Abstract
Invertebrate neuroscience has provided a number of very informative model systems that have been extensively utilized in order to define the neurobiological bases of animal behaviours (Sattelle and Buckingham in Invert Neurosci 6:1–3, 2006). Most eminent among these are a number of molluscs, including Aplysia californica, Lymnaea stagnalis and Helix aspersa, crustacean systems such as the crab stomatogastric ganglion and a wide-range of other arthropods. All of these have been elegantly exploited to shed light on the very important phenomenon of the molecular and cellular basis for synaptic regulation that underpins behavioural plasticity. Key to the successful use of these systems has been the ability to study well-defined, relatively simple neuronal circuits that direct and regulate a quantifiable animal behaviour. Here we describe the pharyngeal system of the nematode C. elegans and its utility as a model for defining the genetic basis of behaviour. The circuitry of the nervous system in this animal is uniquely well-defined. Furthermore, the feeding behaviour of the worm is controlled by the activity of the pharynx and this in turn is regulated in a context-dependent manner by a simple nervous system that integrates external signals, e.g. presence or absence of food, and internal signals, e.g. the nutritional status of the animal to direct an appropriate response. The genetics of C. elegans is being effectively exploited to provide novel insight into genes that function to regulate the neuronal network that controls the pharynx. Here we summarise the progress to date and highlight topics for future research. Two main themes emerge. First, although the anatomy of the pharyngeal system is very well-defined, there is a much poorer understanding of its neurochemistry. Second, it is evident that the neurochemistry is remarkably complex for such a simple circuit/behaviour. This suggests that the pharyngeal activity may be subject to exquisitely precise regulation depending on the animal’s environment and status. This therefore provides a very tractable genetic model to investigate neural mechanisms for signal integration and synaptic plasticity in a well-defined neuronal network that directs a quantifiable behaviour, feeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Caenorhabditis elegans is widely used as a model animal for the study of nematode behavioural and physiological processes (de Bono and Maricq 2005). This has provided insight into the mode of action of drugs which are used to treat infection with parasitic nematodes. Furthermore, since it has been shown that over 40% of the predicted protein products from the C. elegans genome have significant matches with proteins in other organisms, including human (The C. elegans Sequencing Consortium 1998) it is also widely exploited as a tractable genetic system to define the role of genes for which the functions are conserved throughout the animal phyla (Culetto and Sattelle 2000). Much progress has been made, by comparing the phenotypes of wild type and mutant animals and thereby deducing gene function.
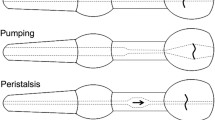
Despite its many advantages, one hindrance for neurobiologists seeking to use C. elegans as a model system has been its small size. Until relatively recently this has precluded more detailed physiological analysis. However, one tissue that has proven more amenable to physiological, including electrophysiological analysis, is the pharynx. This is a muscular pump which sucks bacteria into its lumen, grinds them and then passes them to the gut (Fig. 1). The dimensions of the pharynx, approximately 100 μm long with a diameter of up to 20 μm, have made it accessible for electrophysiological recording techniques. Furthermore, as during some phases of the activity of the muscle it synchronously contracts and relaxes, this generates a large enough electrical signal to be monitored by extracellular recording electrodes. This has led to the development of the so-called ‘electropharyngeogram’ (EPG) by Leon Avery and colleagues (Avery et al. 1995), an extremely useful approach which has been widely adopted by a number of C. elegans laboratories to provide a functional readout of the activity of the pharynx in wild type and mutant animals.
The pharynx of C. elegans is a muscular pump. It can be subdivided into three main sections based on function. The metacorpus and procorpus function together to ingest bacteria, the isthmus acts as a peristaltic valve and passes food to the terminal bulb where it is crushed. The right-hand side of the figure shows a mercator projection, detailing the arrangement of muscle cells. The pharynx has a tri-radiate symmetry, with three main muscle fields, two sub-ventral and one dorsal. The muscle cells (pm1–pm8) are linked via gap-junctions in the longitudinal axis, but also form gap-junctions with neighbouring marginal cells (mc1–mc3) whose longitudinal dimensions define the main functional units within the pharynx. They can receive input from motoneurones. The expression pattern of four innexins is also shown. Loss of function mutations in these gap-junction subunits have been shown to disrupt coupling between muscle cells (Starich et al. 1996, 2001, 2003). Marginal cells are shown in dark grey and muscle cells in light grey. inx is innexin
The pharynx, which is derived from the ectoderm, can pump at two rates. In the absence of food it pumps at a rate of around one pump per second while in the presence of food the rate increases to around four pumps per second. There is intrinsic myogenic activity (Avery and Horvitz 1989) and this is regulated by a simple pharyngeal nervous system, consisting of just 20 neurones. The neurones are embedded within the pharynx and isolated from the rest of the animal by a basement membrane. The only link with the somatic nervous system is through a pair of neurones, the RIP neurones (which in turn receive input from four pairs of extrapharyngeal neurones, IL1, IL2, URA and RME; White et al. 1986). A set of anterior sensilla, the inner labials, provide the sensory input onto these neurones (Ward et al. 1975).
A number of laboratories have focused their studies on the pharynx of C. elegans and there is now a considerable literature available. We therefore consider a review on the pharynx is timely. The pharynx has been mapped in terms of its different cell types and their major connections (Albertson and Thomson 1976). However, unlike other invertebrate model systems which are composed of a relatively small number of neurones, such as the crustacean stomatogastric or cardiac ganglia (Selverston et al. 1976; Maynard 1955) electrophysiological recordings from individual cell types are still in their infancy.
Anatomy of the pharynx
The anatomy of the pharynx has been comprehensively reconstructed from serial section electron micrographs by Albertson and Thomson (1976) and extensive reference will be made to this admirable paper together with http://www.wormatlas.org (a reference database which is an information resource for C. elegans anatomy) in this section of the review. The pharynx consists of 20 muscle cells, some of which are syncytial (Table 1). There are also 9 marginal cells, 9 epithelial cells, 5 gland cells and 20 neurones. The muscle cells, which surround the lumen, can be divided into eight classes (pm1–8). The neurones can be divided into five classes of motoneurone (M1–5) and six classes of interneurone (I1–6). Albertson and Thomson (1976), also describe two further classes, neurosecretory and motor (NSM) and motor and interneurone (MI). Their final class consists of a pair of neurones, MC, which they report only innervate marginal cells. Certain of these neurones also have mechano- or proprio-ceptive endings, for example, M3, I1, I2, I3, I5, I6, NSM and MC. Most of these endings are in the cuticle but M3s end in muscle while I5 has a similar ending to M3s but makes a connection on to M2. The presence of multifunctional neurones indicates that the pharyngeal nervous system may be primitive (Horridge 1968). Each type of cell will now be reviewed. However, it should be remembered that the following identification, i.e. moto- sensory- or inter- neurone, is based on cell morphology and when electrophysiological recordings are made from these cells the classification of some types may be amended.
Muscle cells
The pharynx of C. elegans connects to the outside by the buccal cavity and is composed of three parts, the corpus, the isthmus and the terminal bulb, Fig. 1 (Albertson and Thomson 1976). The corpus is subdivided into the more anterior procorpus and the metacorpus. These parts are formed from eight muscle layers, pm1-8, Fig. 2. The most anterior muscle cell, pm1, has six nuclei and completely surrounds the triangular lumen and has six thin processes running in a posterior direction, adjacent to the outer edges of the marginal cells. Posterior to pm1 are three wedge-shaped binucleate pm2 cells which have processes which project into the nerve cords, Fig. 2. Pm3, pm4 and pm5 are each made up of three wedge-shaped muscle cells and each cell contains two nuclei. Pm4 cells form the metacorpus and the pm5 cells form the isthmus. Pm1–pm5 have radially orientated filaments attached medially to the cuticle of the lumen and laterally to the pharyngeal basal lamina by hemi-desmosomes. The terminal bulb is formed from two types of muscle cell, pm6 and pm7. There are six cells in total and each cell has three nuclei (i.e. it is formed by the fusion of three cells). The grinder, which is formed through a secretion of a thick, ridged cuticle from pm6 and pm7, is localized in the terminal bulb. Finally a saucer-shaped uninucleate muscle cell, pm8, lines the posterior wall of the pharynx. Pm8 has no direct motor innervation but does have gap junctions to mc3 which are innervated by M5. The pm7 muscle cells contain radial and longitudinal filaments and provide a longitudinal and a transverse component to the motion of the grinder teeth (Albertson and Thomson 1976). The terminal bulb connects to the intestine by the pharyngeal-intestinal valve or cardia. The cellular composition and innervation of the eight muscle types are summarized in Table 1.
