Abstract
Background
Hyperkalemia is prevalent in end-stage renal disease patients, being involved in life-threatening arrhythmias. Although polystyrene sulfonate (PS) is commonly used for the treatment of hyperkalemia, direct comparison of effects between calcium and sodium PS (CPS and SPS) on mineral and bone metabolism has not yet been studied.
Methods
In a randomized and crossover design, 20 pre-dialysis patients with hyperkalemia (>5 mmol/l) received either oral CPS or SPS therapy for 4 weeks.
Results
After 4-week treatments, there was no significant difference of changes in serum potassium (K) from the baseline (ΔK) between the two groups. However, SPS significantly decreased serum calcium (Ca) and magnesium (Mg) and increased intact parathyroid hormone (iPTH) values, whereas CPS reduced iPTH. ΔiPTH was inversely correlated with ΔCa and ΔMg (r = −0.53 and r = −0.50, respectively). Furthermore, sodium (Na) and atrial natriuretic peptide (ANP) levels were significantly elevated in patients with SPS, but not with CPS, whereas ΔNa and ΔANP were significantly correlated with each other in all the patients. We also found that ΔNa and Δ(Na to chloride ratio) were positively correlated with ΔHCO3 −. In artificial colon fluid, CPS increased Ca and decreased Na. Furthermore, SPS greatly reduced K, Mg, and NH3.
Conclusion
Compared with SPS, CPS may be safer for the treatment of hyperkalemia in pre-dialysis patients, because it did not induce hyperparathyroidism or volume overload.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperkalemia is one of the most common complications in end-stage renal disease patients, and could cause serious electronic abnormality in the heart such as cardiac arrhythmias, thereby being involved in heart failure and sudden death in patients with advanced chronic kidney disease (CKD) [1]. Indeed, it has been reported that higher serum potassium (K) levels are associated with the increased risk of mortality in both CKD and non-CKD patients [2, 3]. Although there is a growing body of evidence that renin–angiotensin system (RAS) inhibitors have protected against the progression of renal disease and its associated organ damage in patients with advanced CKD [4, 5], the therapeutic option may be limited due to considerable side effects, such as hyperkalemia [6, 7]. Therefore, strict management of hyperkalemia is desirable for a wide variety of CKD patients to prevent adverse cardio-renal events [8, 9].
Polystyrene sulfonate (PS), a cation-exchange resin, is the most commonly used drug for the treatment of hyperkalemia [10, 11]. There are two types of PS, calcium (Ca) and sodium (Na) PS (CPS and SPS), which are cross-linked polymers to which reactive sulfonic groups are attached and preloaded with Ca and Na, respectively [12, 13]. Although both types of PS exchange their bound cations for K in the lumen of proximal and distal colon and resultantly reduce serum K levels through the excretion of K into the stool, as far as we know, there is no head-to-head comparison prospective study of CPS vs. SPS in pre-dialysis CKD patients.
Hypocalcemia and secondary hyperparathyroidism are also prevalent in advanced CKD patients [14, 15], both of which are associated with vascular calcification and osteoporotic bone fracture [16, 17], partly contributing to the increased risk of cardiovascular disease in these patients [18, 19]. Moreover, exposure to Na-containing formulations of medicines has been shown significantly to increase the risk of cardiovascular events in humans compared with standard formulations of those same drugs [20, 21]. Therefore, in this study, we performed an open-labeled, randomized, prospective, and crossover trial to directly compare the effects of CPS and SPS on mineral and bone metabolism, including serum K, Ca, phosphate (P), magnesium (Mg), intact parathyroid hormone (iPTH) and Na levels in 20 pre-dialysis outpatients (estimated glomerular filtration rate (eGFR) <30 ml/min/1.73 m2) with hyperkalemia (serum K > 5.0 mmol/l). We further examined the effects of CPS and SPS on plasma natriuretic peptide (ANP) levels in our patients and investigated whether change of ANP after PS treatments (ΔANP) was correlated with that of ΔNa.
