Abstract
Background
Kidney injury molecule-1 (KIM-1) is a sensitive biomarker for proximal tubular injury. Recently, a few studies have shown that urinary KIM-1 has clinical implications in IgA nephropathy (IgAN). We performed this study to determine whether tissue KIM-1 has clinical implications for predicting long-term outcome and whether urinary KIM-1 is correlated with tissue KIM-1 and kidney injury in IgAN patients.
Methods
We assessed the prognostic prediction capability of tissue KIM-1 expression in 69 adult patients with IgAN retrospectively. Renal biopsies from all patients were scored by a pathologist who was blinded to the clinical data for the pathologic variables. The primary outcome was the composite of a 50 % reduction in eGFR or end-stage renal disease. Tissue KIM-1 expression was assessed semiquantitatively by counting the stained tubules per 100× power field; 0 tubule indicates grade 0; 1–5 tubules, grade 1; 6–10 tubules, grade 2; and more than 10 tubules, grade 3. Comparing urinary KIM-1 and tissue KIM-1 expression, 50 consecutive IgAN patients were prospectively enrolled to measure urinary KIM-1 levels and examine their biopsy specimens by KIM-1 immunohistochemistry.
Results
Univariate analysis showed that tissue KIM-1 expression was associated with the renal outcome in IgAN. Multivariate regression analysis, as the relationship of tissue KIM-1 with prognosis, was consistent. Proteinuria at biopsy and tissue KIM-1 grade 3 were shown to have a prognostic value. The concentration of urinary KIM-1/Cr in patients with IgAN was significantly higher than that in the normal controls.
Conclusion
Tissue KIM-1 expression is an independent predictor of adverse renal outcomes in IgA nephropathy patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with IgAN have a variable clinical course, with 6–43 % progressing to end-stage renal disease (ESRD) within 10 years [1–4]. Previous studies have identified clinical features as predictors of adverse outcomes, and histological changes are also recognized as an important prognostic factor in IgAN [1, 5, 6]. Since tubulointerstitial damage is correlated with a rapid decline in renal function, even when controlling for other clinical parameters [7, 8], specific markers to detect tubulointerstitial damage could allow for early intervention in order to prevent renal failure in IgAN.
Kidney injury molecule-1 (KIM-1), a type 1 membrane protein, is not expressed in normal kidney, but is markedly upregulated in injured proximal tubular epithelial cells. Recently, a few reports have indicated that urinary KIM-1 has clinical implications in IgAN [9, 10]. Since this new biomarker should be validated in various populations, we investigated whether tissue KIM-1 has clinical implications for predicting long-term outcome in IgAN and whether urinary KIM-1 is associated with kidney injury and tissue KIM-1 expression.
Materials and methods
Patients and controls
The institutional review boards of the participating centers approved this investigation. This study included retrospective analysis and prospective enrollment. First, to investigate prognostic prediction with KIM-1 in IgAN patients, we did a retrospective survival analysis. Patients with IgAN who were followed for more than 3 years were included in the study. From January 1994 to December 2007, data were collected from renal biopsies of native kidneys performed and/or processed in our unit. The diagnosis of IgAN was defined by the predominant mesangial deposition of IgA. Clinical and laboratory data of patients were collected at the time of renal biopsy. Patients were excluded if the clinical diagnosis suggested rapidly progressive IgAN and if secondary causes, such as liver disease, diabetes and Henoch-Schönelin purpura, were founded. Second, to investigate whether urinary KIM-1 is correlated with tissue KIM-1 expression and tubulointerstitial damage, we prospectively enrolled 50 consecutive patients with IgAN starting in September 2009. Clinical data at the time of biopsy were recorded. The normal control group consisted of 27 healthy volunteers who had no known health problems and were not taking medication. In our practice, we have treated high blood pressure and proteinuria with angiotensin-converting enzyme (ACE) inhibitor or angiotensinogen receptor blocker (ARB) in IgAN patients. Corticosteroids were considered if proteinuria above 1 g/day persisted on maximal ACE inhibitors or ARB therapy. We tried steroids and cyclophosphamides if renal biopsy showed crescentic glomerulonephritis and renal function declined rapidly.