A mercator projection of the pharynx shows the general location of neuronal somata and their main processes (data taken from Albertson and Thomson 1976 and http://www.wormatlas.org). Somata are located in either the metacorpus or terminal bulb, however, all of the neurons send processes through the posterior part of the corpus where the majority are involved in the formation of a radial nerve ring located within the metacorpus. Marginal cells are shown in dark grey and muscle cells in light grey. Pairs of neurones are shown in the same colour
Gap junctions
The muscle cells of the pharynx communicate with each other via a network of gap-junctions made up of innexins (Fig. 1), a family of gap-junction proteins predominantly found in invertebrates (Phelan 2005). The proteins are expressed as hexameric hemi-channels which cooperate in the formation of an open pore between adjacent muscle cells. In the pharynx this confers a high degree of electrical connectivity which is likely to play a central role in coordinating waves of myogenic excitation and the responses to neuronal modulation. Adult hermaphrodite worms express at least four innexins in the pharynx. INX-2 and INX-3 co-express in the terminal bulb and isthmus (Starich et al. 1996, 2001, 2003) and may interact with each other in the formation of functional gap-junctions. In inx-3 mutants all but a small proportion of offspring die as embryos, a phenotype that has been primarily associated with gross developmental defects that occur as a result of losing functional INX-3. However, in animals that develop to maturity contractions of the corpus, which expresses EAT-5 in addition to INX-3, are coordinated. Terminal bulb muscle cells, that express INX-3 but not EAT-5, are unable to contract together (Starich et al. 2003). EAT-5 is expressed in the metacorpus and isthmus (Starich et al. 1996) and INX-6 is expressed in the metacorpus, procorpus and marginal cells of the isthmus (Li and Dent 2003). Loss of function mutations in eat-5 and inx-6 do not lead to any obvious anatomical defects, but the worms have a starved appearance throughout development (Starich et al. 1996; Li and Dent 2003; Avery 1993b). This starvation phenotype has been associated with reduction in food intake as a direct result of lost coordination in muscle contractions. Mutants in inx-6 show a loss of synchrony between the metacorpus and pro-corpus (Li and Dent 2003) and mutations in eat-5 leads to a failure in coordination between cells of the terminal bulb and the corpus (Starich et al. 2003). An indication that individual innexin species may confer different physiological characteristics to the gap-junctions that they make-up come from the work of Li and Dent (2003). They rescued the inx-6 mutant phenotype by expressing inx-6 under its own promoter sequence. However, when they substituted the rescue construct for eat-5 expressed under the inx-6 promoter there was only a partial rescue of the phenotype.
The role of gap-junctions in mediating communication between pharyngeal neurons is less well understood. However, ultrastructural and anatomical data collected by Albertson and Thomson (1976) indicates that, with the exception of NSM and I4, all of the pharyngeal neurons form gap-junctions with at least one other neurone. The only innexin so far known to subserve a function in the network is UNC-7. The pharyngeal interneurone I1 forms a gap-junction with RIP an extra-pharyngeal neurone. This is the only synaptic connection between the pharynx and the central nervous system of the worm and is involved in modulating pharyngeal pumping in response to sensory information. For example, pharyngeal pumping is reduced in response to a light tail tap in wild-type worms, however this response is lost in unc-7 mutants (Keane and Avery 2003).
Marginal cells
There are nine marginal cells (Fig. 1) (Albertson and Thomson 1976). The first three, mc1, extend from the most anterior muscle cell in the procorpus to the level of the nerve ring in the metacorpus. The second three, mc2, extend from the nerve ring to the anterior end of the terminal bulb while the final three marginal cells, mc3, in the terminal bulb surround the posterior part of the pharyngeal cuticle and are syncytial. The second set of marginal cells are innervated by the MC cells while M5 terminals ramify deeply into the third set of marginal cells. M2 may innervate the subdorsal two most anterior marginal cells. These marginal cells constitute an interesting wedge-shaped structure sandwiched between the muscle cells and extending the entire length of the pharynx (Fig. 2). One possibility for their function may be that they provide a type of fast conducting system to assist in co-ordinated contraction of parts of the pharynx, rather like the Purkinje fibres of the mammalian heart. Marginal cells also contain a relatively large number of mitochondria indicating that they are likely to be metabolically very active. This, combined with the fact that the marginal cells form gap junctions with the muscle, presents the intriguing possibility that they may have a functional role analogous to interstitial cells in the smooth muscle tissues of higher animals (Cambova et al. 2003) and be involved in generating rhythmic activity. It would be of particular interest to know whether the marginal cells express specific ion channels as this may provide some insight into the origin of the pharyngeal myogenic activity which has proved rather elusive to date.
Epithelial cells
Nine epithelial cells surround the anterior margin of the pharynx (Albertson and Thomson 1976). The first three, e1, are arranged on the dorsal and subventral sides of the triangular lumen. The second set of cells, e2, is placed with one cell at each apex of the lumen. The third set of dorsally and subventrally disposed cells, e3, joins the second set slightly posteriorly.
Gland cells
There are two sets of gland cells. One set, g1, has two cells but three nuclei while the other set, g2, has two cells with two nuclei and has clearer cytoplasm. Both types of gland cell open into the lumen via a short duct (Albertson and Thomson 1976). The g1 cell bodies fill a large part of the terminal bulb. Albertson and Thomson suggest that the gland cells may be innervated by M4 and M5 while the cell bodies of M2 attach to the gland cells by desmosome-like junctions. It is not known what is released from the gland cells but the secretions may possibly contain mucoids.
Nervous system
Most pharyngeal neurones are unbranched, unipolar or bipolar (Albertson and Thomson 1976). Of the five motoneurones, M2 and M3, are paired while the other three are single neurones. Of the six interneurones, I1 and I2, are paired while the rest are single neurones. The neurosecretory motoneurones, NSMs, are paired, as are the marginal cell neurones, MCs. The motor-interneurone, MI, is a single neurone. A number of these neurones send processes to the pharyngeal nerve ring, including M1, MCs, NSMs, I5 and I6. The majority of pharyngeal neurones make synapses en passant (Albertson and Thomson 1976). Furthermore, a single neuronal process can have both presynaptic and postsynaptic compartments and therefore the majority of neurones do not have axons and dendrites in the conventional sense. However, an exception is M3 which sends a process to the most posterior projection of pm4 where it makes a synapse. The chemical and electrical (gap junction) connections are summarized in Table 2 while Table 3 summarizes the classical transmitters and neuropeptides synthesized in, and the receptors expressed on each cell type. The position of neuronal somata and their major projections are shown in Fig. 2.
Motoneurones
The cell body of M1 is located in the terminal bulb and synapses onto pm1, pm2 and pm3 of the procorpus. M1 is cholinergic (Rand et al. 2000) and expresses GLR-2 and AVR-14 glutamate receptor subunits (Brockie et al. 2001; Aptel et al. 2001). M1 receives chemical synaptic input from I1, I2, I5 and M5 and synapses onto I3. It is also possible that M1 makes electrical connections with RIPs (Albertson and Thomson 1976). M2 motoneurones are unipolar and also have their cell bodies in the terminal bulb. Their axons run forward to the metacorpus and synapse onto pm4 and pm5. M2 motoneurones are cholinergic and also express FLP-18, FLP-21, NLP-3 and NLP-13 peptides (Rand et al. 2000; Kim and Li 2004; Nathoo et al. 2001). They terminate in gap junctions with each other. M2s have reciprocal chemical synapses with I1 and electrical synapses with M1, M2, M3 and M5. M3 motoneurones are bipolar, with their cell bodies at the posterior end of the metacorpus. M3s may also be sensory neurones since they have free subcuticular endings which may be mechanosensory. They only synapse onto pm4. These neurones contain glutamate and express FLP-18 and NLP-3 (http://www.wormatlas.org; Kim and Li 2004; Nathoo et al. 2001). M3s express the glutamate receptor subunit GLR-8 (Brockie et al. 2001) and a Neuropeptide Y-like protein, NPR-1 (Coates and de Bono 2002). M3s receive chemical synaptic inputs from I1, I3, I4, I5, M1 and NSM. They do not appear to have strong synaptic inputs onto any neurones. The cell body for M4 lies at the level of the nerve ring and synapses on the posterior portion of the isthmus muscle, pm5 and gland cells, g1. M4 is cholinergic (Chiang et al. 2006) and expresses FLP-2, FLP-5 and FLP-21 (Rand et al. 2000; Kim and Li, 2004). M4 expresses the 5-HT receptor, SER-7b and the glutamate receptor subunit, GLR-8 (Hobson et al. 2003; Brockie et al. 2001). It has also been suggested that it may express the muscarinic receptor, ACM-2 (http://www.wormatlas.org). M4 receives chemical inputs from I3, I5 and I6 and has an electrical link with I6. The cell body of M5 is posterior to pm7 and has processes which remain in the terminal bulb. M5 is cholinergic and expresses FLP-1, FLP-13 and FLP-17 (Rand et al. 2000; Kim and Li 2004). It synapses with pm6 and pm7 and with both gland cells marginal cells and receives a chemical input from I5.
