Abstract
Background
Anti-neutrophil cytoplasmic antibody-associated glomerulonephritis (ANCA-GN) is a critical kidney disease that sometimes results in an unfavorable renal outcome. Cellular crescent formation is a hallmark of ANCA-GN and is associated with renal prognosis, response to treatment, and it was reportedly associated with podocyte detachment. Because there is a need to explore non-invasive biomarkers for the evaluation of ANCA-GN activity, we tested whether urinary podocyte mRNA might be a potent non-invasive biomarker.
Methods
We measured two different types of urinary podocyte mRNA, including podocin mRNA in relation to urine creatinine concentration (U-PodCR) and urinary podocin mRNA in relation to nephrin mRNA (U-PNR), which were reportedly associated with the activity of various glomerular diseases.
Results
In ANCA-GN patients (n = 19), we discovered that U-PodCR was positively correlated with the percent of crescent formation until 50% crescent was reached because of podocyte depletion; U-PNR was correlated with the percent of crescent formation in all patients. Furthermore, patients with high levels of urinary podocyte mRNA exhibited a favorable renal outcome compared with the outcomes of patients with low levels of urinary podocyte mRNA. The levels of urinary podocyte mRNA were correlated with the rate of improvement in estimated glomerular filtration rate.
Conclusions
U-PodCR, U-PNR or a combination of these parameters might serve as a non-invasive potential biomarker in patients with ANCA-GN to predict the percent of crescent formation and renal prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anti-neutrophil cytoplasmic antibody-associated glomerulonephritis (ANCA-GN) is a critical disease that typically involves microscopic hematuria, moderate proteinuria, and a rapidly declining glomerular filtrate rate over days or weeks [1]. Because untreated ANCA-GN results in unfavorable renal and life outcomes [1, 2], prompt diagnosis and treatment based on an evaluation of disease activity are important. Histological analysis from renal biopsy is a well-established method [3] that provides prognostic data, including glomerular extracapillary cell proliferation, known as crescent formation. Notably, crescent formation is a hallmark of ANCA-GN [1, 4, 5], and its percentage is associated with prognosis [3] and response to treatment [6]. However, renal biopsy is invasive and involves a risk of complications [7]. Therefore, non-invasive biomarkers of ANCA-GN are needed.
Podocytes, a visceral epithelial cell type covering the surface of glomerular capillaries, are critical for the maintenance of healthy kidney function [8]; however, these cells exhibit a limited ability to proliferate [9,10,11]. Therefore, severe podocyte depletion is commonly accompanied by manifestations of various glomerular diseases [12]. Because podocytes are detached by persistent or severe injury [10] and then passed into urine [13, 14], the amount of podocytes in urine is a potent biomarker that directly reflects the progression of podocyte depletion [11, 12, 14, 15]. In ANCA-GN, podocyte depletion was reportedly associated with unfavorable renal prognosis [16]. It is reasonable that podocyte detachment might be related to crescent formation because crescents are derived from necrotizing inflammation of glomerular capillaries [17] where podocytes are located. Indeed, Fujita et al., reported that increased WT1-positive cells in urine, derived from detached podocytes or activated parietal cells, predicted crescent formation in humans [18].
There are various methods to evaluate podocytes in urine; we previously reported the usefulness of urinary podocyte mRNA analysis [11]. Urinary podocyte counting by podocalyxin-immunostaining is a well-established methodology to estimate glomerular podocyte detachment [15]; however, the methodology is not accurate because both podocalyxin-positive cells and podocalyxin-positive granules are detectable in urinary sediment, and which cells are counted as true podocytes varies between individual investigators. Real-time PCR is relatively easy to perform [11] and provides reproducible measurements, avoiding investigator’s bias. Notably, the rate of podocin mRNA excretion in relation to urine creatinine concentration (U-PodCR) and aquaporin2 mRNA as a tubular marker were shown to reflect glomerular disease activity in various rodent models [14, 19,20,21], and were positively correlated with glomerular podocyte loss per day [19]; as well as human IgA nephropathy [22]. Relative expression levels of podocin and nephrin mRNA (urine podocin to nephrin mRNA ratio; U-PNR), modified by the relative downregulation of nephrin versus podocin in pathological conditions, might also be a biomarker of glomerular disease [14, 23].