Materials and methods
Patients
A total of 20 pre-dialysis CKD 4–5 outpatients with hyperkalemia (K > 5 mmol/l) not treated with PS were enrolled in this study. The patients were followed up at Kurume University Hospital, Oita Prefectural Hospital, and Munakata Suikokai General Hospital from October 2013 to November 2014. We excluded patients who were already given PSs, who had acute kidney injury. The etiology of renal disease was as follows: diabetic nephropathy (n = 6), glomerulonephritis (n = 5), nephrosclerosis (n = 3), lupus nephritis (n = 2), membranous nephropathy (n = 1), IgA nephropathy (n = 1), and unknown (n = 2). More than a half of the patients received RAS inhibitors and Ca blockers for the treatment of hypertension (55, 65%, respectively). No patients received diuretics or phosphate binders during the study period.
Study design
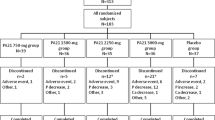
This study was designed as a prospective, open-labeled, randomized, and crossover study (Fig. 1). Twenty hyperkalemic patients were randomly assigned to CPS group (n = 10) or SPS group (n = 10) by an envelope method. Patients were orally administered CPS (ARGAMATE® 89.29% GRANULE 5.6 g; powder 5 g) or SPS (KAYEXALATE DRY SYRUP 76%® 6.54 g; powder 5 g) after each meal. After 4-week treatment (period 1), each PS was immediately switched to another PS without washout interval, and followed up for further 4 weeks (period 2). Blood pressure was measured in the sitting position using an upright standard sphygmomanometer.
Study design. CKD patients were randomized to CPS and SPS groups. After the first 4 weeks of the therapy phase (period 1, n = 20), CPS and SPS were immediately switched to another PS, and followed for further 4 weeks (period 2, n = 35). CKD chronic kidney disease, PS polystyrene sulfonate, CPS calcium polystyrene sulfonate, SPS sodium polystyrene sulfonate
Data collection
Before and after the switching to PS, blood was drawn from antecubital veins for determination of serum blood urea nitrogen, creatinine, K, albumin, Ca, P, Mg, Na, ammonia (NH3), iPTH, and plasma ANP. Urine was collected for evaluation of urinary K and Na excretion levels. Blood and urine chemistries were measured at a commercially available laboratory (SRL Inc., Hachioji, Japan) as described previously [22]. We calculated corrected Ca levels by the following Ca correction formula (Payne): Ca (mg/dl) + [4 − serum albumin (g/dl)] [23]. Venous blood gas was taken to analyze the plasma bicarbonate (HCO3 −) levels [24].
In vitro study
To investigate the cation-absorption capacity of CPS and SPS, we constructed an artificial colon fluid (ACF) based on the data of human diarrhea as described previously [25]. One gram of CPS or SPS was added into 50 ml of ACF (n = 6, respectively) and the solution were stirred at room temperature for 120 min. After filtration, the concentrations of K, Ca, Na, Mg, and NH3 in the supernatant were determined.
Statistical analysis
Unless otherwise, data were expressed as mean ± SD. Distribution of iPTH is, in general, heavily skewed; therefore, data on iPTH were presented as the median value (interquartile range). A one-way analysis of variance for repeated measures was used to assess the differences in baseline characteristics. To examine the difference of serum K, Ca, P, Na, chloride (Cl), Mg, iPTH, and ANP levels before and after the treatment with CPS or SPS in the period 1 and period 2, a paired t test was performed. Unpaired t test was performed in the comparison between CPS (n = 20) and SPS (n = 15) group. A Wilcoxon rank sum test and a Mann–Whitney U test were used for within-group differences and between-group differences of serum iPTH, respectively. All statistical analyses were performed by Graph Prism 5.0 for windows (GraphPad Software Inc. La Jolla, CA, USA) except for stepwise multiple regression analysis, which was performed to explore the independent determinants of ΔiPTH using IBM SPSS statistics ver.20 (IBM, Chicago, IL, USA). Statistical significance was defined as p < 0.05.
Results
Clinical characteristics of the patients
Clinical characteristics of 20 pre-dialysis outpatients with hyperkalemia are shown in Table 1. Overall, 55% of the patients were women and mean age was 69.7 ± 11.5 years. Six patients (30%) had diabetes mellitus. The mean eGFR was 15.9 ± 5.9 ml/min/1.73 m2 and 45% had an eGFR < 15 ml/min/1.73 m2. The mean serum K levels were 5.50 ± 0.51 mmol/l. Nutritional conditions of all subjects were almost normal. There was no significant difference of clinical data between the two groups at baseline. Medications except PS were not changed during the study period. As shown in Fig. 1, 5 patients treated with SPS dropped out due to drug-related adverse events, such as edema (3 patients), headache (1 patient), and diarrhea (1 patient).