Interneurones
I1, I2 and I3 are unbranched unipolar neurones which are associated with the corpus (Albertson and Thomson 1976). I1 neurones have their cell bodies immediately anterior to the metacorpus and their axons project onto pm1 and pm2. These neurones also have proprioceptive-like endings at the anterior end. The posterior axon projections run within the subvental nerve cords and then into the pharyngeal nerve ring. The somatic nervous system RIP (ring/pharynx interneurone) neurones make gap junctions with I1 neurones and so represent a pathway for the modulation of pharyngeal pumping from, for example, the sensory system (Avery and Thomas 1997). When the touch cells receive a light stimulus pharyngeal pumping is briefly inhibited (Chalfie et al. 1985). The I1 neurones express FLP-6 and NLP-3 (Kim and Li 2004; Nathoo et al. 2001). These interneurones also express GLR-7 and GLR-8 glutamate subunits (Brockie et al. 2001) and possibly the muscarinic acetylcholine receptor ACM-2 (http://www.wormatlas.org). I1s make chemical synapses with M2, M3, MC and NSM and either chemical or electrical synapses with I5. I2s have their cell bodies very slightly posterior to the I1 neurones and their axons project to pm1. I2 neurones have proprioceptive-like free subcuticular endings slightly anterior to those of I1. The I2 neurones express FLP-15, NLP-3 and NLP-8 peptides, GLR-7 and GLR-8 glutamate receptor subunits and ACM-2 muscarinic acetylcholine receptors (Kim and Li 2004; Nathoo et al. 2001; Brockie et al. 2001; http://www.wormatlas.org). I2s make chemical synapses with I4, I6 and NSM and both electrical and chemical synapses with M1. It is possible that I2s could excite M1 and initiate opening of the procorpus to begin the pump cycle. I3 cell body lies at the same level as the I2 interneurones in the anterior metacorpus. Its axon extends forwards and is inserted into pm1 and pm2 and terminates with proprioceptive-like endings. I3 expresses NLP-3 and GLR-7 and GLR-8 glutamate subunits (Nathoo et al. 2001; Brockie et al. 2001). It makes chemical synapses with M3 and NSM. I4 interneurone’s cell body is at the anterior end of the terminal bulb and its two axons run anterior to the nerve ring in the subventral nerve cords. I4 expresses FLP-5, FLP-6, NLP-3 and NLP-13 (Kim and Li 2004; Nathoo et al. 2001). It makes chemical synapses with M1, M3 and NSM and receives inputs from M1 and I2. I5 has a large cell body on the ventral side of the terminal bulb. Its axons extend forward to the terminal bulb and terminate in the nerve ring. I5 contains 5-HT and probably glutamate (Sawin et al. 2000; Lee et al. 1999). I5 expresses FLP-2, FLP-4 and FLP-13 (Kim and Li 2004) and makes chemical synapses with M1, M3, M4 and electrical synapses with M5. It also makes a chemical synapse with the isthmus muscle pm5 and g1 gland cells. I5 receives both chemical and electrical inputs from I1. I6 is bipolar with its cell body in the terminal bulb and a free subcuticular ending between pm5 and pm6. Its longer axon runs anterior to the nerve ring from the dorsal cord. I6 is probably cholinergic and expresses FLP-4 and NLP-3 (Rand et al. 2000; Kim and Li 2004; Nathoo et al. 2001) and makes chemical synapses with M4 and receives chemical synaptic inputs from I2, I3 and NSM. It has electrical synapses with M4 and M5.
Neurosecretory neurones
There is a pair of neurosecretory motoneurones, NSMs, with cell bodies at the level of the metacorpus. These cells are bipolar and their axons run in a posterior direction through the isthmus but terminate before reaching the terminal bulb and makes contact with pm5. They probably function as sensory-motor neurones. Processes from NSMs run very close to the pseudocoelom. The axons are swollen with varicosities filled with large lightly- and darkly-stained membrane bound vesicles and also small clear vesicles. These neurones contain 5-HT and glutamate (Sze et al. 2000; Lee et al. 1999) and express FLP-4, NLP-13, NLP-18 and NLP-19 (Kim and Li 2004; Nathoo et al. 2001). NSMs have a transporter for 5-HT, MOD-5 (Ranganathan et al. 2001). NSMs express GLR-7 and GLR-8 glutamate receptor subunits, a tyramine receptor SER-2, and probably AEX-2, a protein related to the G-protein-coupled receptor family (Brockie et al. 2001; Tsalik et al. 2003). NSMs make a chemical synaptic contact with I6 and M3 and receive a chemical input from I1, I2, I3, I4 and I6.
Motor-interneurones
The cell body of MI is located in the dorsal nerve cord at the level of the metacorpus. It is unipolar and its axon processes around the nerve ring and synapses with pm4. MI makes chemical synapses with M1, M2, M3, MC, NSM and I5, and electrical synapses with I3, I4, I5, M1, M2, MC and M3. It receives a chemical input from M1. MI expresses GLR-2 glutamate receptor subunits and NLP-3 (Brockie et al. 2001; Nathoo et al. 2001).
Marginal cell neurones
The MC cell bodies are at the level of the metacorpus and have short axons, the posterior ones terminating at the very anterior end of the isthmus. They have subcuticular, possibly proprioceptive, nerve endings between pm3 and pm4. MCs synapse onto the c2 marginal cells. They contain acetylcholine and express FLP-2 and FLP-21 (Niacaris and Avery 2003; Kim and Li 2004) and the glutamate receptor subunit, GLR-8 (Brockie et al. 2001). MCs make electrical connections with M2 and receive chemical synaptic inputs from M1 and I1.
Muscle physiology
Feeding mechanisms in the Rhabditina were first analysed in detail from ciné film by Doncaster (1962) and later extended by Avery and his colleagues. In his paper, Doncaster refers to the pharynx as the oesophagus. C. elegans feed almost continuously through their life cycle, apart from when they moult or enter the dauer larval form. During these periods there must be a mechanism for inhibition of pumping. Normal pharyngeal pumping represents a contraction–relaxation cycle and involves the corpus, the anterior isthmus and the terminal bulb (Avery and Shtonda 2003) Fig. 1. In wild type animals pharyngeal muscle contraction does not normally last much more than 200 ms. The corpus and anterior isthmus have two functions, one to trap food and the other to transport food. The incorporation of the anterior isthmus into pumping greatly increases the efficiency of food transport by the corpus (Avery and Shtonda 2003). The role of the terminal bulb is to grind and break up bacteria while that of the posterior isthmus is to regulate the passage of bacteria from the corpus to the terminal bulb by a peristaltic wave. The muscles of the corpus and anterior isthmus are radially orientated, so that when they contract, the lumen opens. During this period of the cycle the posterior isthmus is closed. The cycle begins with the almost simultaneous contraction of the muscles. When the lumen opens, it is filled with liquid sucked in by the mouth which also brings in bacteria. When the muscles relax, the pharyngeal lumen is closed and the liquid expelled. Some of the bacteria remain in the pharynx, i.e. ‘trapping’. When the muscles next contract, these bacteria are carried in a posterior direction by the influx of liquid. When the muscles relax, the bacteria are now at a more posterior position and with repeated pumps, the bacteria are carried back in the isthmus, i.e. transport. Videotape analysis by Avery and Shtonda (2003) has shown that the motions of the anterior pharyngeal muscles during relaxation are not precisely the reverse of those during contraction, for example, the anterior isthmus muscles. The actions of these muscles, which are just posterior to the corpus, are slightly delayed, such that the anterior isthmus is relaxed when the corpus begins to contract but contracted when the corpus relaxes. Furthermore, the anterior isthmus contracts in a wave from anterior to posterior. This small deviation from perfectly reciprocal motion is sufficient to produce a small amount of net particle transport in a posterior direction, assuming the particles move at the same average speed as the fluid (Avery and Shtonda 2003). In addition the geometry of the pharyngeal lumen causes particles to be pushed to the centre of the lumen during relaxation. These authors also found that small bacteria provide a better source of food than large ones. Contraction of the terminal bulb rotates the grinder plates which break up the bacteria. The food is then returned to the lumen. Following contraction, the muscles relax and the grinder returns to its resting position. Relaxation at this point closes the lumen of the corpus and anterior isthmus, resulting in the expulsion of liquid. The broken bacteria are transferred through the pharyngeal-intestinal valve. The muscles of the posterior isthmus are relaxed and the lumen here closed throughout pumping, isolating the corpus from the terminal bulb (Avery 1993a). Contraction of the posterior isthmus muscle conveys food from the anterior isthmus backwards to the terminal bulb (Avery and Shtonda 2003).
Although pharyngeal pumping rate is under the control of MCs (Avery and Horvitz 1989), the pharynx continues to pump at a reduced rate when MCs are ablated and continues to pump slowly when all 20 pharyngeal neurones have been ablated, providing evidence that the pharynx may be myogenic (Avery and Horvitz 1989). For example, the normal pumping rate is around 200 pumps per minute but in animals where MCs have been ablated, this value drops to around 50 pumps per minute (Shtonda and Avery 2005). The term myogenic means that the timing of each pump is controlled by the muscle and one or more neurones modulate the excitability of the muscle. In contrast, the term neurogenic means that the timing of each pump is controlled directly by one or more neurones. Whether pharyngeal pumping is myogenic or neurogenic has been investigated in detail by Raizen et al.(1995). These authors used electropharyngeogram (EPG) recordings first developed by Raizen and Avery (1994). An example of an EPG is shown in Fig. 3a. However, the form of the EPGs can vary between wild type animals, depending at least in part on the relative position of the recording electrode to the pharynx and this is shown in Fig. 3b–d. Thus interpretation of differences in the shape of the EPG requires some caution when making comparisons between different animals. The E (excitatory or contraction) and the R (relaxation) phases indicate the beginning and end respectively of the EPG which provides a read-out of the activity of the pharynx during a single muscle pump. The E phase is normally composed of two components, the initial part (E1), which is usually smaller in amplitude compared to the second part (E2). A small negative potential (R2) follows the R1 phase and is associated with the repolarization of the terminal bulb. During the EPG, negative going potentials (I potentials) of varying amplitudes are often observed. It has been proposed that these potentials are generated by glutamate released from M3 and activating ligand-gated chloride channels. The evidence for this is provided by the following data. M3 neurones are likely to be glutamatergic as they stain with antibodies against glutamate (http://www.wormatlas.org) and they express both synaptic and vesicular glutamate transporters (Lee et al. 1999). Furthermore, exogenous glutamate mimics the action of M3s on the pharynx by shortening the duration of the EPG (Dent et al. 1997). In mutants that have a loss of function mutation in the glutamate-gated chloride channel, GluCl-α2 (AVR-15), the negative going potentials are absent and in these mutants ionophoretic glutamate has no effect on the muscle (Dent et al. 1997). Lastly, EPGs recorded from animals in which the M3 neurones have been laser ablated do not exhibit negative going potentials between the E and R phase (Li et al. 1997). Overall, this supports the contention that these are glutamatergic potentials generated by the M3 neurone.