Here, we hypothesized that the elevation of urinary podocyte mRNA is a non-invasive biomarker of ANCA-GN, especially in terms of predicting crescent formation. Thus, we conducted the current study to compare urine mRNAs, including U-PodCR and U-PNR, with histologic findings in ANCA-GN patients.
Materials and methods
Histological analysis
On the basis of the statement by the European Vasculitis Study Group [3], the following histological definitions of ANCA-associated nephritis were used. Normal glomerulus had no vasculitic lesions (including synechia, segmental sclerosis, and extensive ischemic changes) or global sclerosis; a cellular crescent was composed of purely or partial cellular components; and global sclerosis indicated that > 80% of the glomerulus showed sclerosis. Using periodic acid–Schiff-stained sections stored as virtual slides, total numbers of normal, sclerotic, and cellular crescent glomeruli were counted for each patient and the percent of normal glomeruli (%normal), percent of global sclerosis (%sclerosis), and percent of glomeruli with cellular crescent (%crescent) were calculated. Histology slides were blinded and then evaluated by three observers and their median values were calculated.
Immunostaining
Kidney specimens were Dubosq-Brazil liquid-fixed and paraffin-embedded prior to sectioning, and 1.5-µm-thick unstained sections were used. After deparaffinization, each section was treated with the Retrieve-All Antigen Unmasking System 1 (BioLegend, San Diego, CA, USA) at 90 °C for 2 h, and then blocked by using horse serum. An anti-human Wilums tumor protein (WT-1) monoclonal rabbit antibody (ab89901; Abcam, Cambridge, UK) was applied as a primary antibody and incubated at 4 °C overnight. An immunoperoxidase reaction was performed using biotinylated anti-rabbit IgG at room temperature for 1 h, followed by avidin and biotinylated enzyme complex (VECTASTAIN ABC kit; Vector Laboratories, Burlingame, CA, USA) at room temperature for 1 h, and then by treatment with 3, 3′-diaminobenzidine with hydrogen peroxide (SIGMAFAST; Sigma-Aldrich, St. Louis, MO, USA). Post-staining was performed with hematoxylin. Brownish cells on glomerular capillaries were regarded as podocyte nuclei. Mean podocyte nuclear caliper diameter, podocyte nuclear count per glomerulus, and glomerular area were measured using WinROOF imaging software (Mitani Corporation, Tokyo, Japan). These data were then used to estimate the podocyte number and density with Venkatareddy’s methodology, as previously described [22, 24]. In addition to ANCA-GN patients, histological specimens from patients with minor change nephrotic syndrome (MCNS) were used as controls for podocyte number and density.
RNA extraction and real-time PCR to evaluate urine mRNA
Urine samples were collected in the morning of the renal biopsy and centrifuged at 4 °C for 15 min at 800×g. Urine sediment was suspended in 1.5 mL of diethyl pyrocarbonate-treated phosphate-buffered saline, then centrifuged at 4 °C for 5 min at 13,200×g. The washed sediment was resuspended in buffer RLT with beta mercaptoethanol (RNeasy Mini kit, Qiagen, Germantown, MD, USA) and then frozen at − 80 °C until RNA extraction. Total urine RNA was purified by using an RNeasy Mini Kit. Complementary DNA (cDNA) was transcribed using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA). Quantitation of podocin (NPHS2) and nephrin (NPHS1) mRNA abundance was performed with a 7300HT fast real-time PCR system (Applied Biosystems) using TaqMan Fast universal PCR master mix at a final volume of 10 µL per reaction. The TaqMan probes (Applied Biosystems) used were specific to human NPHS1 (Hs00190446_m1) and human NPHS2 (Hs00922492_m1). All data were generated using cDNA samples, measured in duplicate. cDNA standards were used for each assay as previously described [22, 24]. Subsequently, we calculated U-PodCR, nephrin mRNA in relation to urine creatinine concentration (U-NepCR), and U-PNR as previously described [22, 23]. In addition to the ANCA-GN patients, urine samples were collected from healthy volunteers who had no known kidney disease, for use as controls in analyses of urinary podocyte mRNA.