Biopsy sections were stained with hematoxylin-eosin, periodic acid-Schiff, and silver methanamine. Light microscopic assessment of renal biopsies was performed in accordance with the Oxford classification of IgAN by a single experienced renal pathologist [7].
Immunohistochemical studies
Sections measuring 4 µm were deparaffinized in 96 % ethanol and xylene. The sections were blocked for endogenous peroxidase in a mixture of 1 ml of 3 % H2O2 in 250 ml methanol for 10 min and subsequently hydrated in solutions of decreasing ethanol concentration. Antigen retrieval was performed using target retrieval solution at pH 6.0 (Dako, Glostrup, Denmark). Sections were rinsed with phosphate-buffered saline between each step. Monoclonal mouse anti-KIM-1 (R&D Systems, Minneapolis, MN, USA) primary antibody was applied for 60 min at room temperature. Binding was detected with appropriate peroxidase-labeled secondary antibody. Kidney tissue from acute pyelonephritis served as the positive control. KIM-1 staining was scored semi-quantitatively by counting the stained tubules per 100× power field; 0 tubule indicates grade 0; 1–5 tubules, grade 1; 6–10 tubules, grade 2; and more than 10 tubules, grade 3.
Urinary KIM-1 measurement by sandwich enzyme-linked immunosorbent assay
The level of KIM-1 protein in urine was measured in duplicate by ELISA using a commercial kit (R&D Systems) in accordance with the manufacturer’s guidelines. The lowest limit of detection for this assay was 0.009 pg/ml. The inter- and intra-assay variability was <10 %. The urinary KIM-1 level was standardized with urine creatinine (ng/mg).
Statistical analysis
Data are presented as mean ± standard deviation or median with interquartile range. Comparisons of continuous and categorical variables between groups were performed using nonparametric tests and a two-sided chi-square test or a two-sided Fisher's exact test, respectively, where applicable. Clinical outcomes were studied to address the predictive values of KIM-1. Adverse outcome was defined as a 50 % reduction in renal function or ESRD. Kaplan–Meier cumulative survival curves were plotted for each category. The log-rank test was used to examine differences in the survival curves among the groups. Multivariate survival analysis using Cox regression was performed to test the association between pathological categories and clinical outcomes; p < 0.05 was considered statistically significant. Statistical analysis was performed using the SPSS 14 software package (SPSS, Chicago, IL, USA).
Results
Patient characteristics of the retrospective analysis
To evaluate the clinical implication of KIM-1, we analyzed 69 patients. The mean eGFR (modification of diet in renal disease, MDRD, equation) at biopsy was 90.8 ± 38.0 ml/min/1.73 m2. The median 24-h proteinuria in the patient group was 1.2 (0.4–1.9) g/day, and 63 % of the IgAN patients had tubular lesions of T0 classification.
Prospective comparison of urinary KIM-1/creatinine between healthy controls and IgAN patients
For the comparison of urinary KIM-1 and renal pathology, we prospectively collected data for 50 IgAN patients with a mean age of 39.3 ± 11.4 years; 50 % were male, and the eGFR was 83.1 ± 30.8 ml/min/1.73 m2. The median 24-h proteinuria in the patient group was 1.2 (0.3–1.5) g/day.
The concentration of urinary KIM-1/creatinine in IgAN patients and healthy controls was 1.32 ± 0.18 and 0.56 ± 0.19 (ng/mg), respectively. The concentration of urinary KIM-1/creatinine in IgAN patients was significantly higher than that of the normal controls (p < 0.0001).
Retrospectively studied tissue KIM-1 expression in IgAN patients
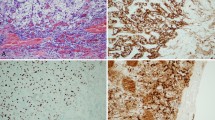
Tissue KIM-1 expression was localized to the apical sides of dilated tubules, mainly proximally, in fibrotic areas. KIM-1 was heterogeneously expressed within individual tubular cross sections, with KIM-1-positive and -negative tubular cells adjacent to each other. Completely atrophic tubules did not express KIM-1 (Fig. 1). According to the KIM-1 grade, there were significant differences in eGFR and proteinuria at biopsy (Table 1). Among the Oxford classification variables, E1 increased significantly as the KIM-1 score increased (p < 0.0001; Fig. 2).