Electropharyngeogram (EPG) recordings. The recordings were made from cut heads as described in Papaioannou et al. (2005). Each recording is from an adult hermaphrodite wild type animal made within 1 min of achieving the suction pipette seal. a The EPG consists of several components. The first excitatory component, E1, is associated with activation of the MC motoneurone. E2 is a reflection of muscle cell depolarization while repolarization of corpus muscle has a close temporal association with the R1 phase. R2 may be a reflection of terminal bulb repolarization. The exact shape of the EPG recorded can vary between wild type animals. b E1 and R2 phase are often reduced in amplitude or partially masked by a much larger E2 or R1 phase. c the I potentials can also be unmeasureable and indistinct. E1, R2 and I potentials can change amplitude over time, in the absence of changes in recording conditions. d E potentials associated with excitatory synaptic events, can be recorded in the inter-EPG interval
During the inter-pump period, or I phase, small positive potentials are sometimes observed. Raizen et al. (1995) demonstrated that in wild type animals the E phase of each EPG was composed of two components and the small positive potentials were present during the I phase. However, when MCs were ablated both events disappeared, suggesting they were associated with an excitatory input from MCs. In the absence of MC neurones the pharyngeal muscle is still capable of pumping, albeit at a much reduced rate. Raizen et al. (1995) also demonstrated that MCs were able to induce excitatory potentials in the absence of muscle contraction by using the synaptotagmin mutant, snt-1(md290). In these mutants synaptic transmission is reduced but not eliminated (Nonet et al. 1993) and the pump rate is very low.
The resting membrane potential of pharyngeal muscle cells is around −75 mV and is due mainly to the potassium gradient plus a contribution from a ouabain-sensitive electrogenic pump (Franks et al. 2002). A gene encoding a putative α subunit for a Na,K-ATPase, eat-6, has been identified which may underly the sensitivity of the pharynx to ouabain (Davis et al. 1995). The amplitude and duration of the muscle action potentials is dependent on the external concentration of calcium. In low calcium the action potential exhibits an extended duration. A likely explanation for this is that a calcium-dependent potassium channel plays a role in muscle repolarization. Interestingly, action potentials persist in zero calcium/sodium external solution but are abolished in zero external sodium/calcium external solution. Whilst the persistence in zero calcium may readily be explained by the known permeability of many calcium channels to sodium when calcium ions are not present, the latter observation of a dependence on external sodium is more difficult to explain as it would be expected that calcium ions would be able to carry the current. This would suggest that sodium plays a role in the action potential although there are no obvious candidate genes for a voltage-gated sodium channel in the genome of C. elegans. In support of this, a voltage-activated inward current which is reduced in the absence of external sodium ions has been identified in the pharynx (Vinogradova et al. 2006).
The currents underlying pharyngeal muscle action potentials have been investigated (Lee et al. 1997; Davis et al. 1999; Shtonda and Avery 2005; Steger et al. 2005). Three channel currents have been identified as playing key roles in the initiation and termination of the muscle action potential, viz, CCA-1, EGL-19 and EXP-2. CCA-1 is a T-type calcium channel, EGL-19 an L-type calcium channel while EXP-2 is a potassium channel. Avery and co-workers have suggested the following sequence of events for the pharyngeal action potential (summarized in Fig. 4). When the nicotinic acetylcholine receptor subunit EAT-2 is activated by acetylcholine released from MC, the muscle membrane potential depolarizes to around −30 mV. At this potential the CCA-1 channel protein is activated. This results in a large, quickly inactivating inward current due to the entry of calcium into the muscle cells. This depolarizes the muscle cells further and when this depolarization reaches −10 mV, the EGL-19 channel protein is activated. This calcium current triggers muscle contraction and maintains the plateau component of the action potential. The membrane potential of the muscle cells gradually hyperpolarizes as this current slowly inactivates. When the membrane potential reaches the threshold value for re-activation of the potassium channel, EXP-2, a large current is generated which rapidly hyperpolarizes the membrane potential towards its “resting” level. Inhibitory junction potentials, generated through the release of glutamate from M3 enhance the decline of the plateau phase of the action potential towards the value at which EXP-2 channel is re-activated. Interestingly, when CCA-1 is not functional, the depolarizing phase of the action potential tends to plateau around −30 mV (Steger et al. 2005). This model for the sequence of events that underly the pharyngeal action potential provides a useful framework in which to incorporate the activity of further ionic channels that are likely to play a role, such as calcium-activated potassium channels.
A diagram illustrating currents that underlie the initiation and generation of the muscle action potential have been identified (Lee et al. 1997; Davis et al. 1999; Shtonda and Avery 2005; Steger et al. 2005). It is likely that activation EAT-2 containing nAChRs can mediate excitatory post-synaptic events that cause CCA-1 T-type calcium channels to activate and initiate an action potential. The action potential plateau is shaped by the activation of EGL-19, an L-type calcium channel, AVR-15 a glutamate-gated chloride channel and EXP-2 a inward-rectifying potassium channel. EXP-2 contributes to full repolarization of the membrane potential
Optical imaging of calcium transients in pharyngeal muscle and neurones of wild-type and mutant C. elegans was first attempted using cameleon proteins (Kerr et al. 2000). Using unc-36 mutants these authors found that loss of UNC-36 function resulted in an increased size of pharyngeal calcium transients which suggested this calcium channel α2 subunit might negatively regulate the calcium influx to pharyngeal muscle. In a later series of experiments the spatio-temporal patterns of intracellular calcium dynamics in pharyngeal muscle during feeding were analysed using a ratiometric fluorescent calcium indicator (Shimozono et al. 2004). Fast, repetitive and synchronous spikes in calcium concentrations were observed in the corpus, anterior isthmus and terminal bulb, which indicated electrical coupling between the muscles. Broad calcium transients were only seen in the posterior isthmus during peristalsis and these were delayed compared with the calcium transients from the anterior isthmus and terminal bulb. Evidence was found that the calcium dynamics in the posterior isthmus were often decoupled during fast pumping. A single gene, unc-68, encodes a ryanodine receptor in C. elegans (Sakube et al. 1993; Maryon et al. 1996) which is localized to the sarcoplasmic reticulum in the posterior isthmus and terminal bulb (Maryon et al. 1998). Using unc-68 mutants Shimozono et al. (2004) found evidence for a role for calcium-induced calcium release from internal stores in the random decoupling of calcium spikes in the posterior isthmus. These authors suggest that M4 may modulate the excitation–calcium coupling in the posterior isthmus.
Pharmacology of the pharynx: classical transmitters and neuropeptides
Eight of the pharyngeal neurones probably contain acetylcholine, viz, M1, M2s, M4, M5, MCs and I6. Three of these, the MCs and M4, play key roles in pharyngeal function (Avery and Horvitz 1987, 1989) and so acetylcholine receptors are important for its normal physiology. There are at least 27 nicotinic acetylcholine receptor subunits in C. elegans (Jones and Sattelle 2004). Of these, EAT-2 (McKay et al. 2004) has been identified in the pharynx, while mutations in another (lev-8) have been shown to affect pharyngeal pumping (Towers et al. 2005). Although Albertson and Thomson (1976) originally proposed that MCs synapse onto c2 and not directly onto pharyngeal muscle, Shtonda and Avery (2005) consider there may be a direct connection from MCs onto pm4. When MCs are activated, they induce excitatory junction potentials in the muscle through activation of an EAT-2/EAT-18 nicotinic acetylcholine receptor complex, increasing the pumping rate (McKay et al. 2004; Raizen et al. 1995). EAT-2 is localized near the junction of pm4 and pm5 which is the area of the MC synapse. EAT-18 is expressed in pharyngeal muscle and M5. When MCs are stimulated, EAT-2 channels open which allows current to flow, opening voltage-gated calcium channels (Lee et al. 1997). acr-7 encodes an α-7-like subunit which is a member of the nicotinic acetylcholine receptor family and ACR-7 is also expressed in pharyngeal muscle (http://www.wormbase.org).
GAR-3 is a muscarinic receptor in pharyngeal muscle which is activated by arecoline and which plays a key role in regulating muscle activity by altering calcium levels or calcium processes to optimize pumping (Steger and Avery 2004). Activation of GAR-3 receptors in pharyngeal muscle has two distinct functions, one affecting excitation–contraction coupling and the other affecting membrane potential. Steger and Avery (2004) suggest that the GAR-3 pathway may converge with intracellular calcium signalling following calcium influx through the L-type voltage-gated calcium channel, EGL-19. This channel contributes to fast depolarization of pharyngeal muscle. EGL-19 probably contributes to excitation–contraction coupling as well as membrane depolarization. In mutants over expressing gar-3, pharyngeal muscle relaxation is inhibited by altering the entry of calcium through EGL-19. Loss of gar-3 expression causes shortening of action potentials and speeds up terminal bulb repolarization. Loss of gar-3 expression also increases pumping rate which appears to be independent of pump duration but may be linked with enhanced excitability of the muscle or a shortening of the refractory period between pumps. Steger and Avery (2004) propose that GAR-3 activates a previously unidentified signalling cascade which regulates membrane potential and excitation–contraction coupling through two different mechanisms. These involve changes in intracellular calcium signalling which adjusts the kinetics of pharyngeal muscle function to allow optimal feeding.
Ablation studies suggest the remaining acetylcholine-containing neurones probably only contribute minor roles in the normal physiology of the pharynx (Avery and Horvitz 1989). However, M1, M2 and M5 have excitatory inputs onto pm3/pm4, pm5/pm6 and pm7, respectively, Fig. 5. Of these, M1 and M5 receive inputs from I5 which probably contains two classical transmitters, viz, 5-HT and glutamate. In addition, I5 contains three FLPs, viz, FLP-2, FLP-4 and FLP-13 (Kim and Li 2004). Of these, FLP-13 has a potent inhibitory action on pharyngeal pumping, FLP-2 is excitatory while the response to the application of FLP-4 is variable, sometimes exciting and sometimes inhibiting preparations (Papaioannou et al. 2005). However, the actions of these peptides on M1 and M5 are unknown.