Statistical analysis
Statistical analyses were performed using GraphPad PRISM software, version 7 (GraphPad Software Inc., La Jolla, CA, USA), and IBM SPSS Statistics, version 22 (IBM Corp., Armonk, NY, USA). All clinical, pathology and urinary data are shown as the median and interquartile range. Comparisons of two groups were performed with the Mann–Whitney U-test; comparisons of three or more groups were performed with the Kruskal–Wallis test and Dunn’s test; correlations between parameters were assessed with Pearson’s test; comparisons of categorical data in two groups were performed with Pearson’s Chi-square test; survival analysis was performed with the Mantel–Cox log-rank test; and the reproducibility of histology among three observers was assessed using Intraclass Correlation Coefficients. p values < 0.05 were considered statistically significant.
Results
Characteristics of patients and controls
From 2010 to 2016, patients at the University of Miyazaki Hospital who were diagnosed with ANCA-GN based on immunological and histological evidence were enrolled in this study. Of the original 22 patients, two were excluded because of inadequate histological data, and one was excluded due to disagreement with participation in the study. Thus, a total of 19 ANCA-GN patients were analyzed. The clinical characteristics of the patients are shown in Table 1, and the histological profiles evaluated by three observers are shown in Table 2. A total of 28 urine samples were collected from healthy volunteers and 8 histological specimens were collected from patients with MCNS. Follow-up data were obtained from 19 patients during the 6-month follow-up period after renal biopsy.
WT-1 immunostaining shows podocyte depletion associated with sclerotic and crescentic lesions in patients with ANCA-GN
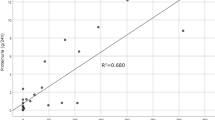
To evaluate the podocyte number and density in patients with ANCA-GN and make a comparison with patients with MCNS, WT-1 immunostaining was performed. Although some WT-1-positive cells were present in crescent cells and on Bowman’s capsule, we regarded WT-1-positive cells on glomerular capillaries as podocyte nuclei. In patients with MCNS (Fig. 1a), as well as in patients with ANCA-GN, podocyte nuclei were globally observed among normal glomeruli (Fig. 1b). They were partially observed in crescentic glomeruli separate from crescent cells (Fig. 1c) and were generally absent from sclerotic glomeruli (Fig. 1d). The median podocyte number in patients with ANCA-GN was approximately 2.6 times lower (p < 0.01) than that in patients with MCNS, whereas the median podocyte density in patients with ANCA-GN was approximately 2.3 times lower (p < 0.01) than that in patients with MCNS (Fig. 2a, b). In a comparison of histologic findings and WT-1 immunostaining results, the percent of normal glomeruli was positively correlated with podocyte number (Fig. 2c; R2 = 0.60, p < 0.01) and density (Fig. 2d; R2 = 0.48, p < 0.01). Moreover, in a comparison of lesions between patients with ANCA-GN and patients with MCNS, sclerotic lesions showed the lowest podocyte number (Fig. 2e) and density (Fig. 2f) in patients with ANCA-GN, which were significantly lower than those in patients with MCNS (p < 0.01 and p < 0.01, respectively), followed by patients with crescentic lesions (p < 0.01 and p < 0.05, respectively). These findings suggest that patients with ANCA-GN showed various degrees of podocyte depletion, which was characterized mainly by sclerotic lesions, followed by crescentic lesions.