Effects of CPS and SPS on serum and urinary levels of K
We examined serum K levels before and after the CPS and SPS treatments in period 1 (n = 10, respectively). As shown in Table 2, serum K and urinary K corrected for urinary creatinine (Cr) values were significantly decreased by both treatments. The change in serum K and urinary K/Cr from baseline (serum and urinary ∆K) was almost equal between the CPS and SPS group.
Effects of CPS and SPS on other mineral and bone metabolism
We next examined whether CPS or SPS treatment could affect serum Ca, P, Mg, and iPTH levels in our patients. As shown in Table 3, although serum P levels were not affected by either PS treatment, SPS, but not CPS treatment significantly decreased serum Ca and Mg levels. Furthermore, SPS treatment significantly increased serum iPTH levels, whereas CPS decreased it. In univariate linear regression analysis, ΔCa and ΔMg were positively associated with ΔiPTH values during the periods 1 and 2 (n = 35, r = −0.53, p < 0.001 and r = −0.50, p < 0.01, respectively) (Fig. 2a, b). Stepwise multiple regression analysis revealed that ΔCa (adjusted β = −0.33, p < 0.05), ΔMg (adjusted β = −0.37, p < 0.01), and ΔP (adjusted β = 0.35, p < 0.05) were independent determinants of ΔiPTH values in our patients (Table 4). Multicollinearity in these three values was not recognized.
Effects of CPS and SPS on serum and urinary Na, plasma ANP, and acid–base equilibrium
We further investigated whether the administration of CPS or SPS could affect serum and urinary Na and plasma ANP levels. As shown in Table 5, serum Na levels were significantly increased by SPS treatment, whereas decreased by CPS. Urinary Na/Cr values were modestly, but not significantly increased by SPS, whereas decreased by CPS (Table 5). Moreover, SPS, but not CPS treatment significantly increased plasma ANP levels. In univariate linear regression analysis, ΔNa was positively associated with ΔANP values during the periods 1 and 2 (Fig. 2c). Mean blood pressure and body weight were tended to increase by SPS treatment (95.3 ± 17.5 to 97.5 ± 13.4 mmHg, p = 0.14, 54.6 ± 13.1 to 55.0 ± 12.8 kg, p = 0.33, respectively). There was a positive correlation between ΔNa and Δbody weight (p < 0.05, r = 0.45, n = 20) (Fig. 2d).
It has been reported that SPS induces metabolic alkalosis via the absorption of NH3 in the colon fluid [26]. So, we next compared the effects of SPS on serum NH3 and plasma bicarbonate (HCO3 −) levels with those of CPS (Table 5). SPS, but not CPS treatment significantly increased plasma HCO3 − and serum Na levels, while serum NH3 levels were not changed by either PS treatment. As shown in Fig. 3a–c, ΔNa and Δ(Na to Cl ratio) but not ΔNH3 were positively correlated with ΔHCO3 − (r = 0.75, p < 0.0001, r = 0.84, p < 0.0001, and r = 0.06, p = 0.75, respectively).
Effects of CPS or SPS on cation-exchange capacity in ACF
As shown in Table 6, K levels in the ACF were significantly decreased by CPS or SPS; the effect of the latter was larger than that of the former. CPS and SPS had opposite effects on Ca and Na levels in the ACF; CPS increased Ca and decreased Na, and SPS vice versa. Furthermore, SPS treatment greatly reduced Mg and NH3.
Discussion
In our human study, although K-decreasing capacity of CPS and SPS was almost equal, serum Ca and Mg levels were significantly decreased by SPS treatment compared with CPS. These findings are consistent with the previous report showing that CPS did not affect serum Ca or Mg levels in patients with chronic renal failure [10]. Furthermore, in this study, SPS treatment significantly increased iPTH levels, whereas CPS decreased it. There was a significant and independent correlation between ΔCa or ΔMg and ΔiPTH values. Since SPS greatly exchanged their bound Na for K, Mg, Ca, and NH3 in the ACF, higher cation-exchange capacity of SPS could be involved in lower serum levels of Ca and Mg, thereby worsening secondary hyperparathyroidism. In addition, although there was no significant difference of serum NH3 between the two groups, SPS may increase intraluminal pH by absorbing NH3. Because higher intestinal pH has been reported to inhibit an activity of TRPM6, a major transporter of Mg in the colon [27], which could suppress the absorption of Mg, it might partly contribute to the decrease in serum Mg levels by SPS.