Schematic diagram showing the main motoneuron inputs onto the pharynx (after Avery 1993a)
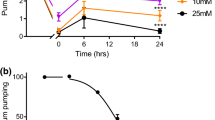
The indolealkylamine, 5-HT (serotonin, enteramine), is present in the paired NSM neurones in the pharyngeal nervous system (Horvitz et al. 1982) which synapse onto the isthmus muscle, pm5. Application of 5-HT stimulates pumping, e.g. from a resting level of around 40 pumps per minute to over 250 pumps per minute (Avery and Horvitz 1990; Niacaris and Avery 2003). A reduction in the level of endogenous 5-HT decreases pumping rate (Sze et al. 2000). There is one tryptophan hydroxylase gene, tph-1, which encodes for the rate limiting enzyme in the synthesis of 5-HT (Sze et al. 2000). tph-1 mutants therefore cannot synthesize 5-HT from tryptophan though if 5-hydroxytryptophan is present in the bacteria they ingest, then these mutants could still make 5-HT through an aromatic amino acid decarboxylase enzyme. This might explain the low levels of 5-HT immunoreactivity which have been reported in the tph-1 null. tph-1 mutants are viable though their behaviours involved in feeding are altered, e.g. their average pumping rate is low in the presence of bacteria, while in the absence of food their pumping rate is comparable to wild-type animals (Sze et al. 2000). A closer analysis of the feeding behaviour of tph-1 mutants showed that they can pump at a rate comparable to wild type animals on bacteria, but their pumping behaviour is erratic and they are unable to sustain a fast pump rate (Hobson et al. 2006). This is consistent with the idea that 5-HT is not required for basal pumping but is required in order to maintain a sustained high pumping rate observed in the presence of food.
Four 5-HT receptors have been identified in C. elegans, viz, SER-1, SER-4, SER-7B and MOD-1 (Hamdan et al. 1999; Olde and McCombie 1997; Hobson et al. 2003; Ranganathan et al. 2000) (summarized in Fig. 6). The first three are expressed in the pharyngeal system together with two tyramine receptors, SER-2 and TYRA-2 (Rex and Komuniecki 2002; Tsalik et al. 2003). SER-1, a 5-HT2-like receptor is expressed in the nerve ring and on most of the larger pharyngeal muscles, viz, pm1, pm2, pm3, pm5 and pm7. SER-4, a 5-HT1-like receptor is expressed on a single unidentified pharyngeal neurone. SER-7B, a 5-HT7-like receptor was initially found to be expressed on only M4 and over expression of this receptor was toxic, suggesting this site might form the basis for an anthelmintic (Hobson et al. 2003). However, a subsequent study found that SER-7 was also expressed consistently in MCs, I2s, I3, and M5 and occasionally in M2s, M3s, I4 and I6 (Hobson et al. 2006). The tyramine receptor, SER-2, is expressed in the nerve ring and on pm1 and pm6 and on NSM (Tsalik et al. 2003). Another tyramine receptor, TYRA-2, is also expressed on NSMs and on MCs and so activation of this receptor is likely to play a role in the physiology of the pharynx. Octopamine inhibits pharyngeal pumping and the firing rate of muscle action potentials (Niacaris and Avery 2003; Rogers et al. 2001) and may act on tyramine receptors. If it acts on either SER-2 or TYRA-2 then it will alter the actions of NSMs and MCs on pharyngeal activity.
The actions of 5-HT and octopamine on the pumping rate of the pharynx have been analysed in depth using mutant animals by Niacaris and Avery (2003). Using an eat-18 mutant, in which MCs were proposed to be functionally decoupled from the pharyngeal muscle, they found that 1 μM 5-HT enhanced M3 activity and decreased the EPG duration while 100 μM octopamine suppressed M3 activity and increased the EPG duration. In an eat-18;avr-15 double mutant, neither 5-HT nor octopamine affected the EPG duration, from which Niacaris and Avery concluded that the regulation of EPG duration by 5-HT in eat-18 mutants is largely through its action on M3 activity. In the avr-15 single mutant 5-HT increased the EPG duration indicating that EAT-18 activation also mediated the action of 5-HT on EPG duration. Niacaris and Avery (2003) also studied the effect of 5-HT on eat-2;avr-15 double mutants and found that 5-HT did not modulate their EPG duration. This provided further evidence that 5-HT was acting via an MC-dependent system. In contrast, octopamine suppressed M3 activity of wild-type animals and increased the EPG duration. Niacaris and Avery (2003) also investigated the effect of depleting 5-HT levels by using the tph-1 mutant. An eat-18;tph-1 double mutant had reduced M3 activity and failed to respond to octopamine. Interestingly, the EPG duration was similar between eat-18;tph-1 double mutants and eat-18 mutants, which suggests there is a 5-HT and M3-independent mechanism for shortening EPG duration. Overall, it would appear that 5-HT alters the physiology of the pharynx to allow for rapid contraction-relaxation cycles. The reciprocal action of 5-HT and octopamine provides a mechanism for adapting to a range of food availability. When food is abundant, 5-HT increases the speed and efficiency of feeding. On the basis of these observations it is proposed that when food levels fall, 5-HT levels also fall and octopamine levels may rise, switching the pharynx to an inactive state, with long duration EPGs, and a slow rate of pumping.
The experiments of Niacaris and Avery (2003) assume 5-HT inputs onto both MC and M3 but currently there is only evidence for an input from NSM onto M3. However, it is also possible that 5-HT circulating in the pseudocoelomic fluid excites MCs as a neurohormone (Komuniecki et al. 2004). To support this possibility 5-HT is present in the perienteric fluid of the parasitic nematode Ascaris suum at around 1 μM (Johnson et al. 1996). Octopamine-containing neurones have not yet been identified in the pharyngeal nervous system but this amine could also act as a neurohormone and it does inhibit EPGs and muscle action potentials (Rogers et al. 2001). Interestingly, this paper showed that in synaptobrevin, snb-1, mutants which are defective in neurotransmission, the inhibitory action of octopamine was enhanced, suggesting that octopamine might act on an excitatory pharyngeal neurone to reduce the release of its transmitter, possibly acetylcholine or 5-HT since both MCs and NSMs express TYRA-2 receptor subunits and NSM also expresses SER-2 subunits.
The role of SER-7 in the stimulation of pharyngeal pumping by 5-HT has been investigated using mutants with a loss of function in ser-7 (Hobson et al. 2006). These authors found that 5-HT did not stimulate pumping in ser-7 mutants although SER-1 receptors were still present on the muscle. Therefore SER-1 receptors are not sufficient to confer pharyngeal sensitivity to exogenous 5-HT. However, bacteria still stimulated pumping in ser-7 mutants though this stimulation was more variable compared with wild-type animals. Together, these two observations, namely that 5-HT cannot stimulate pumping in ser-7 mutants but bacteria can, suggest that 5-HT is not the sole signal for food. Interestingly, in mutants where pumping was activated in the presence of bacteria, 5-HT inhibited pumping, possibly suggesting a feedback system mediated through another 5-HT receptor subunit. Hobson et al. (2006) also presented evidence that SER-7 regulation of pumping might involve MCs since the initial excitatory component of the EPG E phase (1 in Fig. 3), was absent in ser-7 mutants. They concluded that while 5-HT does not appear to be necessary for the stimulation of pumping it may have a role in “fine tuning” the increased rate observed in the presence of bacteria.
The C. elegans inositol 1,4,5-triphosphate (IP3) receptor, ITR-1, is expressed in the pharynx, particularly in the terminal bulb and isthmus (Baylis et al. 1999). The role of IP3 in signalling the pharyngeal up-regulation in response to bacteria and to 5-HT has been compared by the use of a number of different approaches to disrupt the IP3 signal (Walker et al. 2002). One study used the expression of a dominant negative construct, consisting of the IP3 binding domain (sponge treatment). Although the basal level of pumping in the absence of food was unchanged in the sponge-treated animals, the response to food was significantly reduced and the pumping rate was more variable. The response to 5-HT was unaffected. Baylis et al. (1999) also made use of an itr-1 mutant and RNAi for itr-1. The itr -1 mutants exhibited a decreased response to food compared to wild type, i.e. similar to the result observed with the IP3 sponge. Furthermore, although the pumping rate in itr-1 was increased in the presence of 5-HT, it did not achieve wild-type levels. However, the authors note that these animals were rather sick. Overall, they conclude that IP3 receptors are required for the up-regulation of pumping in response to food and that at least part of the 5-HT effect is not dependent on IP3 signalling.
