the participants had previously drunk alcohol, they were asked about the reason for giving up drinking. Ex-drinkers were asked the questions “Why did you stop drinking alcohol? ‘illness or ill health’, ‘doctor’s advice’, ‘health precaution’, ‘financial reasons’, ‘other reason’, ‘do not know’, or ‘prefer not to answer’.” A total of 48.6 % reported stopping drinking for reasons of illness, doctor’s advice, or as a health precaution.
The number of drinks per week was calculated on the basis of the summed total of reported weekly consumption of UK standard units of red wine, champagne or white wine, beer or cider, spirits, fortified wine, or other alcoholic drinks. There are 8 g (10 ml) of alcohol in a standard drink in the UK, equal to one “unit”(House Of Commons Science and Technology Committee 2012). A medium-sized glass of wine or champagne is 2.3 units. One pint of full-strength beer or cider is 3 units, while light beer or cider is 2 units. In the present study, one serve of beer or cider was taken as being equal to 2.5 units. One shot of spirits or fortified wine is 1 unit. Alcopops and other forms of alcohol count as 1.5 units. The alcohol content in grams of each type of drink was calculated by multiplying the number of units by 8. Grams of ethanol for each type of drink were summed to provide the overall total grams of ethanol consumed per week. The total grams of ethanol consumed per week were then classified according to five categories: never drinkers (i.e. those who have never regularly drunk alcohol), ex-drinkers (those who have given up consumption alcohol), the lowest 25 % of alcohol drinkers (the first 25th percentile; 1 to 118.4 g of ethanol per week), the middle 50 % (middle range between the 25th percentile and 75th percentile; 118.4 to 196.8 g of ethanol per week), and the highest 25 % (the top 25 percentile; greater than 196.8 g of ethanol per week). The “highest” range includes levels of alcohol consumption that are considered “hazardous” to general health (The Royal College of Psychiatrists 2011).
Cardiovascular Disease, Cholesterol, Hypertension, and Diabetes
Cardiovascular disease was identified on the basis of self-report of any cardiovascular problem, including angina, heart attack, heart failure, stroke, transient ischemic attack, intermittent claudication, arterial embolism, or deep venous thrombosis. High cholesterol was identified if the participant reported that they had high cholesterol or that they were currently taking medication for high cholesterol. Hypertension was identified if the participant reported that they had hypertension, currently took medication for high blood pressure, or had a measured systolic blood pressure greater than 140 mm Hg or diastolic pressure greater than 90 mm Hg. Diabetes was identified if the participant reported that they had type 1 or type 2 diabetes or that they currently look insulin for diabetes.
Pulse Wave Arterial Stiffness Index and BMI
Pulse wave arterial stiffness index was calculated as the time between peaks of the pulse waveform measured at the finger via infrared sensor divided by the participant’s height. Pulse wave measurement was performed with a PulseTrace PCA2 (CareFusion, USA; for details of pulse wave measurement, see http://biobank.ctsu.ox.ac.uk/crystal/field.cgi?id=21021). BMI was calculated as the participants’ weight (in kilograms) divided by the height squared (in meters).
Physical Activity, Ototoxic Medication, and Occupation- and Music-Related Noise Exposure
Participants were classified as active if they reported doing over 30 min of moderate physical activity on the day prior to assessment, in response to the question “Yesterday, about how long did you spend doing activities that needed moderate effort, making you somewhat short of breath? For example, walking upstairs, going to the gym, jogging, energetic dancing, aerobics, most sports, using heavy power tools, and other physically demanding DIY and gardening.” The participants were classified as “inactive” if they reported doing less than 10 min or no physical activity. Work noise exposure was identified on the basis of any reported noise exposure in response to the question “Have you ever worked in a noisy place where you had to shout to be heard?” Music noise exposure was identified on the basis of any reported exposure in response to the question “Have you ever listened to music for more than 3 h per week at a volume of which you would need to shout to be heard or, if wearing headphones, someone else would need to shout for you to hear them?” The criterion for work- and music-related noise roughly corresponds to exposure exceeding 85 dB(A) (Health and Safety Executive 1989). All medications that were currently being taken regularly (daily, weekly, or monthly) were recorded, not including short-term medications (e.g., a 1-week course of antibiotics) or prescribed medications that were not taken. All medications with known ototoxicity were coded as ototoxic, including loop diuretics, aminoglycoside antibiotics, quinine derivatives, non-steroidal anti-inflammatories, and salicylates.