Parathyroid cells have calcium-sensing receptors (CaRs) on cell surface, which enable them to respond to changes in extracellular Ca concentration [28, 29]. Although Ca is the main CaR agonist, Mg is also able to activate the CaRs [30]. An increased extracellular Mg has been shown to inhibit PTH secretion by parathyroid cells [31]. Low Ca, low Mg, or high iPTH levels are associated with progression of diabetic kidney disease [32] and cardiovascular mortality in patients with dialysis [33]. So our present findings may raise the safety concern of SPS for the treatment of hyperkalemia in pre-dialysis patients due to its detrimental effects of Ca, Mg, and iPTH [18]. On the other hand, since a meta-analysis revealed that calcium supplements were associated with an increased risk of myocardial infarction [34], Ca load by CPS may also increase the risk of vascular calcification and cardiovascular events in CKD patients. Therefore, excess Ca intake and Ca-based phosphate binders may be avoided in CKD patients, especially those with vascular calcification.
In this study, we also found that serum Na and plasma ANP levels were significantly elevated by the treatment with SPS. Further, ΔNa was positively correlated with ΔANP values during the study (Fig. 2c). The changes in serum Na concentration are determined not only by intestinal Na absorption, but also by water intake. Although we did not evaluate water intake in our patients, there was a positive correlation between ΔNa and Δbody weight (p < 0.05, r = 0.45, n = 20) (Fig. 2d), thus suggesting that the increase of Na level by SPS might be associated with increased extracellular fluid. Recent retrospective study has shown that SPS causes inter-dialytic weight gain and increases blood pressure in dialysis patients in Dialysis Outcomes and Practice Patterns Study [35]. Exposure to sodium-containing formulations of drugs are associated with adverse cardiovascular events in patients compared to sodium-free formulation ones [20]. Therefore, intake of sodium should be strictly controlled in patients who were receiving SPS.
In the present study, although K-exchanging capacity of SPS was higher than that of CPS, serum K-reducing ability of both drugs was nearly the same. It has been reported that K concentration in lower intestine was higher than that in upper one, and the highest in distal colon [36, 37]. SPS has mainly absorbed K, Mg, Ca, and NH3 in the fluid of upper digestive tract, where K concentration is low. On the other hand, CPS has absorbed K in the distal colon rather than upper digestive tract due to the lower cation-exchange capacity, where K concentration is highest. This might be a possible reason for the discrepant results between ACF experiments and pre-dialysis patients.
Treatment of SPS has been reported to increase HCO3 − levels due to absorption of NH3 in the digestive tract [26]. Consistent with the previous reports, we found that SPS, but not CPS, dramatically increased plasma HCO3 − levels. Furthermore, although ΔNH3 was not associated with ΔHCO3 − values, ΔNa and Δ(Na to Cl ratio) were positively correlated with ΔHCO3 −. A close relationship between sodium as well as potassium balance and renal response to a chronic acid load was first reported in 1977 [38]. Further, Lindner et al. have shown that rising serum Na levels could cause a concurrent development of metabolic alkalosis in critically ill patients [39]. Recently Stewart approach is proposed as a new idea of acid–base equilibrium [40, 41].
There are several limitations in this study. First, the sample size was small and the study duration was short. Second, due to the ethical concern, we could not set a washout period in this crossover study. Furthermore, as many as 5 patients were dropped out during the phase of SPS. Impaired capacity of electrolyte excretion by the kidney may affect the present findings.
Conclusion
In summary, although the present study demonstrated that K-reducing capacity of CPS and SPS was almost equal in pre-dialysis CKD patients with hyperkalemia, SPS treatment decreased Ca and Mg levels in association with increase in serum iPTH, Na, or plasma ANP values, which might lead to hyperparathyroidism and volume overload. Our present findings suggest that CPS might be better than SPS to control hyperkalemia in pre-dialysis patients. Further longitudinal studies are needed to clarify whether CPS treatment could exert more beneficial effects on bone metabolism and cardiovascular events in advanced CKD patients compared with SPS.