Glutamate is synthesized in five pharyngeal neurones, viz, M3s, NSMs and I5 (Lee et al. 1999) and three ionotropic glutamate receptor genes, viz, glr-2, glr-7 and glr-8 are expressed in 16 neurones, viz, all the motoneurones except M2s, all the interneurones except I4 and I5, and the NSMs and MCs (Brockie et al. 2001; Aptel et al. 2001). Two areas of the pharynx respond strongly to glutamate, viz, pm4 cells in the metacorpus and the junction between pm5 in the isthmus and pm6 in the terminal bulb (Li et al. 1997). Thus glutamate could play a key role in pharyngeal feeding and the role of M3 has already been discussed. Of these neurones, M1, M3, M4 and I6 receive an input from the glutamate-containing neurones. Glutamate released from M3 acts on pm4 and acts through GluCl-α2 subunits encoded by avr-15 (Dent et al. 1997). These glutamate-gated chloride channels are also sensitive to the anthelmintic, ivermectin. The pharynx of avr-15 mutants is insensitive to ivermectin (Pemberton et al. 2001). Another glutamate-gated chloride channel, GluCl-β, is activated by glutamate but not by ivermectin (Cully et al. 1994). This GluCl-β subunit is expressed in pm4 of the pharynx (Laughton et al. 1997). Two other glutamate-gated chloride channels, GluCl-α3A and B, which are encoded by avr-14, are absent in pharyngeal muscle but occur in M1 and M4. However, avr-14 is not required for the paralytic effect of ivermectin on the pharyngeal muscle as a putative null mutant still responds to ivermectin in a similar fashion to wild type (Pemberton 2001). In total, there are eight glutamate/ivermectin chloride channels in C. elegans. Exogneous glutamate depolarizes pharyngeal muscle and inhibits action potential activity by increasing the permeability to chloride ions (Pemberton et al. 2001). This chloride effect is a depolarization because ECl in this muscle is more positive than the resting membrane resting potential. It would be interesting to investigate the effect of glutamate on the neurones which express glutamate receptor subunits. I5, which contains glutamate, synapses onto both M3s and M4, Fig. 5, and both neurones express glutamate receptors. Thus glutamate released onto pharyngeal neurones could influence the timing of the contraction of the posterior isthmus.
The work of Kim and Li (2004) and Nathoo et al. (2001) and summarized by Li (2005) clearly shows that a number of genes that encode precursors for FLP and NLP neuropeptides are expressed in pharyngeal neurones, indicating that these peptides probably play key roles in the physiology of the pharynx (Table 3). A number of FLPs have excitatory or inhibitory effects on pharyngeal pumping (Rogers et al. 2001; Papaioannou et al. 2005). These authors identified two most potent peptides, FLP-17A and FLP-13A. The genes that encode the precursors for these peptides are expressed in pharyngeal neurones; flp-13 in M5, I5 and M3 and flp-17 in M5. It is therefore likely that FLP-13 and FLP-17 peptides have a role in pharyngeal physiology. Twelve further flp genes are expressed in pharyngeal neurones, two of which, flp-5 and flp-15, are also expressed in pharyngeal muscle. Five flp genes, flp-5, flp-4, flp-2, flp-13 and flp-21 are expressed in at least three types of neurone. These include neurones with important roles in the regulation of pharyngeal activity, viz, M3s, M4, I5, MCs and NSMs. Thus a cocktail of neuropeptides have to be considered when determining the role of pharyngeal neurones in the regulation of pharyngeal activity.
Role of pharyngeal nervous system in the regulation of pharyngeal activity
A number of laboratories have been involved in the analysis of pharyngeal function in C. elegans but the major contribution to our knowledge on this topic comes from the work of Leon Avery. There are 20 neurones in the pharyngeal nervous system but the role of a number of these neurones is unknown, these include I2s, I3, I4, I6, M1, M2s, M5 and MI, which make up half the neurones in the pharyngeal nervous system. Currently these neurones are described as redundant (http://www.wormatlas.org) although future work may indicate a role. Both laser ablation experiments and the extensive use of mutations have indicated roles for certain key neurones in feeding, including M3, M4, MC and I5. Removal of any one of these neurones from the pharyngeal circuit results in inefficient feeding. Table 4 lists some of the mutants which show abnormal pharyngeal behaviour.
The first ablation experiments involving pharyngeal neurones were performed on M4 (Avery and Horvitz 1987). When these authors ablated M4 in newly hatched animals the posterior isthmus remained closed while the corpus and terminal bulb muscles still contracted in synchrony. However, since the isthmus was closed the corpus quickly filled with bacteria and the terminal bulb had nothing to grind. Thus no food entered the intestine and the animals starved and arrested as larvae though they continued to be active for up to two weeks. When the other 19 neurones were ablated, the corpus never became engorged with bacteria and the animals grew to the adult stage and produced progeny. When M4 was ablated at the time of the second larval moult (21 h) the animals were either stunted or developed completely normally. Avery and Horvitz (1987) proposed that either in older animals the neurone could be functional in the absence of the cell body or by that age the isthmus muscle had acquired the ability to contract myogenically. It is unlikely that M4 has a direct role in pharyngeal pumping even though in the absence of M4 pumping becomes slow and irregular (Avery and Horvitz 1987). These authors suggest this effect is indirect due to lack of isthmus peristalsis since animals which lack M4 are starved and starvation affects pumping. In the absence of isthmus pumping the corpus fills with bacteria and the terminal bulb becomes empty. Several neurones, for example, MC, M3 and NSM have free nerve endings in the corpus and I5 and I6 have endings in the terminal bulb. If one of the roles of the nerve endings is to sense bacteria, then these cells would fail to function correctly in animals where M4 had been ablated.
In a further series of ablation experiments, Avery and Horvitz (1989) showed that MCs are required for optimal pharyngeal pumping. When MCs alone were ablated, pharyngeal pumping slowed and became irregular and resulted in starved adults. MCs have free nerve endings in the corpus and might be able to sense bacteria. MCs have gap junction connections with M2 and chemical synapses onto c2 marginal cells. Since M2s are not necessary for MC function, it can be concluded that MCs act on c2 directly. However, later experiments suggest that MCs may also synapse directly on pharyngeal pm4 muscles (Shtonda and Avery 2005). Ablating any single type of neurone, apart from M4 and MC, did not produce an obvious effect on pumping or isthmus peristalsis. However, in animals where all 12 types of neurone, termed the GREEN neurones by Avery and Horvitz, that is, apart from MC and M4, are ablated, bacteria no longer moved in a posterior direction efficiently in the lumen of the corpus and isthmus. When all the GREEN neurones were ablated, bacteria were swept in a posterior direction during contraction but instead of remaining where they were, were swept forward during relaxation, a condition termed “slippery pharynx” (Avery and Horvitz 1989). Bacterial trapping may depend on the precise timing of relaxation of the pharyngeal muscles and later Avery (1993a) set out to investigate the role of individual GREEN neurones on the timing of relaxation. Avery and Horvitz (1989) concluded that the posterior half of the isthmus does not contract in synchrony with the anterior half and the rest of the pharyngeal muscles. Peristalsis of the posterior isthmus occurs approximately following each fourth pump. This sequence of events is interesting since pm5 extends the whole length of the isthmus but the halves differ in their excitability. It is probable that electrical activity in the posterior isthmus is graded and that CCA-1 (a T-type calcium channel) and EXP-2 (a potassium channel) currents are absent (Shtonda and Avery 2005), providing an example of subcellular specialization (Avery 1993a).
Apart from the posterior portion of pm5, all pharyngeal muscles contract in synchrony and this synchrony remains following ablation of all 20 pharyngeal neurones (Avery and Horvitz 1989). Since no mechanosensory structures are obvious in the non-neuronal cells of the pharynx, Avery and Horvitz conclude that pharyngeal co-ordination is likely to be due to electrical coupling.
Avery (1993b) discusses the possible function of the pharyngeal nervous system and suggests that it is primarily sensory. Rather than directly inducing pharyngeal contractions, he suggests the pharyngeal nervous system might allow the contractions to change in response to a changing environment. Avery considers the MCs are good examples of pharyngeal sensory neurones and are required for a response to bacteria, viz, MCs can signal whether bacteria are present and increase pharyngeal pumping. Avery notes that if this system had not evolved then the pharynx would have to pump at a fast rate all the time, whether bacteria were present or not. This would be wasteful in terms of energy expenditure by the animal. Avery (1993b) also suggests that M4 and the GREEN neurones might regulate isthmus peristalsis and bacterial trapping in response to changes in the environment. I5, with its sensory endings in the terminal bulb, might sense when the terminal bulb is becoming too full for effective grinding and inhibit M3s and M4.
Avery (1993a) demonstrated that when M3s were ablated the pump duration was increased by around 15%. In animals where I5 alone was ablated the pump duration was decreased but when both I5 and M3s were ablated, pump duration increased. I5 synapses onto M3s and so Avery proposed that I5 increases pump duration by inhibiting M3s. He concluded this from experiments where he measured the time taken by the grinder to move from the contraction to the relaxation position in the absence of M3s. M3s decrease pump duration by increasing the rate of relaxation, i.e. M3s increase rate of terminal bulb relaxation. Avery concluded that both I5 and M3s were necessary for trapping bacteria in the anterior isthmus. Neither neurone could provide normal functioning on its own. The inhibition of M3s by I5 could be due to the release of either glutamate or FLP13A (Lee et al. 1999; Kim and Li 2004; Papaioannou et al. 2005). In summary, defects associated with the transport of food are linked with M3s while defects associated with the rate of feeding are linked with MCs.
Conclusions
In this review the synaptic connections between neurones and pharyngeal muscle cells of C. elegans have been described. However, it should be noted that the synaptic organization of the pharyngeal nervous system is based on the electron micrographs of Albertson and Thomson who obtained serial sections from the entire pharynx of only one animal. Although their findings were confirmed using the major portion of the pharynx from an additional animal, individual variations occur between animals in their morphology and so it is likely that subsequent work may identify additional synaptic connections. Nonetheless, their work forms an excellent basis for further morphological and physiological studies. When it becomes possible to routinely record from the pharyngeal neurones it is possible that more definitive physiological roles will be identified for them and the nomenclature of individual neurones may need to be amended.