Data Analysis
Analyses were performed with IBM SPSS version 20. Logistic regression was used to model the effects of alcohol and smoking and other covariates on hearing loss. Logistic regression models provide odds ratios (ORs), which are measures of association between an exposure (e.g., smoking) and an outcome (e.g., hearing loss). The OR is the odds that the outcome will occur given the exposure compared to the odds of the outcome occurring without the exposure. An OR greater than 1 for an exposure indicates increased odds of the outcome, while an OR less than 1 indicates reduced odds of the outcome. If the 95 % CI for the OR crosses the 0 point, this indicates that the OR is not statistically significantly different from 0 at a level of α = 0.05. In linear regression models, the coefficient of determination r 2 indicates the proportion of the variance in the outcome variable that is associated with the predictor variable(s). Larger r 2 values suggest that more variation is explained by the model. For logistic regression models, it is not possible to compute an r 2 statistic that is directly comparable to the r 2 in a linear regression model. In the present study, a pseudo-r 2 (Nagelkerke r 2) was calculated as an approximation. Pseudo-r 2 measures tend to be lower than the r 2 statistic used with linear regression models.
As shown in Table 2, for some measures such as pulse wave stiffness and physical activity, there were missing data. The primary reason for these missing data is that measures were added to the study protocol at different time points over the course of data collection. As the reason for missing data was not systematically related to hearing or to any other variable, it was assumed that the data are missing completely at random. Missing variable analysis did not identify any pattern to the missing data.
Potential confounders (Table 2) were selected on the basis of having been implicated with hearing loss in previous research (Gates and Mills 2005; Cruickshanks et al. 2010). Variables included SES (Townsend index; first, second, third, and fourth quartile), BMI, pulse wave stiffness index, ethnicity (White/non-White), hypertension (yes/no), cardiovascular disease (yes/no), high cholesterol (yes/no), ototoxic medication (yes/no), diabetes (yes/no), physical activity (yes/no), occupational noise exposure (yes/no), music noise exposure (yes/no), alcohol consumption (never drinker/ex-drinker/lowest/middle/highest drinkers), and smoking status (never/ex-smoker/current). To evaluate the main effects of smoking and alcohol consumption, all variables were entered simultaneously. Non-significant contributors from that multivariable regression were excluded, and the regression rerun retaining only those variables that were important effect modifiers. The variables that were excluded from the multivariable final model were pulse wave stiffness index, BMI, hypertension, music noise exposure, and physical activity.
In order to evaluate dose-response effects for smoking, current and ex-smokers were selected. Regression with all covariates was rerun for this subset of participants with the pack-year categorical variable (lowest/middle/highest number of pack-years) substituted for smoking status. To test for effects of passive exposure to tobacco smoke and dose-response effects of passive exposure in non-smokers, a regression model was rerun with all covariates, and the passive exposure variables were substituted for the smoking status variable. For these analyses, non-significant contributing variables were dropped from the final regression model. The final models for each analysis differed slightly from the model for all participants since those variables not significantly contributing to the model were excluded.
RESULTS
Table 2 shows the characteristics of normal hearing and hearing-impaired participants, according to demographic variables age, sex, SES and ethnicity, as well as alcohol consumption and smoking status and covariates. Each variable was entered into a logistic regression along with age and sex with hearing status as the dependent measure to provide a p value for its association with hearing loss independent of age and sex. Significant p values in Table 2 suggest that the variable is a potential confounder. All variables were significantly associated with hearing loss except sex.
Smoking and Alcohol Consumption
To evaluate the main effects of smoking and alcohol consumption, all variables were entered simultaneously into a multivariable logistic regression model. Non-significant contributors from the initial model were excluded, and the regression model was rerun retaining only those variables that were important effect modifiers in the multivariable model. The variables that were excluded from the final model were pulse wave stiffness index, BMI, hypertension, music noise exposure, and physical activity. Table 3 shows the final multivariable regression model for hearing loss. Nagelkerke r 2 for the model was 0.10. Both alcohol consumption status and smoking status were significantly associated with hearing loss. Current smokers were at higher odds of hearing loss than never smokers, although ex-smokers were at slightly less odds of hearing loss than never smokers. Compared to lifetime non-drinkers, all categories of current drinkers were similarly less likely to have hearing loss.