Typical glomeruli in ANCA-associated glomerulonephritis (ANCA-GN). Left images in a–d show periodic acid-Schiff staining and the right images show anti-Wilums tumor protein (WT1) immunoperoxidase staining. Brownish cells on capillaries are considered to be podocytes. a Minor change nephrotic syndrome (control). b Normal glomeruli in ANCA-GN. c Crescentic glomeruli in ANCA-GN. d Sclerotic glomeruli in ANCA-GN. Bar = 50 µm
Podocyte numbers of crescent glomeruli are partly reduced. Results of anti-Wilums tumor protein (WT1) immunoperoxidase staining. a, c and e show podocyte number and b, d and f show podocyte density. a, b Comparison of podocyte number and density between ANCA-associated glomerulonephritis (ANCA-GN) (n = 20) and minor change nephrotic syndrome (MCNS) (n = 8), assessed by using the Mann–Whitney U-test. c, d Correlations between podocyte number and percent of normal glomeruli, and between podocyte density and percent of normal glomeruli, assessed by using Pearson’s test. e, f Comparison of podocyte number and density between MCNS and apparently normal, crescentic and sclerotic glomeruli of ANCA-GN, assessed by using the Kruskal–Wallis test and Dunn’s test
Urinary podocyte mRNA analysis shows that U-PodCR and U-PNR predict the percent of crescent formation in patients with ANCA-GN
To clarify whether urinary podocyte mRNA could be used to diagnose ANCA-GN and to predict %crescent, we performed urinary podocyte mRNA analysis using quantitative PCR. Compared with healthy controls, patients with ANCA-GN had a median U-PodCR 133 times higher (Fig. 3a, p < 0.01), median U-NepCR 28.3 times higher (Fig. 3b, p < 0.01), and median U-PNR 3.7 times higher (Fig. 3c, p < 0.01). Subsequently, we compared U-PodCR, U-NepCR, and U-PNR with %crescent, stratified by quartiles, in patients with ANCA-GN. U-PodCR and U-NepCR showed prominent increases along with %crescent until 50% crescent was reached (Fig. 3d, e) and U-PNR was increased with %crescent in all patients (Fig. 3f). Furthermore, U-PodCR and U-NepCR were positively correlated with %crescent, except in patients with > 50% crescent (Fig. 3g, h). U-PNR was increased with %crescent in all patients and was significantly correlated with %crescent (Fig. 3i; R2 = 0.39, p < 0.01). However, there was no significant correlation between %normal and U-PNR, U-PodCR or U-NepCR (R2 = 0.05, p = 0.37; R2 = 0.03, p = 0.50; and R2 = 0.07, p = 0.26, respectively), as well as between %sclerosis and U-PNR, U-PodCR or U-NepCR (R2 = 0.01, p = 0.81; R2 = 0.06, p = 0.32; and R2 = 0.10, p = 0.19, respectively). Additionally, in the group with a low level of U-PodCR (below the median value of U-PodCR), patients with high U-PNR showed higher %crescent than patients with low U-PNR (Fig. 3j). These findings suggest that U-PodCR, U-PNR, or a combination of these parameters might be used as a non-invasive biomarker of ANCA-GN, especially in terms of predicting %crescent.
U-PodCR and U-NepCR increase with increased crescent formation except in patients with severe crescent formation, whereas U-PNR is increased in all patients. Results of urine mRNA analysis. a–b Comparison of U-PodCR, U-NepCR, and U-PNR between ANCA-GN (n = 19) and healthy controls (n = 28), assessed by using the Mann–Whitney U-test. d–f Comparison of U-PodCR, U-NepCR, and U-PNR among quartiles of %crescent, assessed by using the Kruskal–Wallis test and Dunn’s test. g–i Correlations between U-PodCR and %crescent, between U-NepCR and %crescent, and between U-PNR and %crescent, assessed by using Pearson’s test. Correlations between U-PodCR (up to 50%) and %crescent, and between U-NepCR (up to 50%) and %crescent, are shown next to the original graphs. j Comparison of %crescent between high and low U-PNR in the low U-PodCR group, divided by the median value and assessed by using the Mann–Whitney U-test
High levels of U-PodCR and U-PNR are associated with favorable short-term renal prognosis
To evaluate the association between renal prognosis and urinary podocyte mRNA analysis, we performed 6-month survival analysis in which the endpoint was > 30% reduction in estimated glomerular filtration rate (eGFR) or dialysis induction. In this analysis, 19 patients with ANCA-GN were divided into groups with high and low levels of U-PodCR, as well as groups with high and low levels of U-PNR, based on the respective median values. The clinical and histological characteristics of the U-PodCR groups are shown in Table 3, and those of U-PNR groups are shown in Table 4. Kaplan–Meier curve analyses stratified by U-PodCR and U-PNR are shown in Fig. 4a, b, respectively. Both high-level groups showed favorable renal prognosis by 6 months (both p < 0.05). In addition, U-PodCR was positively correlated with the rate of change in eGFR over 6 months (Fig. 4c; R2 = 0.29, p < 0.05), and U-PNR was positively correlated with the rate of change in eGFR over 1 month (Fig. 4e; R2 = 0.35, p < 0.01) and 6 months (Fig. 4f; R2 = 0.44, p < 0.01). These findings suggested that U-PodCR and U-PNR predicted the response to treatment reflecting %crescent, and that high levels of U-PNR and U-PodCR indicated favorable short-term renal prognosis. In addition, the group with high levels of U-NepCR had a significantly favorable renal prognosis compared with the group with low levels of U-NepCR. However, the percent of eGFR change during one month or six months did not correlate with U-NepCR (p = 0.28 and p = 0.40, respectively).