In order to better define the pharmacological properties of the pharynx it is important to determine the classical transmitters and neuropeptides synthesized by each pharyngeal neurone together with the receptors which they express. It is interesting that the major neuromuscular excitatory transmitter in both pharyngeal and body wall muscle in C. elegans is acetylcholine. In contrast, the major inhibitory transmitter onto pharyngeal muscle is glutamate while that onto body wall muscle is γ-aminobutyric acid (GABA). From the current literature the major neurones modulating pharyngeal activity are MC, M4, both releasing acetylcholine, and M3 which releases glutamate. It also clear that 5-HT, present in the NSM neurones, has a major role in pharyngeal function and may be involved, at least in part, in signalling the presence of food. Whilst there is comprehensive information on the expression pattern of neuropeptides, little is known concerning their roles and modes of action. Nonetheless, it is clear that a number of neuropeptides have potent biological activity when applied to the pharyngeal muscle in a pharmacological assay, and therefore it is likely that they play a physiological role in regulating pharyngeal function. As a cautionary note, it is worth highlighting that the majority of the statements made concerning neurotransmitter content and receptor localization for individual neurones are drawn from experiments that defined the expression pattern of gene promoter::reporter constructs and have not always been confirmed using immunocytochemistry. Their accuracy therefore depends on the promoter region used for the experiment and may not always be an accurate reflection of the native expression pattern.
Overall, the elegant studies on the anatomy of the C. elegans pharyngeal system, combined with the publication of the animal’s genome and the isolation of many mutants with abnormal pharyngeal properties continues to provide an excellent model for defining the genetic basis of behaviour controlled by a simple network composed of a small number (20) of neurones.
References
Albertson DG, Thomson JN (1976) The pharynx of Caenorhabditis elegans. Phil Trans Roy Soc Lond B 275:299–325
Aptel N, Cook A, Pemberton D, Portillo V, Rogers C, Holden-Dye L, Wolstenholme A (2001) The physiological roles of AVR-14 in C. elegans and the parasite Haemonchus contortus. Intl Worm Meeting Abstr 690
Avery L (1993a) Motor-neuron M3 controls pharyngeal muscle-relaxation timing in Caenorhabditis elegans. J Exp Biol 175:283–297
Avery L (1993b) The genetics of feeding in Caenorhabditis elegans. Genetics 133:897–917
Avery L, Horvitz HR (1987) A cell that dies during wild type Caenorhabditis elegans development can function as a neuron in a ced-3 mutant. Cell 51:1071–1078
Avery L, Horvitz R (1989) Pharyngeal pumping continues after laser killing of the pharyngeal nervous-system of C. elegans. Neuron 3:473–485
Avery L, Horvitz HR (1990) Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. J Exp Zool 253:263–270
Avery L, Shtonda BB (2003) Food transport in the C. elegans pharynx. J Exp Biol 206:2441–2457
Avery L, Thomas JH (1997) Feeding and defecation. In: Riddle DL, Blumenthal T, Meyer BJ, Preiss JR (eds) C. elegans II. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 679–716
Avery L, Raizen D, Lockery S (1995) Electrophysiological methods. Meth Cell Biol 48:251–269
Baylis HA, Furuichi T, Yoshikawa F, Mikoshiba K, Sattelle DB (1999) Inositol 1,4,5-triphosphate receptors are strongly expressed in the nervous system, pharynx, intestine, gonad and excretory cell of Caenorhabditis elegans and are encoded by a single gene (itr-1). J Mol Biol 294:467–476
Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77:71–94
Brockie PJ, Mellem JE, Hills T, Masden DM, Maricq AV (2001) The C. elegans glutamate receptor subunit NMR-1 is required for slow NMDA-activated currents that regulate reversal frequency during locomotion. Neuron 31:617–630
Cambova P, Hubka P, Sulkova I, Hulin I (2003) The pacemaker activity of interstitial cells of Cajal and gastric electrical activity. Physiol Res 52:275–284
Chalfie M, Sulston JE, White JG, Southgate E, Thomson JN, Brenner S (1985) The neural circuit for touch sensitivity in Caenorhabditis elegans. J Neurosci 5:956–974
Chiang JTA, Steciuk M, Shtonda B, Avery L (2006) Evolution of pharyngeal behavior and neuronal function in free-living soil nematodes. J Exp Biol 209:1859–1873
Coates JC, de Bono M (2002) Antagonistic pathways in neurons exposed to body fluid regulate social feeding in Caenorhabditis elegans. Nature 419:925–929
Culetto E, Sattelle DB (2000) A role for Caenorhabditis elegans in understanding the function and interactions of human disease genes. Hum Mol Genet 9:869–877
Cully DF, Vassilatis DK, Liu KK, Paress PS, Van der Ploeg LHT, Schaeffer JM, Arena JP (1994) Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature 371:707–711
Davis MW, Somerville D, Lee RyN, Lockery S, Avery L, Fambrough DM (1995) Mutations in the Caenorhabditis elegans Na,K-ATPase alpha-subunit gene, eat-6, disrupt excitable cell-function. J Neurosci 15:8408–8418
Davis MW, Fleischauer R, Dent JA, Joho RH, Avery L (1999) A mutation in the C. elegans EXP-2 potassium channel that alters feeding behavior. Science 286:2501–2504
de Bono M, Maricq AV (2005) Neuronal substrates of complex behaviors in C. elegans. Ann Rev Neurosci 28:451–501
Dent JA, Davis MW, Avery L (1997) avr-15 encodes a chloride channel subunit that mediates inhibitory glutamatergic neurotransmission and ivermectin sensitivity in Caenorhabditis elegans. EMBO J 16:5867–5879
Doncaster CC (1962) Nematode feeding mechanisms. I. Observations on Rhabditis and Pelodera. Nematologica 8:313–320
Franks CJ, Pemberton D, Vinogradova I, Walker RJ, Holden-Dye L (2002) The ionic basis of the resting membrane potential and action potential in the pharyngeal muscle of Caenorhabditis elegans. J Neurophysiol 87:954–961
Hadju-Cronin YM, Chen WG, Patikoglou G, Koelle MR, Sternberg PW (1999) Goa and Gqa in Caenorhabditis elegans the RGS protein EAT-16 is necessary for Goa signaling and regulates Gqa activity. Genes Dev 13:1780–1793
Hamdan FF, Ungrin MD, Abramovitz M, Ribiero P (1999) Characterization of a novel serotonin receptor from Caenorhabditis elegans: cloning and expression of two splice variants. J Neurochem 72: 1372–1383
Harris TW, Hartweig EA, Horvitz HR, Jorgensen EM (2000) Mutations in synaptojanin disrupt synaptic vesicle recycling. J Cell Biol 150:589–599
Hobson RJ, Geng J, Gray AD, Komuniecki RW (2003) Ser-7b, a constitutively active G alpha coupled 5-HT7 like receptor expressed in the Caenorhabditis elegans M4 pharynx motorneuron. J Neurochem 87:22–29
Hobson RJ, Hapiak VM, Xiao H, Buehrer KL, Komuniecki PR, Komuniecki RW (2006) SER-7 a Caenorhabditis elegans 5HT7-like receptor is essential for the 5-HT stimulation of pharyngeal pumping and egg-laying. Genetics 172:159–169
Horridge GA (1968) The origins of the nervous system. In: Bourne GH (ed) The structure and function of nervous tissue, vol. 1. Academic, New York, pp 1–31
Iwasaki K, Staunton J, Saifee O, Nonet M, Thomas JH (1997) aex-3 encodes a novel regulator of presynaptic activity in C. elegans. Neuron 18:613–622
Horvitz HR, Chalfie M, Sulston JE, Evans PD (1982) Serotonin and octopamine in the nematode C. elegans. Science 216:1012–1014
Johnson CD, Reinitz CA, Sithigorngul P, Stretton AOW (1996) Neuronal localization of serotonin in the nematode Ascaris suum. J Comp Neurol 367:352–360
Jones AK, Sattelle DB (2004) Functional genomics of the nicotinic acetylcholine receptor gene family of the nematode, Caenorhabditis elegans. BioEssays 26:39–49
Keane J, Avery L (2003) Mechanosensory inputs influence Caenorhabditis elegans pharyngeal activity via Ivermectin sensitivity genes. Genetics 164:153–162
Kerr R, Lev-Ram V, Baird G, Vincent P, Tsein RY, Schafer WR (2000) Optical imaging of calcium transients in neurons and pharyngeal muscle. Neuron 26:583–594
Kim K, Li C (2004) Expression and regulation of an FMRFamide-related neuropeptide gene family in Caenorhabditis elegans. J Comp Neurol 475:540–550
Komuniecki RW, Hobson RJ, Rex EB, Hapiak VM, Komuniecki PR (2004) Biogenic amine receptors in parasitic nematodes: what can be learned from Caenorhabditis elegans. Mol Biochem Parasitol 137:1–11
Koushika SP, Richmond JE, Hadwiger G, Weimer RM, Jorgensen EM, Nonet ML (2001) A post-docking role for active zone protein RIM. Nature Neurosci 4:997–1005
Landell SJ, Gee VJ, Harkness PC, Doward AI, Baker ER, Gibb AJ, Millar AS (2005) RIC-3 enhances functional expression of multiple nicotinic acetylcholine receptor subtypes in mammalian cells. Mol Pharmacol 68:1431–1438