Smoking Dose-Response Analysis
Compared to the bottom 25 % of smokers by pack year, those in the middle 50 % and top 25 % had greater odds of hearing loss in a final regression model (Table 4) that included age, cholesterol, occupation-related noise exposure, ethnicity, alcohol consumption, and SES. Higher ORs for those with a higher “dose” of smoking (represented by pack-years) indicate that higher doses of smoking are associated with increased odds of hearing loss. This is consistent with a dose-response effect for smoking.
Passive Exposure to Tobacco Smoke
Non-smokers who were exposed to tobacco smoke were more likely to have hearing loss than non-smokers with no exposure in a final regression model (Table 5) that included age, sex, ethnicity, cardiovascular disease, diabetes, hypertension, occupation-related noise exposure, and alcohol consumption. Regression modeling of dose effects revealed that those who reported 1 h or less weekly passive exposure to tobacco smoke were at no additional risk compared to non-smokers with no exposure (OR 1.00, 95 % CI 0.94–1.07), while those that reported between 2 and 9 h of weekly exposure and over 10 h per week were at progressively higher odds of hearing loss (OR 1.28, 95 % CI 1.18–1.39; OR 1.39, 95 % CI 1.19–1.61). Increasing odds of hearing loss with increasing amounts of passive exposure to tobacco smoke were consistent with a dose-dependent effect.
DISCUSSION
Smoking
In the present study, current smokers were at 15.1 % higher odds of hearing loss than non-smokers. The most recent survey estimated the proportion of smokers in the UK adult population at 20 % (Office for National Statistics 2012), and rates of up to 60 % are reported in other countries (World Health Organisation 2013). Given such high levels of exposure and evidence of a substantial association between smoking and hearing loss, smoking may represent a significant contributor to hearing loss worldwide. Note that the association between smoking and hearing loss was observed in a regression model that included cardiovascular disease. This might suggest that smoking has an impact on hearing via causal pathways in addition to cardiovascular ones, such as via direct ototoxic effect of tobacco smoke (Maffei and Mianil 1962; Guth and Norris 1996). Alternatively, the measures of cardiovascular disease in the present study may not have been sensitive to microvascular changes that could impact on hearing and not have fully captured the variance due to cardiovascular factors on hearing.
In addition to elevated risk associated with smoking, there was evidence of a dose-response effect, with the risk of hearing loss higher for those with higher dose, measured in pack years of smoking. The present study provided the novel finding that passive exposure to tobacco smoke among non-smokers was associated with a 28 % elevated risk of hearing loss and that this association was dose-dependent. Note that the association between hearing loss and passive smoking appears stronger than the association between hearing loss and smoking. This may be partly because the odds for smoking were determined by comparing smokers with non-smokers. Some non-smokers may be exposed to tobacco smoke, and so, the association between smoking and hearing loss may be underestimated.
One unexpected result not reported in previous research was that ex-smokers had slightly reduced risk of hearing loss than non-smokers. If this is a reliable result, it could perhaps be a reflection of a tendency for ex-smokers to adopt healthier lifestyles; the decision to stop smoking may be only one of several healthy lifestyle changes that may also impact upon hearing. With respect to cardiovascular disease, there is inconsistent evidence for residual risks for ex-smokers; some studies suggest little or no residual risk of smoking while others show some residual risk (Critchley and Capewell 2003). The overall pattern identified by Critchley and Capewell’s (2003) review was that there is a substantial and reliable reduction in risk for cardiovascular disease associated with quitting smoking. The present study suggests that the benefit of quitting or reducing smoking may extend to a reduction in the risk of hearing loss.