Distribution of Oxford pathological variables according to KIM-1 grade. a Distribution of M1 by tissue KIM-1 grade (19 % in grade 0; 60 % in 1; 58 % in 2; 59 % in 3). b Distribution of S1 by tissue KIM-1 grade (none in grade 0; 88 % in 1; 78 % in 2; 81 % in 3). c Distribution of E1 by tissue KIM-1 grade (none in grade 0; 16 % in 1; 34 % in 2; 74 % in 3; p < 0.0001). d Distribution of T1 and T2 by tissue KIM-1 grade (11.1 % as T1 in 0; 17 % as T1 and 4 % T2 in 1; 52 % as T1 and 11 % T2 in 2; 43 % T1 and 43 % T2 in grade 3). M mesangial hypercellularity, S1 segmental glomerulosclerosis, E1 endocapillary hypercellularity, T tubular atrophy/interstitial fibrosis
Prospectively evaluated tissue KIM-1 and urinary KIM-1 in IgAN
As the severity of the tubulointerstitial lesion according to the Oxford classification increased, the tissue KIM-1 expression also increased (T0, 1.10 ± 0.64; T1, 1.95 ± 0.72; and T2, 2.63 ± 0.51, p < 0.0001). However, the urinary KIM-1 level did not correspond to the tubular lesion according to the Oxford classification, and the level of urinary KIM-1/creatinine did not depend on the tissue KIM-1 grade (grade 0, 0.47 ± 0.12; grade 1, 1.85 ± 1.63; grade 2, 1.12 ± 1.10; and grade 3, 1.96 ± 1.04, p = 0.203).
Tissue KIM-1 expression and renal outcomes in the retrospective analysis
During a median follow-up of 85 (60–114) months, 6 patients (8 %) achieved a 50 % reduction in eGFR, and 10 patients (14 %) progressed to ESRD. Kaplan–Meier cumulative survival curves showed the survival of patients with tissue KIM-1 grade 3 was significantly worse than that of patients with grade 1 and grade 2 (p = 0.003; Fig. 3). Multivariate analysis (backward selection), measured by various clinical predictors—proteinuria, eGFR at biopsy, age, sex and the tissue KIM-1 grade—showed that tissue KIM-1 grade 3 and proteinuria were independent predictors of adverse renal outcome (Table 2).
Discussion
In this study, we assessed the clinical implications of the KIM-1 molecule in IgAN using urinary KIM-1 and KIM-1 immunohistochemistry. Our research indicates that tissue KIM-1 expression correlates with poor prognostic clinical parameters, and univariate analyses show that KIM-1 has significant meaning. This relationship was also significant in the multivariate analyses. However, the urinary KIM-1 was not associated with tissue KIM-1 expression or tubulointerstitial damage according to the Oxford classification in our cohort.
The pathological systems previously used to classify renal lesions in IgAN, can be divided into two types: lumped and split. These classifications depend on the histological damage. KIM-1 is markedly unregulated in the proximal tubule after various kidney injuries [11–13]. As tubular KIM-1 expression is specific to ongoing tubular cell damage and differentiation [12, 13], we presumed the tissue KIM-1 could have a clinical implication when compared to simple histological damage. In the present study, we proposed a relatively simplified histological grade scheme with a new biomarker, KIM-1, in IgAN and tested for clinical relevance in a retrospective analysis with a long-term follow-up of patient groups. Although our classification of KIM-1 grade is very simple, our definition of tissue KIM-1 expression correlated with clinical parameters such as eGFR and proteinuria at biopsy. Our results suggest that tissue KIM-1 represents kidney injury in IgAN. However, completely atrophic tubules did not express KIM-1, revealing the limitation of tissue KIM-1 expression. This may implicate the poor prognostic value of tissue KIM-1 in severe cases of IgAN. Especially when acute tubular necrosis with excessive hematuria, usually resulting in severe atrophic tubules, or rapid progressive crescentic glomerulonephritis, omitted during our study, develop in IgAN, tissue KIM-1 expression may have limited prognostic value.