High U-PodCR and U-PNR predict good short-term renal prognosis. a, d Kaplan–Meier curves stratified by U-PodCR and U-PNR, in which the endpoint was > 30% reduction of estimated GFR or dialysis induction, assessed by the Mantel–Cox log-rank test. Censored data are marked in each curve, and asterisks indicate p < 0.05. b, e Correlation between U-PodCR and rate of change in eGFR rate during the first month among 18 surviving patients at that time point, assessed by Pearson’s test. c, f Correlation between U-PodCR and rate of change in eGFR over 6 months in 15 surviving patients at that time point, assessed by Pearson’s test
Discussion
Histological analysis is the gold standard for establishing a diagnosis and evaluating the activity of ANCA-GN [3, 6]. Because the procedure is invasive [7] it should be avoided if the patient has contraindications such as uncorrectable bleeding diathesis or severe systemic illness. Inflammatory markers (e.g., C-reactive protein and erythrocyte sedimentation rate) are widespread, but are nonspecific and cannot differentiate disease activity from other sources of inflammation, such as infection. ANCA against myeloperoxidase and proteinase 3 are useful for diagnosis but are considered less useful for the assessment of disease activity [25]. Thus, there is a need to explore novel biomarkers in patients with ANCA-GN to evaluate disease activity [25].
To the best of our knowledge, the current study is the first report of urinary podocyte mRNA analysis of patients with ANCA-GN. It showed that urinary podocyte mRNAs were positively correlated with %crescent. Furthermore, patients with a high level of urinary podocyte mRNA exhibited a favorable renal outcome at 6 months compared with patients with a low level of urinary podocyte mRNA. Thus, urinary podocyte mRNAs were positively correlated with the rate of improvement in eGFR.
The current study revealed a strong association between podocyte stress or detachment and crescent formation. Similarly, we previously reported that %crescent was positively correlated with U-PodCR in IgA nephropathy [22]. Hara et al., also reported a correlation between urinary podocyte number and %crescent, which paralleled the increasing numbers of glomerular and urinary leukocytes in patients with IgA nephropathy and/or IgA vasculitis [26, 27]. Therefore, it is likely that podocyte detachment is closely related to crescent formation because most crescents are presumed to derive from the necrotizing inflammation of capillary walls where podocytes are located [17]. Furthermore, Le Hir et al. reported that endocapillary inflammation caused podocytes to bridge between the parietal and glomerular basement membrane, leading to the proliferation of the parietal epithelium, which resulted in crescent formation in experimental crescentic glomerulonephritis [28]. In that report, podocytes were proposed to directly contribute to crescent formation and the findings supported the correlation of podocyte detachment with %crescent, as observed in the current study.
Importantly, U-PNR was significantly correlated with %crescent in all patients, whereas U-PodCR was prominently elevated along with %crescent until %crescent reached 45%. Urinary podocyte mRNAs are biomarkers of dynamic podocyte injury and are elevated during early injury and continue to be elevated while the injury remains. In addition, both U-PodCR and U-NepCR tended to decrease due to severe podocyte depletion, as observed in patients with > 50% crescent in the current study (Fig. 3d–f). In contrast, because U-PNR represents podocyte stress related to the relative downregulation of nephrin versus podocin after podocyte insults [14, 23], high levels of U-PNR are maintained, which might be a useful biomarker to evaluate disease activity in ANCA-GN, even during the stage of podocyte depletion. Indeed, as shown in Fig. 3j, U-PNR differentiated mild crescentic cases from severe crescentic cases in the group with a low level of U-PodCR. These findings suggest that a combination of U-PodCR and U-PNR may be useful for the evaluation of disease activity in patients with ANCA-GN.