Alcohol Consumption
Compared to those who have never consumed alcohol, all three levels of alcohol consumption were associated with around 40 % reduced risk of hearing loss. The finding supports the small body of research to date (Popelka et al. 1998; Itoh et al. 2001; Fransen et al. 2008; Gopinath et al. 2010). Previous studies have shown either less or no association with very high levels of alcohol consumption (Itoh et al. 2001) or that very heavy drinking was associated with increased odds of hearing loss (Rosenhall et al. 1993; Popelka et al. 1998). The present study included levels of alcohol consumption that are considered “hazardous” to general health (The Royal College of Psychiatrists 2011). One may therefore have expected a U-shaped effect, with moderate levels of consumption associated with a protective effect and higher levels of consumption with less or no benefit compared to non-drinkers. In the studies by Rosenhall et al. (1993) and Popelka et al. (1998) cited above, “very heavy drinking” was based on a historic measure, a record of having received two or more reports to the Swedish temperance board and a history of consuming more than four drinks per day, for Rosenhall et al. and Popelka et al., respectively. Note that in the study by Popelka et al, all levels of current alcohol consumption were associated with a reduction in risk of hearing loss, similar to the present study. This discrepancy may be due to differences in patterns of alcohol consumption, in addition to the overall volume of consumption. In studies of cardiovascular disease, binge drinking (consuming a whole week’s healthy allowance of alcohol in one or two sittings) was associated with either no benefit or an increased risk of disease (Kauhanen et al. 1997; Murray et al. 2002). No data on binge drinking were available in the UK Biobank, so we were unable to test this possibility in the present study. Extrapolating from previous literature, one would expect that binge drinking would be associated with increased risk of hearing loss.
One variable relating to patterns of alcohol consumption that was available in the UK Biobank data set was “alcohol usually taken with meals.” In the present study, drinking alcohol with meals was associated with marginally reduced risk of hearing loss, compared to those who usually drink alcohol outside meals (data not reported here). A similar association has previously been observed in relation to risk for cardiovascular disease (Rehm et al. 2003), although this finding is difficult to interpret. Hypothesized casual mechanisms for beneficial effects of drinking alcohol with meals include a reduction in blood pressure (Foppa et al. 2002), increased fibrinolysis (Hendriks et al. 1994), increased HDL cholesterol (Veenstra et al. 1990), reduced absorption, and/or increased elimination of alcohol (Lin and Li 1998; Ramchandani et al. 2001). Alternatively, drinking alcohol with meals or drinking outside meals may be a marker of lifestyle, which may include a range of other risk and protective effects. Rehm et al. (2003) suggested that drinking wine with meals is characteristic of middle- and upper-class socioeconomic status, and socioeconomic status is strongly related to a wide range of health outcomes. It is therefore unclear whether drinking alcohol with meals represents a reduced risk of hearing loss or whether it is merely a marker of a lifestyle associated with better hearing.
A strength of the present study was that associations between alcohol consumption and hearing loss were measured with reference to lifetime teetotalers. To our knowledge, all previous research to date has utilized current non-drinkers as the comparison group. This may have resulted in a bias because some non-drinkers may abstain from alcohol due to poor health and so have poorer hearing due to health-related factors that are unrelated to alcohol consumption (Hines and Rimm 2001). The inclusion of “sick quitters” (referring to those who abstain from alcohol because of poor health) in the non-drinker comparison groups may have resulted in overestimates of the benefit associated with alcohol consumption. In the present study, the protective effect of alcohol consumption was evident based on comparisons with lifetime teetotalers and so provides evidence that the protective association between alcohol consumption and hearing loss is reliable. Note that this conclusion rests on the assumption that lifetime teetotalers represent an unbiased comparison group. However, biases may still remain (Wannamethee and Shaper 1998). Lifetime teetotalers are a minority group within society and may have unknown differences in lifestyle that result in increased risk of hearing loss. The benefits of alcohol consumption may therefore be overestimated.
A further novel aspect of the present study was that hearing was measured with a test of speech recognition in noise. The measures in previous studies were predominantly tests of hearing sensitivity. Speech recognition tests arguably provide a more ecologically valid measure of hearing than detection of tones in a quiet environment does (Arlinger et al. 2009). The associations reported in the present study are therefore likely to relate strongly to real-life hearing difficulties.
Limitations
This study utilized a cross-sectional correlational design, and it was not possible to establish causal associations, nor was it possible to examine the time course of exposure to risks and development of hearing loss. A prospective cohort design may provide more convincing evidence of causal links. It is possible that an unmeasured confounder may be responsible for the effects observed in this study or that the results are due to an effect specific to this sample. However, similar associations have been observed in previous studies in different countries and with different age cohorts. Smoking is associated with other risks for hearing loss (e.g. noise exposure), and so the apparent association between smoking and hearing loss may be explained by these other risks. However, the association between smoking and hearing loss was significant in a model that accounted for alcohol, cardiovascular disease, work-related noise exposure, and SES. This suggests that smoking is not merely a marker for these other risks but rather represents a distinct risk in itself. Goodness-of-fit statistics suggested that there was variance in hearing loss that was not explained by the model. Some variance may not have been adequately captured by the predictor measures (as described in the next paragraph) or the measure of hearing loss used in this study. Additionally, hearing loss is known to have a strongly heritable component (Uchida et al. 2011), and there may be interactions between genetic and environmental effects on susceptibility to hearing loss that were not accounted for in the present study.