References
Einhorn LM, Zhan M, Hsu VD, Walker LD, Moen MF, Seliger SL, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169(12):1156–62.
Torlén K, Kalantar-Zadeh K, Molnar MZ, Vashistha T, Mehrotra R. Serum potassium and cause-specific mortality in a large peritoneal dialysis cohort. Clin J Am Soc Nephrol. 2012;7(8):1272–84.
Jarman PR, Kehely AM, Mather HM. Hyperkalaemia in diabetes: prevalence and associations. Postgrad Med J. 1995;71(839):551–2.
Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–9.
Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–60.
Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med. 2004;351(6):585–92.
Takaichi K, Takemoto F, Ubara Y, Mori Y. Analysis of factors causing hyperkalemia. Intern Med. 2007;46(12):823–9.
Sterns RH, Grieff M, Bernstein PL. Treatment of hyperkalemia: something old, something new. Kidney Int. 2016;89(3):546–54.
Watson M, Abbott KC, Yuan CM. Damned if you do, damned if you don’t: potassium binding resins in hyperkalemia. Clin J Am Soc Nephrol. 2010;5(10):1723–6.
Tomino Y, Yamazaki T, Shou I, Tsuge T, Satake K, Takeda Y, et al. Dose-response to a jelly preparation of calcium polystyrene sulfonate in patients with hyperkalemia–changes in serum potassium levels with or without a RAAS inhibitor. Clin Nephrol. 2007;68(6):379–85.
Lepage L, Dufour AC, Doiron J, Handfield K, Desforges K, Bell R, et al. Randomized clinical trial of sodium polystyrene sulfonate for the treatment of mild hyperkalemia in CKD. Clin J Am Soc Nephrol. 2015;10(12):2136–42.
Berlyne GM, Janabi K, Shaw AB. Dangers of resonium A in the treatment of hyperkalemia in renal failure. Lancet. 1966;1(7430):167–9.
Frohnert PP, Johnson WJ, Mueller GJ, Tauxe WN, McCall JT. Resin treatment of hyperkalemia. II. Clinical experience with a cation exchange resin (calcium cycle). J Lab Clin Med. 1968;71(5):840–6.
Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15(8):2208–18.
Block GA, Zaun D, Smits G, Persky M, Brillhart S, Nieman K, et al. Cinacalcet hydrochloride treatment significantly improves all-cause and cardiovascular survival in a large cohort of hemodialysis patients. Kidney Int. 2010;78(6):578–89.
Komaba H, Kakuta T, Fukagawa M. Diseases of the parathyroid gland in chronic kidney disease. Clin Exp Nephrol. 2011;15(6):797–809.
Jadoul M, Albert JM, Akiba T, Akizawa T, Arab L, Bragg-Gresham JL, et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2006;70(7):1358–66.
Taniguchi M, Fukagawa M, Fujii N, Hamano T, Shoji T, Yokoyama K, et al. Serum phosphate and calcium should be primarily and consistently controlled in prevalent hemodialysis patients. Ther Apher Dial. 2013;17(2):221–8.
Fukagawa M, Kido R, Komaba H, Onishi Y, Yamaguchi T, Hasegawa T, et al. Abnormal mineral metabolism and mortality in hemodialysis patients with secondary hyperparathyroidism: evidence from marginal structural models used to adjust for time-dependent confounding. Am J Kidney Dis. 2014;63(6):979–87.
George J, Majeed W, Mackenzie IS, Macdonald TM, Wei L. Association between cardiovascular events and sodium-containing effervescent, dispersible, and soluble drugs: nested case-control study. BMJ. 2013;347:f6954.
Takase H, Sugiura T, Kimura G, Ohte N, Dohi Y. Dietary sodium consumption predicts future blood pressure and incident hypertension in the Japanese normotensive general population. J Am Heart Assoc. 2015;4(8):e001959.
Manabe R, Fukami K, Ando R, Sakai K, Kusumoto T, Hazama T, et al. Effects of switching from calcium carbonate to lanthanum carbonate on bone mineral metabolism in hemodialysis patients. Ther Apher Dial. 2013;17(Suppl 1):35–40.