Furthermore, the current study showed that urinary podocyte mRNA analysis, especially U-PodCR and/or U-PNR was also useful for estimating responses to treatment and renal prognosis in at least the acute phase, as well as for the evaluation of disease activity. Similarly, Hauer et al. reported that segmental and cellular crescent formation was positively correlated with the rate of improvement in GFR, suggesting that crescent formation constitutes a reversible lesion [6]. Because the elevation of urinary podocyte mRNAs might predict %crescent, it is likely that urinary podocyte mRNAs correlate with the rate of improvement in GFR, and might predict renal prognosis. Taken together, these findings indicate that urinary podocyte mRNAs are potential biomarkers for ANCA-GN, and which can be used to predict disease activity and response to treatment.
Although the current study reported novel findings, it also had some limitations. The small number of participants made it difficult to stratify the participants based on treatment type, comorbid conditions, and gender. Thus, the current study did not estimate the influence of confounding factors in the predictive ability of urinary podocyte mRNAs and therefore evidence in the current study is limited and cannot be used to determine the cut-off point to distinguish a patient’s prognosis. In the group with high levels of urinary podocyte mRNAs, more patients were treated with steroid pulse and immunosuppressant therapy, compared with the group with low levels of urinary podocyte mRNAs. This difference in treatment may have influenced the renal prognosis and response to treatment.
Conclusions
In conclusion, the current study is the first report of urinary podocyte mRNA analysis in patients with ANCA-GN. The findings showed that U-PodCR, U-PNR, or a combination of these parameters were non-invasive potential biomarkers in patients with ANCA-GN, especially in terms of predicting %crescent and renal prognosis. However, further studies are needed to overcome the above limitations and to validate the findings observed in the current study.
References
Group KDIGOKGW. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl. 2012;2:139–274.
Berden A, Goceroglu A, Jayne D, Luqmani R, Rasmussen N, Bruijn JA, et al. Diagnosis and management of ANCA associated vasculitis. BMJ. 2012;344:e26.
Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, et al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol. 2010;21(10):1628–36.
Jennette JC, Wilkman AS, Falk RJ. Anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and vasculitis. Am J Pathol. 1989;135(5):921–30.
Jennette JC, Nachman PH. ANCA glomerulonephritis and vasculitis. Clin J Am Soc Nephrol CJASN. 2017;12(10):1680–91.
Hauer HA, Bajema IM, Van Houwelingen HC, Ferrario F, Noel LH, Waldherr R, et al. Determinants of outcome in ANCA-associated glomerulonephritis: a prospective clinico-histopathological analysis of 96 patients. Kidney Int. 2002;62(5):1732–42.
Madaio MP. Renal biopsy. Kidney Int. 1990;38(3):529–43.
Grahammer F, Schell C, Huber TB. The podocyte slit diaphragm–from a thin grey line to a complex signalling hub. Nat Rev Nephrol. 2013;9(10):587–98.
Lasagni L, Lazzeri E, Shankland SJ, Anders HJ, Romagnani P. Podocyte mitosis—a catastrophe. Curr Mol Med. 2013;13(1):13–23.
Nagata M. Podocyte injury and its consequences. Kidney Int. 2016;89(6):1221–300.
Kikuchi M, Wickman L, Hodgin JB, Wiggins RC. Podometrics as a potential clinical tool for glomerular disease management. Semin Nephrol. 2015;35(3):245–55.
Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71(12):1205–14.
Hara T, Matsuo M, Tasaki R, Miyazaki S, Bamba M. Detection of immunodeficiencies and persistent infections by urinary neopterin measurement. Acta Paediatr Jpn. 1995;37(5):575–8.
Sato Y, Wharram BL, Lee SK, Wickman L, Goyal M, Venkatareddy M, et al. Urine podocyte mRNAs mark progression of renal disease. J Am Soc Nephrol. 2009;20(5):1041–52.
Hara M, Yamamoto T, Yanagihara T, Takada T, Itoh M, Adachi Y, et al. Urinary excretion of podocalyxin indicates glomerular epithelial cell injuries in glomerulonephritis. Nephron. 1995;69(4):397–403.