Measures of alcohol consumption and smoking were based on self-report. There may be a tendency for participants to underreport smoking and drinking (Del Boca and Darkes 2003; Gorber et al. 2009). The effect of this would be to bias results toward the null, and so, associations between actual levels of smoking and drinking and hearing loss may therefore be larger than reported here. Occupation- and music-related noise exposure was based on a self-report measure which corresponds to noise levels above 85 dB(A) (Health and Safety Executive 1989) but does not account for noise levels that may substantially exceed this level nor for the use or non-use of ear protection. There was no measure of leisure-related noise exposure (such as use of firearms or power tools). Some variance associated with noise exposure may not be therefore adequately measured. The UK Biobank utilized a proxy measure of socioeconomic status based on the participant’s area of residence. This neighborhood-based estimate may have resulted in an ecological fallacy; i.e., erroneous inferences about individual participant’s socioeconomic status were made based on their area of residence. This procedure may have decreased the standard error of the estimated regression coefficient resulting in overestimation of the significance of socioeconomic status as a correlate of hearing loss.
Some previous studies (utilizing pure tone audiometric measures) suggested a stronger effect of alcohol consumption and smoking on high-frequency than on low-frequency hearing (Popelka et al. 2000; Mizoue et al. 2003), though other studies have not found such frequency-related effects (Fransen et al. 2008). Specific patterns of association with either high- or low-frequency hearing loss could provide evidence from which to infer causal mechanisms. We were not able to distinguish associations with particular patterns of high- versus low-frequency hearing loss with the hearing measure used in the present study.
The response rate in the present study was low, and this may represent a source of bias. However, this bias would only explain the association between smoking and hearing loss if smokers with hearing loss participated more readily than smokers without hearing loss. Likewise for alcohol, alcohol drinkers without hearing loss participated more readily than alcohol drinkers with hearing loss. Neither of these possibilities seems likely. The UK Biobank suggests that as long as there are sufficiently large numbers of participants with different levels of relevant risk factors (as there seem to be in the present study), generalizable associations between risk factors and health outcomes can be made with confidence (Allen et al. 2012). Further reassurance of the generalizability of the associations reported in the present study is that they accord with those reported by other studies with close to 100 % response rates (Nakanishi et al. 2000; Mizoue et al. 2003). The associations between smoking, alcohol consumption, and hearing loss reported in the present study are unlikely to be the result of recruitment bias.
CONCLUSION
In this cross-sectional analysis, alcohol consumption was associated with reduced odds of hearing loss, while smoking and passive smoking was associated with increased odds of hearing loss, all in a dose-dependent manner. Ex-smokers were not associated with increased odds of hearing loss compared to non-smokers. Giving up or reducing smoking and avoiding passive exposure to tobacco smoke may be beneficial in reducing the risk of hearing loss.