Laughton DL, Lunt GG, Wolstenholme AJ (1997) Alternative splicing of a Caenorhabditis elegans gene produces two novel inhibitory amino acid receptor subunits with identical ligand binding domains but different ion channels. Gene 201:119–125
Lee RN, Lobel L, Hengartner M, Horvitz HR, Avery L (1997) Mutations in the alpha 1 subunit of an L-type voltage-activated Ca2+ channel cause myotonia in Caenorhabditis elegans. EMBO J 16:6066–6076
Lee RYN, Chalfie M, Horvitz HR, Avery L (1999) EAT-4, a homolog of a mammalian sodium-dependent inorganic phosphate co-trnasporter, is necessary for glutamatergic neurotransmission in Caenorhabditis elegans. J Neurosci 19:159–167
Li C (2005) The ever-expanding neuropeptide gene families in the nematode Caenorhabditis elegans. Parasitol 131:S109−S127
Li S, Dent R (2003) Regulation of intramuscular electrical coupling by the Caenorhabditis elegans innexin INX-6. Cell 14:2630–2644
Li HY, Avery L, Denk W, Hess GP (1997) Identification of chemical synapses in the pharynx of Caenorhabditis elegans. Proc Natl Acad Sci USA 94:5912–5916
Livingston D (1991) Studies on the unc-31 gene of Caenorhabditis elegans. PhD thesis. University of Cambridge, Cambridge
Long de J, Meng Y, Dent J, Hekimi S (2004) Thiamine pyrophosphate biosynthesis and transport in the nematode Caenorhabditis elegans. Genetics 168:845–854
Maryon EB, Coronado R, Anderson P (1996) unc-68 encodes a ryanodine receptor involved in regulating C. elegans body-wall muscle-contraction. J Cell Biol 134:885–893
Maryon EB, Saari B, Anderson P (1998) Muscle-specific functions of ryanodine receptor channels in Caenorhabditis elegans. J Cell Sci 111:2885–2895
Maynard DM (1955) Activity of a crustacean ganglion. II. Pattern and interaction in burst formation. Biol Bull 109:420–436
McKay JP, Raizen DM, Gottschalk A, Schafer WR, Avery L (2004) eat-2 and eat-18 are required for nicotinic transmission in the Caenorhabditis elegans pharynx. Genetics 166:161–169
Nathoo AN, Moeller RA, Westlund BA, Hart AC (2001) Identification of neuropeptide-like protein gene families in Caenorhabditis elegans and other species. Proc Natl Acad Sci USA 98:14000–14005
Nguyen M, Alfonso A, Johnson CD, Rand JB (1995) Caenorhabditis elegans mutants resistant to inhibitors of acetylcholinesterase. Genetics 140:527–535
Niacaris T, Avery L (2003) Serotonin regulates repolarization of the C. elegans pharyngeal muscle. J Exp Biol 206: 223–231
Nonet ML, Grundahl K, Meyer BI, Rand JB (1993) Synaptic function is impaired but not eliminated in C. elegans mutants lacking synaptotagmin. Cell 73:1291–1305
Nonet ML, Holgado AM, Brewer F, Serpe CJ, Norbeck BA, Holleran J, Wei L, Hartweig EA, Jorgensen EM, Alfonso A (1999) UNC-11, a Caenorhabditis elegans AP 180 homologue, regulates the size and protein composition of synaptic vesicles. Mol Biol Cell 10:2343–2360
Olde B, McCombie WR (1997) Molecular cloning and functional expression of a serotonin receptor from Caenorhabditis elegans. J Mol Neurosci 8:53–62
Papaioannou S, Marsden D, Franks CJ, Walker RJ, Holden-Dye L (2005) Role of a FMRFaimde-like family of neuropeptides in the pharyngeal nervous system of Caenorhabditis elegans. J Neurobiol 65:304–319
Pelham HRB (1993) Neurotransmission and secretion. Nature 364:582
Pemberton DJ (2001) Studies in to the functinoal properties of the pharyngeal muscle of Caenorhaditis elegans. PhD Thesis, University of Southampton, Southampton
Pemberton D, Franks C, Walker R, Holden-Dye L (2001) Characterisation of glutamate-gated chloride channels in the pharynx of wild-type and mutant Caenorhabditis elegans delineates the role of the subunit GluCl-α2 in the function of the native receptor. Mol Pharmacol 59:1037–1043
Phelan P (2005) Innexins: members of an evolutionarily conserved family of gap-junction proteins. Biochim Biophys Acta 1711:225–245
Raizen DM, Avery L (1994) Electrical-activity and behavior in the pharynx of Caenorhabditis elegans. Neuron 12:483–495
Raizen DM, Lee RN, Avery L (1995) Interacting genes required for pharyngeal excitation by motor-neuron mc in Caenorhabditis elegans. Genetics 141:1365–1382
Rand JB, Duerr JS, Frisby DL (2000) Neurogenetics of vesicular transporters in C. elegans. FASEB J 14:2414–2422
Ranganathan R, Cannon SC, Horvitz HR (2000) MOD-1 is a serotonin-gated chloride channel that modulates locomotory behaviour in C. elegans. Nature 408:470–475
Ranganathan R, Sawin ER, Trent C, Horvitz HR (2001) Mutations in the Caenorhabditis elegans serotonin reuptake transporter MOD-5 reveal serotonin-dependent and -independent activities of fluoxetine. J Neurosci 21:5871–5874
Robatzek M, Niacaris T, Steger K, Avery L, Thomas JH (2001) eat-11 encodes GPB-2, a G-beta(5) ortholog that interacts with G(o)alpha and G(q)alpha to regulate C. elegans behaviour. Curr Biol 11:288–293
Rex E, Komuniecki RW (2002) Characterization of a tyramine receptor in Caenorhabditis elegans. J Neurochem 82:1352–1359
Rogers CM, Franks CJ, Walker RJ, Burke JF, Holden-Dye L (2001) Regulation of the pharynx of Caenorhabditis elegans by 5-HT, octopamine and FMRFamide-like neuropeptides. J Neurobiol 49:235–244
SakubeY, Ando H, Kagawa H (1993) Cloning and mapping of a ryanodine receptor homologue gene of Caenorhabditis elegans. Ann NY Acad Sci 707:540–545
Sattelle DB, Buckingham SD (2006) Invertebrate studies and their ongoing contributions to neuroscience. Invert Neurosci 6:1–3
Sawin ER, Ranganathan R, Horvitz HR (2000) C. elegans locomotion rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26:619–631
Schafer WR, Kenyon CJ (1995) A calcium channel homologue required for adaptation to dopamine and serotonin in Caenorhabditis elegans. Nature 375:73–78
Schafer WR, Sanchez BM, Kenyon CJ (1996) Genes affecting sensitivity to serotonin in Caenorhabditis elegans. Genetics 143:1219–1230
Selverston AI, Russell DF, Miller JP, King DG (1976) The stomatogastric nervous system: structure and function of a small neural network. Prog Neurobiol 7:215–290
Shibata Y, Fujii T, Dent JA, Fujisawa H, Takagi S (2000) EAT-20, a novel transmembrane protein with EGF motifs, is required for efficient feeding in Caenorhabditis elegans. Genetics 154:635–646
Shimozono S, Fukano T, Kimura KD, Mori I, Kirino Y, Miyawaki A (2004) Slow calcium dynamics in pharyngeal muscle in Caenorhabditis elegans during fast pumping. EMBO Rep 5:521–526
Shtonda B, Avery L (2005) CCA-1, EGL-19 and EXP-2 currents shape action potentials in the Caenorhabditis elegans pharynx. J Exp Biol 208:2177–2190
Starich T, Lee R, Panzarella C, Avery L, Shaw J (1996) eat-5 and unc-7 represent a multi-gene family in Caenorhabditis elegans involved in cell-cell coupling. J Cell Biol 134:537–548
Starich T, Sheehan M, Jadrich J, Shaw J (2001) Innexins in C. elegans. Cell Comm Adhes Res 8:311–314
Starich T, Miller A, Nguyen R, Hall D, Shaw J (2003) The Caenorhabditis elegans innexin INX-3 is localized to gap-junctions and is essential for embryonic development. Dev Biol 256:403–417
Steger KA, Avery L (2004) The GAR-3 muscarinic receptor cooperates with calcium signals to regulate muscle contraction in the Caenorhabditis elegans pharynx. Genetics 167:643
Steger KA, Shtonda BB, Thacker C, Snutch TP, Avery L (2005) The C. elegans T-type calcium channel CCA-1 boosts neuromuscular transmission. J Exp Biol 208:2191–2203
Sze JY, Victor M, Loer C, Shi Y, Ruvkun G (2000) Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature 403:560–564
Towers PR, Edwards B, Richmond JE, Sattelle DB (2005) The Caenorhabditis elegans lev-8 gene encodes a novel type of nicotinic acetylcholine receptor alpha subunit. J Neurochem 93:1–9
Tsalik EL, Niacaris T, Wenick ASPK, Avery L, Hobert O (2003) LIM homeobox gene-dependent expression of biogenic amine receptors in restricted regions of the C. elegans nervous system. Dev Biol 263:81–102
Vinogradova I, Cook A, Holden-Dye L (2006) The ionic dependence of voltage-activated inward currents in the pharyngeal muscle of Caenorhabditis elegans. Inv Neurosci (E pub ahead of print)
Walker DS, Gower NJ, Ly S, Bradley GL, Baylis HA (2002) Regulated disruption of inositol 1,4,5-triphosphate signalling in Caenorhabditis elegans reveals new functions in feeding and embryogenesis. Mol Biol Cell 13:1329–1337
Ward S, Thomson N, White JG, Brenner S (1975) Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J Comp Neurol 160:313–338
White J, Southgate E, Thomson JN, Brenner S (1986) The structure of the nervous system of the the nematode Caenorhabditis elegans. Philos Trans Roy Soc Lond B Biol Sci 314:1–340
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Franks, C.J., Holden-Dye, L., Bull, K. et al. Anatomy, physiology and pharmacology of Caenorhabditis elegans pharynx: a model to define gene function in a simple neural system. Invert Neurosci 6, 105–122 (2006). https://doi.org/10.1007/s10158-006-0023-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10158-006-0023-1