Zou R, Wang SX, Liu G, Yu F, Chen M, Zhao MH. Podocyte detachment is associated with renal prognosis in ANCA-associated glomerulonephritis: a retrospective cohort study. Medicine (Baltimore). 2016;95(15):e3294.
Hauer HA, Bajema IM, de Heer E, Hermans J, Hagen EC, Bruijn JA. Distribution of renal lesions in idiopathic systemic vasculitis: a three-dimensional analysis of 87 glomeruli. Am J Kidney Dis. 2000;36(2):257–65.
Fujita T, Sofue T, Moritoki M, Nishijima Y, Tokuhara Y, Wakisaka H, et al. Urinary WT1-positive cells as a non-invasive biomarker of crescent formation. Cytopathology. 2017;28(6):524–30.
Fukuda A, Minakawa A, Sato Y, Iwakiri T, Iwatsubo S, Komatsu H, et al. Urinary podocyte and TGF-beta1 mRNA as markers for disease activity and progression in anti-glomerular basement membrane nephritis. Nephrol Dial Transplant. 2017;32(11):1818–30.
Fukuda A, Chowdhury MA, Venkatareddy MP, Wang SQ, Nishizono R, Suzuki T, et al. Growth-dependent podocyte failure causes glomerulosclerosis. J Am Soc Nephrol. 2012;23(8):1351–63.
Wiggins JE, Goyal M, Sanden SK, Wharram BL, Shedden KA, Misek DE, et al. Podocyte hypertrophy, "adaptation," and "decompensation" associated with glomerular enlargement and glomerulosclerosis in the aging rat: prevention by calorie restriction. J Am Soc Nephrol. 2005;16(10):2953–66.
Fukuda A, Sato Y, Iwakiri T, Komatsu H, Kikuchi M, Kitamura K, et al. Urine podocyte mRNAs mark disease activity in IgA nephropathy. Nephrol Dial Transplant. 2015;30(7):1140–50.
Fukuda A, Wickman LT, Venkatareddy MP, Wang SQ, Chowdhury MA, Wiggins JE, et al. Urine podocin:nephrin mRNA ratio (PNR) as a podocyte stress biomarker. Nephrol Dial Transplant. 2012;27(11):4079–87.
Venkatareddy M, Wang S, Yang Y, Patel S, Wickman L, Nishizono R, et al. Estimating podocyte number and density using a single histologic section. J Am Soc Nephrol. 2014;25(5):1118–29.
Draibe JB, Fulladosa X, Cruzado JM, Torras J, Salama AD. Current and novel biomarkers in anti-neutrophil cytoplasm-associated vasculitis. Clin Kidney J. 2016;9(4):547–51.
Hara M. Urinary podocytes in childhood IgA nephropathy. Nephrology (Carlton). 2001;6:179–84.
Hara M, Yanagihara T, Kihara I. Cumulative excretion of urinary podocytes reflects disease progression in IgA nephropathy and Schonlein–Henoch purpura nephritis. Clin J Am Soc Nephrol CJASN. 2007;2(2):231–8.
Le Hir M, Keller C, Eschmann V, Hahnel B, Hosser H, Kriz W. Podocyte bridges between the tuft and Bowman's capsule: an early event in experimental crescentic glomerulonephritis. J Am Soc Nephrol. 2001;12(10):2060–71.
Acknowledgements
We acknowledge the staff of the Pathology Division, University of Miyazaki Hospital for their help in pathological diagnosis and cutting paraffin-embedded sections: Ms. Ofuku for her technical help; Ms. Tahara for her secretarial assistance; and Ryan Chastain-Gross, Ph.D., from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
AM and AF planned the study, conducted the study, and drafted the manuscript. AM, YS and YS evaluated the histology. MK and YS revised the manuscript. KK and SF critically reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee at which the studies were conducted (Ethics Committee of the University of Miyazaki approval numbers #950 and #1073) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study in the form of a document or opt-out on the web-site.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Minakawa, A., Fukuda, A., Kikuchi, M. et al. Urinary podocyte mRNA is a potent biomarker of anti-neutrophil cytoplasmic antibody-associated glomerulonephritis. Clin Exp Nephrol 24, 242–252 (2020). https://doi.org/10.1007/s10157-019-01823-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-019-01823-5