References
Agrawal Y, Platz EA, Niparko JK (2008) Prevalence of hearing loss and difference by demographic characteristics among US adults. Arch Intern Med 168:1522–1530
Allen N, Sudlow C, Downey P, Peakman T, Danesh J, Elliott P, Gallacher J, Green J, Matthews P, Pell J, Sprosen T, Collins R (2012) UK Biobank: current status and what it means for epidemiology. Health Policy Technol 1
Arlinger S, Lunner T, Lyxell B, Pichora-Fuller KM (2009) The emergence of cognitive hearing science. Scand J Psychol 50:371–384
Barone JA, Peters JM, Garabrant DH, Bernstein L, Krebsbach R (1987) Smoking as a risk factor in noise-induced hearing loss. J Occup Environ Med 29:741–745
Baum-Baicker C (1985) The health benefits of moderate alcohol consumption: a review of the literature. Drug Alcohol Depend 15:207–227
Brant LJ, Gordon-Salant S, Pearson JD, Klein LL, Morrell CH, Metter EJ (1996) Risk factors related to age-associated hearing loss in the speech frequencies. J Am Acad Audiol 7:152–160
Browning G, Gatehouse S, Lowe G (1986) Blood viscosity as a factor in sensorineural hearing impairment. Lancet 327:121–123
Burr H, Lund SP, Bügel Sperling B, Kristensen TS, Poulsen OM (2005) Smoking and height as risk factors for prevalence and 5-year incidence of hearing loss. A questionnaire-based follow-up study of employees in Denmark aged 18-59 years exposed and unexposed to noise. Int J Audiol 44:531–539
Cocchiarella L, Sharp D, Persky V (1995) Hearing threshold shifts, white-cell count and smoking status in working men. Occup Med 45:179–185
Collins R (2012) What makes UK Biobank special? Lancet 379:1173–1174
Criqui MH (1987) The roles of alcohol in the epidemiology of cardiovascular diseases. Acta Med Scand 221:73–85
Critchley JA, Capewell S (2003) Mortality risk reduction associated with smoking cessation in patients with coronary heart disease. J Am Med Assoc 290:86–97
Cruickshanks KJ, Klein R, Klein B, Wiley TL, Nondahl DM, Tweed TS (1998a) Cigarette smoking and hearing loss: the epidemiology of hearing loss study. J Am Med Assoc 279:1715–1719
Cruickshanks KJ, Wiley TL, Tweed TS, Klein BEK, Klein R, Mares-Perlman JA, Nondahl DM (1998b) Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin The Epidemiology of Hearing Loss Study. Am J Epidemiol 148:879–886
Cruickshanks KJ, Zhan W, Zhong W (2010) Epidemiology of age-related hearing impairment. The Aging Auditory System:259-274
Curhan SG, Eavey R, Shargorodsky J, Curhan GC (2011) Prospective study of alcohol use and hearing loss in men. Ear Hear 32:46
Davis AC (1989) The prevalence of hearing impairment and reported hearing disability among adults in Great Britain. Int J Epidemiol 18:911–917
Dawes P, Fortnum H, Moore DR, Emsley R, Norman P, Cruickshanks KJ, Davis AC, Edmondson-Jones M, McCormack A, Lutman ME, Munro K (2014) Hearing in middle age: a population snapshot of 40-69 year olds in the UK. Ear hear
Del Boca FK, Darkes J (2003) The validity of self-reports of alcohol consumption: state of the science and challenges for research. Addiction 98:1–12
Foppa M, Fuchs FD, Preissler L, Andrighetto A, Rosito GA, Duncan BB (2002) Red wine with the noon meal lowers post-meal blood pressure: a randomized trial in centrally obese, hypertensive patients. J Stud Alcohol Drugs 63:247
Fransen E, Topsakal V, Hendrickx J-J, Van Laer L, Huyghe JR, Van Eyken E, Lemkens N, Hannula S, Mä ki-Torkko E, Jensen M (2008) Occupational noise, smoking, and a high body mass index are risk factors for age-related hearing impairment and moderate alcohol consumption is protective: a European population-based multicenter study. JARO J Assoc Res Otolaryngol 9:264–276
Gates GA, Mills JH (2005) Presbycusis. Lancet 366:1111–1120
Gates GA, Cobb JL, D’Agostino RB, Wolf PA (1993) The relation of hearing in the elderly to the presence of cardiovascular disease and cardiovascular risk factors. Arch Otolaryngol Head Neck Surg 119:156–161
Gopinath B, Flood V, McMahon C, Burlutsky G, Smith W, Mitchell P (2010) The effects of smoking and alcohol consumption on age-related hearing loss: the Blue Mountains Hearing Study. Ear Hear 31:277–282
Gorber SC, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M (2009) The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tobacco Res 11:12–24
Guth PS, Norris CH (1996) The hair cell acetylcholine receptors: a synthesis. Hear Res 98:1–8