High-grade expression of KIM-1 increased in elderly patients (mean age; 49.6 vs. 36.5 or 34.9 in 3 grades vs. 1 or 2 grades of KIM-1, respectively), probably meaning the prognostic factor of old age influenced increased tissue KIM-1 expression in our IgAN patients. Also, age was correlated with decreased eGFR (p < 0.0001), but not proteinuria (p = 0.564), in the univariate correlation analysis. However, multivariable analysis showed that age did not affect adverse renal outcomes, but proteinuria and tissue KIM-1 did. According to these results, old age did not seem to affect poor prognosis in our study.
According to the Oxford classification, as tubulointerstitial lesions and endocapillary lesions increase, tissue KIM-1 expression also increases. Even if an early report described endocapillary hypercellularity [14] in IgAN patients as a prognostic factor, it was not extensively studied until the introduction of the Oxford classification of IgAN. There is not enough evidence of a correlation between endothelial hypercellularity and tissue KIM-1 expression to reach a reasonable conclusion. Recently, Xu et al. [10] reported that the urinary KIM-1 level was significantly higher in IgAN patients with endocapillary proliferation than in those without. Endothelial proliferation may results in severe tubular damage in IgAN patients. Differences from the data of 206 IgAN patients [10] were that we did not include cases of rapid progressive glomerulonephritis and had a small number of patients in our study. Interestingly, in that study the level of urinary KIM-1 did not increase in grade 2 compared to grade 1 tubular atrophy and interstitial fibrosis, just like our results, even indicating the significant prognostic value of urinary KIM-1 in IgAN [10].
These may indicate the significance of tissue KIM-1 in Kaplan–Meier analysis and Cox regression analyses. In addition, we found an interesting result. Among 13 patients, biopsy specimens were KIM-1 grade 3 with Oxford tubular minimal and moderate lesions such as T1 and T2 lesions; 4 (33 %) patients reached primary renal outcomes. Considering 23 % among our cohort had adverse renal outcomes, this increased expression of tissue KIM-1 can help identify patients at risk of progression who would not have been identified anywhere else.
Our results support the previous studies of chronic kidney disease including IgAN that showed a clinical implication of KIM-1 [9, 10]. Peter et al. [9] showed that urinary KIM-1 excretion is an independent predictor of end-stage renal disease in IgAN. Another study reported that urinary KIM-1 represents the tubulointerstitial damage in IgAN [10]. Tubulointerstitial injury is an important mediator and final common pathway to end-stage renal disease [1, 2, 15]. If urinary KIM-1 could be used to detect and monitor tubulointerstitial damage, this would be very useful in clinical settings. Our results failed to show a correlation between urinary KIM-1 and histological damage. However, Peng et al. [10] argued that urinary KIM-1 is closely associated with severe tubulointerstitial injury based on a larger cohort (202 patients) than ours. Given that well-known prognostic factors such as proteinuria are controversial [9, 16, 17], this new molecule should be validated in variable large cohorts. It would be exciting to study the prognostic impact of urinary KIM-1 relative to conventional clinical parameters.
Tissue KIM-1 was significantly correlated with tubular lesion's grading according to the Oxford classification, but urinary KIM-1, despite its increase when compared with controls, is not correlated with the degree of tissue injury in IgAN. This implies a discrepancy between urinary KIM-1 and tissue KIM-1 in IgAN patients. The cleavage of KIM-1 is an active process mediated by a matrix metalloproteinase (MMP), confirmed by the action of MMP inhibitors the blocking release of soluble KIM-1 [18]. In the human glomerulosclerosis and the hypoxia animal models of chronic kidney disease, the expression of TIMPs (tissue inhibitors of metalloproteinase) was increased, suggesting the decreased activity of MMP [19]. This decreased MMP activity in IgAN probably cause to excrete urinary KIM-1 non-significantly despite of increased tissue expression of KIM- 1. Also, the corrected creatinine could be the limit in predicting the true levels of urinary KIM-1. More importantly, urinary KIM-1 is a preclinical assay; there is need for standardization for measuring.
This study had several limitations. As in all single-center studies, the prognostic results may not be reproducible in other settings. In addition, compared to other reported populations, renal impairment and proteinuria were less severe in the patient sample in our study. Since patients with advanced renal disease are less likely to be biopsied in our hospital, a selection bias may exist. The results of our study should be validated and confirmed in a larger, multicenter population.