Clase CM, Norman GL, Beecroft ML, Churchill DN. Albumin-corrected calcium and ionized calcium in stable haemodialysis patients. Nephrol Dial Transplant. 2000;15(11):1841–6.
Hynes D, Bates S, Loughman A, Klim S, French C, Kelly AM. Arteriovenous blood gas agreement in intensive care patients with varying levels of circulatory compromise: a pilot study. Crit Care Resusc. 2015;17(4):253–6.
Yamada T, Alpers D, Laine L, Owyang C, Powell D. Approach to the patient with diarrhea. Textb Gastroenterol. 2003;1:858–64.
Evans BM, Jones NC, Milne MD, Yellowlees H. Ion-exchange resins in the treatment of anuria. Lancet. 1953;265(6790):791–5.
Li M, Jiang J, Yue L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol. 2006;127(5):525–37.
Kumar R, Thompson JR. The regulation of parathyroid hormone secretion and synthesis. J Am Soc Nephrol. 2011;22(2):216–24.
Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, et al. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366(6455):575–80.
Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81(1):239–97.
Shoback DM, Thatcher JG, Brown EM. Interaction of extracellular calcium and magnesium in the regulation of cytosolic calcium and PTH release in dispersed bovine parathyroid cells. Mol Cell Endocrinol. 1984;38(2–3):179–86.
Sakaguchi Y, Shoji T, Hayashi T, Suzuki A, Shimizu M, Mitsumoto K, et al. Hypomagnesemia in type 2 diabetic nephropathy: a novel predictor of end-stage renal disease. Diabetes Care. 2012;35(7):1591–7.
Sakaguchi Y, Fujii N, Shoji T, Hayashi T, Rakugi H, Isaka Y. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int. 2014;85(1):174–81.
Bolland MJ, Avenell A, Baron JA, Grey A, MacLennan GS, Gamble GD, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ. 2010;341:c3691.
Jadoul M, Karaboyas A, Goodkin DA, Tentori F, Li Y, Labriola L, et al. Potassium-binding resins: associations with serum chemistries and interdialytic weight gain in hemodialysis patients. Am J Nephrol. 2014;39(3):252–9.
Powell DW, Berschneider HM, Lawson LD, Martens H. Regulation of water and ion movement in intestine. Ciba Found Symp. 1985;112:14–33.
Kamel KS, Schreiber M. Asking the question again: are cation exchange resins effective for the treatment of hyperkalemia? Nephrol Dial Transplant. 2012;27(12):4294–7.
van de Strihou CY, Dieu JP. Potassium deficiency acidosis in the dog: effect of sodium and potassium balance on renal response to a chronic acid load. Kidney Int. 1977;11(5):335–47.
Lindner G, Schwarz C, Grüssing H, Kneidinger N, Fazekas A, Funk GC. Rising serum sodium levels are associated with a concurrent development of metabolic alkalosis in critically ill patients. Intensive Care Med. 2013;39(3):399–405.
Luke RG, Galla JH. It is chloride depletion alkalosis, not contraction alkalosis. J Am Soc Nephrol. 2012;23(2):204–7.
Seifter JL. Integration of acid-base and electrolyte disorders. N Engl J Med. 2014;371(19):1821–31.
Acknowledgement
This work was supported, in part, by Grants-in-Aid for Welfare and Scientific Research (C) (no. 16k09637) (K.F) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Fukami has received honoraria such as lecture fees from Sanwa (Sanwa Kagaku Kenkyusyo). This paper has not been published previously in whole or part.
Human rights
(with IRB approval number) All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional ethics committee at which the studies were conducted (Approval No. 13170) and with the 1964 Helsinki Declaration and its later amendments of comparable ethical standards. This trial was registered with the University Hospital Medical Information Network clinical trials database (UMIN 000021955).
Informed consent
Informed consent was obtained from all individual participants included in the study.
About this article
Cite this article
Nakayama, Y., Ueda, K., Yamagishi, Si. et al. Compared effects of calcium and sodium polystyrene sulfonate on mineral and bone metabolism and volume overload in pre-dialysis patients with hyperkalemia. Clin Exp Nephrol 22, 35–44 (2018). https://doi.org/10.1007/s10157-017-1412-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-017-1412-y