Health and Safety Executive (1989) Noise at work. Guidance on regulation. HMSO, London
Helzner EP, Cauley JA, Pratt SR, Wisniewski SR, Zmuda JM, Talbott EO, Rekeneire N, Harris TB, Rubin SM, Simonsick EM (2005) Race and sex differences in age‐related hearing loss: The Health, Aging and Body Composition Study. J Am Geriatr Soc 53:2119–2127
Hendriks HF, Veenstra J, Velthuis-te Wierik EJ, Schaafsma G, Kluft C (1994) Effect of moderate dose of alcohol with evening meal on fibrinolytic factors. BMJ Br Med J 308:1003
Hines LM, Rimm EB (2001) Moderate alcohol consumption and coronary heart disease: a review. Postgrad Med J 77:747–752
Hoffman HJ, Dobie RA, Ko C-W, Themann CL, Murphy WJ (2012) Hearing threshold levels at age 70 years (65-74 years) in the unscreened older adult population of the United States, 1959-1962 and 1999-2006. Ear Hear 33:437
Horikawa C, Kodama S, Tanaka S, Fujihara K, Hirasawa R, Yachi Y, Shimano H, Yamada N, Saito K, Sone H (2013) Diabetes and Risk of hearing impairment in adults: a meta-analysis. J Clin Endocrinol Metabol 98:51–58
House Of Commons Science and Technology Committee (2012) Alcohol guidelines. The Stationery Office Limited, London
Hull RH, Kerschen SR (2010) The influence of cardiovascular health on peripheral and central auditory function in adults: a research review. Am J Audiol 19:9
Itoh A, Nakashima T, Arao H, Wakai K, Tamakoshi A, Kawamura T, Ohno Y (2001) Smoking and drinking habits as risk factors for hearing loss in the elderly: epidemiological study of subjects undergoing routine health checks in Aichi, Japan. Publ Health 115:192–196
Johnsson L (1973) Vascular pathology in the human inner ear. Adv Otorhinolaryngol 20:197
Rehm J, Sempos CT, Trevisan M (2003) Average volume of alcohol consumption, patterns of drinking and risk of coronary heart disease—a review. Eur J Cardiovasc Risk 10:15–20
Kauhanen J, Kaplan GA, Goldberg DE, Salonen JT (1997) Beer binging and mortality: results from the Kuopio ischaemic heart disease risk factor study, a prospective population based study. BMJ Br Med J 315:846
Lin RC, Li T-K (1998) Effects of isoflavones on alcohol pharmacokinetics and alcohol-drinking behavior in rats. Am J Clin Nutr 68:1512S–1515S
Lowe G, Drummond M, Forbes C, Barbenel J (1980) The effects of age and cigarette-smoking on blood and plasma viscosity in men. Scott Med J 25:13
Maffei G, MianiI P (1962) Experimental tobacco poisoning: resultant structural modifications of the cochlea and tuba acustica. Arch Otolaryngol Head Neck Surg 75:386
Makishima K (1978) Arteriolar sclerosis as a cause of presbycusis. Otolaryngology 86, ORL322
McKee M, Britton A (1998) The positive relationship between alcohol and heart disease in eastern Europe: potential physiological mechanisms. J R Soc Med 91:402
Mizoue T, Miyamoto T, Shimizu T (2003) Combined effect of smoking and occupational exposure to noise on hearing loss in steel factory workers. Occup Environ Med 60:56–59
Moore RD, Pearson TA (1986) Moderate alcohol consumption and coronary artery disease: a review. Medicine 65:242–267
Murray RP, Connett JE, Tyas SL, Bond R, Ekuma O, Silversides CK, Barnes GE (2002) Alcohol volume, drinking pattern, and cardiovascular disease morbidity and mortality: is there a U-shaped function? Am J Epidemiol 155:242–248
Nakanishi N, Okamoto M, Nakamura K, Suzuki K, Tatara K (2000) Cigarette smoking and risk for hearing impairment: a longitudinal study in Japanese male office workers. J Occup Environ Med 42:1045–1049
Nomura K, Nakao M, Morimoto T (2005) Effect of smoking on hearing loss: quality assessment and meta-analysis. Prev Med 40:138–144
Noorhassim I, Rampal KG (1998) Multiplicative effect of smoking and age on hearing impairment. Am J Otolaryngol 19:240–243
Norman P (2010) Identifying change over time in small area socio-economic deprivation. Appl Spat Anal Pol 3:107–138
Office for National Statistics (2012) General Lifestyle Survey 2010. In
Palmer K, Griffin M, Syddall H, Coggon D (2004) Cigarette smoking, occupational exposure to noise, and self reported hearing difficulties. Occup Environ Med 61:340–344
Pearson TA (1996) Alcohol and heart disease. Circulation 94:3023–3025
Plomp R, Mimpen A (1979) Speech-reception threshold for sentences as a function of age and noise level. J Acous Soc Am 66:1333