In conclusion, tissue KIM-1 staining is a useful biomarker to predict renal outcome in IgAN patients according to our results. Future studies are needed to confirm the clinical implications of KIM-1 in IgAN patients.
References
Alamartine E, Sabatier JC, Guerin C, Berliet JM, Berthoux F. Prognostic factors in mesangial IgA glomerulonephritis: an extensive study with univariate and multivariate analyses. Am J Kidney Dis. 1991;18:12–9.
D’Amico G. Influence of clinical and histological features on actuarial renal survival in adult patients with idiopathic IgA nephropathy, membranous nephropathy, and membranoproliferative glomerulonephritis: survey of the recent literature. Am J Kidney Dis. 1992;20:315–23.
Haas M. Histologic subclassification of IgA nephropathy: a clinicopathologic study of 244 cases. Am J Kidney Dis. 1997;29:829–42.
Johnston PA, Brown JS, Braumholtz DA, Davison AM. Clinico-pathological correlations and long-term follow-up of 253 United Kingdom patients with IgA nephropathy. A report from the MRC Glomerulonephritis Registry. Q J Med. 1992;84:619–27.
Bohle A, Mackensen-Haen S, von Gise H, Grund KE, Wehrmann M, Batz C, et al. The consequences of tubulo-interstitial changes for renal function in glomerulopathies. A morphometric and cytological analysis. Pathol Res Pract. 1990;186:135–44.
Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76:546–56.
Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–45.
Coppo R, Troyanov S, Camilla R, Hogg RJ, Cattran DC, Cook HT, et al. The Oxford IgA nephropathy clinicopathological classification is valid for children as well as adults. Kidney Int. 2010;77:921–7.
Peters HP, Waanders F, Meijer E, van den Brand J, Steenbergen EJ, van Goor H, et al. High urinary excretion of kidney injury molecule-1 is an independent predictor of end-stage renal disease in patients with IgA nephropathy. Nephrol Dial Transpl. 2011;26:3581–8.
Xu PC, Zhang JJ, Chen M, Lv JC, Liu G, Zou WZ, et al. Urinary kidney injury molecule-1 in patients with IgA nephropathy is closely associated with disease severity. Nephrol Dial Transpl. 2011;26:3229–36.
de Borst MH, van Timmeren MM, Vaidya VS, de Boer RA, van Dalen MB, Kramer AB, et al. Induction of kidney injury molecule-1 in homozygous Ren2 rats is attenuated by blockade of the renin–angiotensin system or p38 MAP kinase. Am J Physiol Renal Physiol. 2007;292:F313–20.
Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286:F552–63.
Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–42.
Katafuchi R, Kiyoshi Y, Oh Y, Uesugi N, Ikeda K, Yanase T, et al. Glomerular score as a prognosticator in IgA nephropathy: its usefulness and limitation. Clin Nephrol. 1998;49:1–8.
Risdon RA, Sloper JC, De Wardener HE. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet. 1968;2:363–6.
Bartosik LP, Lajoie G, Sugar L, Cattran DC. Predicting progression in IgA nephropathy. Am J Kidney Dis. 2001;38:728–35.
Donadio JV, Bergstralh EJ, Grande JP, Rademcher DM. Proteinuria patterns and their association with subsequent end-stage renal disease in IgA nephropathy. Nephrol Dial Transpl. 2002;17:1197–203.
Zhang Z, Humphreys BD, Bonventre JV. Shedding of the urinary biomarker kidney injury molecule-1 (KIM-1) is regulated by MAP kinases and juxtamembrane region. J Am Soc Nephrol. 2007;18:2704–14.
Catania JM, Chen G, Parrish AR. Role of matrix metalloproteinases in renal pathophysiologies. Am J Physiol Renal Physiol. 2007;292:F905–11.
Conflict of interest
The authors have no competing interests.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kwon, S.H., Park, M.Y., Jeon, J.S. et al. KIM-1 expression predicts renal outcomes in IgA nephropathy. Clin Exp Nephrol 17, 359–364 (2013). https://doi.org/10.1007/s10157-012-0707-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-012-0707-2