Popelka MM, Cruickshanks KJ, Wiley TL, Tweed TS, Klein BE, Klein R (1998) Low prevalence of hearing aid use among older adults with hearing loss: the Epidemiology of Hearing Loss Study. J Am Geriatr Soc 46:1075
Popelka MM, Cruickshanks KJ, Wiley TL, Tweed TS, Klein BEK, Klein R, Nondahl DM (2000) Moderate alcohol consumption and hearing loss: a protective effect. J Am Geriatr Soc 48:1273–1278
Pouryaghoub G, Mehrdad R, Mohammadi S (2007) Interaction of smoking and occupational noise exposure on hearing loss: a cross-sectional study. BMC Public Health 7:137
Ramchandani VA, Kwo PY, Li T-K (2001) Effect of food and food composition on alcohol elimination rates in healthy men and women. J Clin Pharmacol 41:1345–1350
Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, Stampfer MJ (1991) Prospective study of alcohol consumption and risk of coronary disease in men. Lancet 338:464–468
Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA (2011) Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ Br Med J 342
Rosenhall U, Sixt E, Sundh V, Svanborg A (1993) Correlations between presbyacusis and extrinsic noxious factors. Int J Audiol 32:234–243
Rubinstein M, Hildesheimer M, Zohar S, Chilarovitz T (1977) Chronic cardiovascular pathology and hearing loss in the aged. Gerontology 23:4–9
Sharabi Y, Reshef-Haran I, Burstein M, Eldad A (2002) Cigarette smoking and hearing loss: lessons from the young adult periodic examinations in Israel (YAPEIS) database. IMAJ 4:1118–1120
Siegelaub AB, Friedman GD, Adour K, Seltzer CC (1974) Hearing loss in adults: relation to age, sex, exposure to loud noise, and cigarette smoking. Arch Environ Health Int J 29:107–109
Smits C, Kapteyn TS, Houtgast T (2004) Development and validation of an automatic speech-in-noise screening test by telephone. Int J Audiol 43:15–28
Susmano A, Rosenbush SW (1988) Hearing loss and ischemic heart disease. Otol Neurotol 9:403–408
The Royal College of Psychiatrists (2011) Alcohol-use disorders: diagnosis, assessment and management of harmful drinking and alcohol dependence. The British Psychological Society, London
Uchida Y, Nakashima T, Ando F, Niino N, Shimokata H (2005) Is there a relevant effect of noise and smoking on hearing? A population-based aging study. Int J Audiol 44:86–91
Uchida Y, Sugiura S, Ando F, Nakashima T, Shimokata H (2011) Molecular genetic epidemiology of age-related hearing impairment. Auris Nasis Larynx 28:657–665
Veenstra J, Ockhuizen T, van de Pol H, Wedel M, Schaafsma G (1990) Effects of moderate dose of alcohol on blood lipids and lipoproteins postprandially and in the fasting state. Alcohol Alcohol 25:371–377
Vlaming MSMG, Kollmeier B, Dreschler WA, Rainer M, Wouters J, Gover B, Mohammadh Y, Houtgast T (2011) HearCom: Hearing in the Communication Society. Acta Acoust United Ac 97:175–192
Wannamethee SG, Shaper AG (1998) Alcohol, coronary heart disease and stroke: an examination of the J-shaped curve. Neuroepidemiology 17:288–295
World Health Organisation (2013) Tobacco Free Initiative (TFI). Tobacco control country profiles. http://www.who.int/tobacco/surveillance/policy/country_profile/en/index.html. Accessed 15 Sept 2013
Zhan W, Cruickshanks KJ, Klein R, Huang G, Pankow JS, Gangnon RE, Tweed TS (2009) Generational differences in the prevalence of hearing impairment in older adults. Am J Epidemiol 171:260–266
Acknowledgments
Thank you to Dr. David Nondahl for statistical advice. DRM was supported by the intramural program of the Medical Research Council [grant U135097130]. KJC was supported by R37AG11099, R01AG021917, and an unrestricted grant from Research to Prevent Blindness. The Nottingham Hearing Biomedical Research Unit is funded by the National Institute for Health Research. This paper presents independent research funded in part by the National Institute for Health Research (NIHR). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health. This research was facilitated by the Manchester Biomedical Research Centre. This research was conducted using the UK Biobank Resource.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dawes, P., Cruickshanks, K.J., Moore, D.R. et al. Cigarette Smoking, Passive Smoking, Alcohol Consumption, and Hearing Loss. JARO 15, 663–674 (2014). https://doi.org/10.1007/s10162-014-0461-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-014-0461-0