Abstract
Introduction
Serum cystatin C was recently proposed as an alternative marker of glomerular filtration rate (GFR), with a suggested better performance than creatinine. However, detailed studies are limited. We evaluated the performance of cystatin C as a GFR marker.
Methods
GFR was measured by inulin clearance in 763 Japanese subjects. Factors other than GFR influencing serum cystatin C or serum creatinine were analyzed by multivariate analyses.
Results
After adjustment for GFR, the value of serum creatinine was 25.2% lower in females than males, and decreased by 5.2% for every 20 years of age. Serum cystatin C was 8.2% lower in females, and did not change significantly with aging. Creatinine but not cystatin C was significantly affected by body weight, height and body mass index after adjustment for GFR, gender and age. The correlation coefficient between GFR and 1/cystatin C was significantly higher than that of 1/creatinine in total subjects (0.866 and 0.810, respectively, p < 0.001). Unlike serum creatinine, serum cystatin C did not increase in association with the reduction of GFR in subjects with very low GFR. The regression line of 1/cystatin C against GFR showed a significantly negative intercept of about −8 ml/min/1.73 m2.
Conclusion
The performance of serum cystatin C was not good in the subjects with very low GFR. Non-renal elimination of cystatin C may contribute to the result. The correlation between reciprocal cystatin C and GFR suggested its superiority in predicting GFR compared to creatinine in subjects with normal and mildly reduced GFR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Serum creatinine level has been used to assess renal function, but is often affected by muscle mass, which is dependent on age, weight, and gender [1]. Serum cystatin C was recently proposed as an alternative marker of glomerular filtration rate (GFR), and its higher performance compared with creatinine has been suggested from a meta-analysis [2]. However, detailed studies on the comparison between cystatin C and serum creatinine as markers of GFR are limited. In the present study, we compared the performance of serum cystatin C and serum creatinine as a GFR marker in subjects stratified by gender and age. We confirmed the better performance of cystatin C but also found an apparent non-renal elimination of cystatin C in subjects with low GFR that may affect the performance of cystatin C as a GFR marker.
Methods
Subjects and measurements
To compare the performance of serum cystatin C with that of serum creatinine, we used same data set from which the GFR equation for Japanese was developed. Details of the subjects have been reported previously [3, 4]. A total of 763 Japanese patients in 80 medical centers were included. We stratified the subjects according to gender and into three age groups (18–39, 40–59 and 60–79 years old). GFR was measured by inulin renal clearance [3], and serum creatinine was measured by the IDMS-traceable enzymatic method [3]. In the present study, we analyzed the serum cystatin C values which were measured with serum creatinine previously in a single laboratory. Cystatin C was measured by nephrometric assay (Dade Behring).
Multivariate analyses
Factors other than GFR influencing serum cystatin C levels were analyzed by multivariate linear regression analyses. Cystatin C levels and GFR were log-transformed. Age, height, weight and body mass index (BMI) were used as the raw data. Gender was expressed as a binary factor. After adjusting for GFR, the percent change in serum level of cystatin C for a change of 20 years of age, female gender and one unit in the variables such as height, weight, and BMI was analyzed. We also examined the same analyses using serum creatinine levels.
Correlation coefficient between GFR and 1/cystatin C
The correlation coefficient between GFR and 1/cystatin C was analyzed in total subjects and in subjects stratified by gender and age groups (18–39, 40–59 and 60–79 years old). To evaluate an apparent non-renal elimination of cystatin C, the intercept of the linear regression line in the reciprocal plot was calculated. The same analyses were performed for reciprocal serum creatinine. The relationship between GFR and reciprocal creatinine curved upwards slightly, and therefore linear regression lines for 1/creatinine and 1/cystatin C were separately calculated in subjects with serum levels higher than 1.5 mg/dl and 1.5 mg/L, respectively.
Statistical analysis
Data were expressed as means ± SD. p < 0.05 was considered statistically significant. Statview version 4.02 (SAS Institute) and JMP 8.01 (SAS Institute) were used for statistical analyses. Smoothed lines fit to the data (Figs. 2, 3, 5) were calculated using spline model of JMP 8.01 (SAS Institute).
Results
Table 1 shows the characteristics of the study subjects. Mean measured GFR of males was significantly lower than that of females (54 ± 34 and 65 ± 36 ml/min/1.73 m2, respectively). Mean measured GFR in the older age group was significantly lower than that in the younger age group in both males and females.
Multivariate analyses
Serum creatinine was 25.2% lower in females than in males, and decreased by 5.2% for every 20 years of age after adjustment for GFR (Table 2). Serum creatinine was significantly increased in association with increase in body weight, height, and BMI after adjustment for GFR, gender and age. Serum cystatin C was 8.2% lower in females than in males, and was not significantly changed by age after adjustment for GFR. Cystatin C was not significantly changed by body weight, height and BMI after adjustment for GFR, gender and age.
Relationship between GFR and serum concentration of cystatin C
The relationship between GFR and serum concentrations of cystatin C is shown in Fig. 1. The plot of serum cystatin C showed a curvilinear pattern that was similar to that of serum creatinine. The relationship between GFR and 1/cystatin C was almost linear, while the plots of 1/creatinine seemed to be curving upwards slightly (Figs. 2, 3). The correlation coefficient in the reciprocal plot of cystatin C was significantly higher compared with that of creatinine in total subjects (r = 0.866 and 0.810, respectively) (Table 3). The correlation coefficients of cystatin C were consistently higher than the values of creatinine in subjects stratified by gender and three age groups, although statistically not significant.
Relationship between GFR and serum concentration of creatinine or cystatin C. Left GFR versus serum concentration of creatinine in male subjects. Dotted lines show upper reference limit of serum creatinine and lower reference limit of GFR (1.04 mg/dl and 60 ml/min/1.73 m2, respectively). Right GFR versus serum concentration of cystatin C in male subjects. Dotted lines show upper limit of serum cystatin C and lower reference limit of GFR (0.95 mg/dl and 60 ml/min/1.73 m2, respectively)
Relationship between serum concentration of creatinine and serum concentration of cystatin C
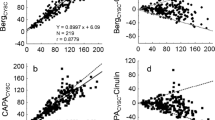
The relationship between serum concentration of creatinine and serum concentration of cystatin C was not linear (Fig. 4). Unlike serum creatinine, serum cystatin C did not increase in association with reduction of GFR in subjects with GFR below 15 ml/min/1.73 m2 (Fig. 5). To evaluate the apparent non-renal elimination of cystatin C, the linear regression line of the reciprocal plots was calculated in subjects with serum cystatin C higher than 1.5 mg/L. Intercepts (95% confidence interval [CI]) of the regression lines in male and female were −8.4 (−12.1, −4.8) ml/min/1.73 m2 and −9.1 (−15.5, −2.6) ml/min/1.73 m2, respectively (Table 4). The values were significantly lower than zero (p < 0.01). The linear regression line of the reciprocal plots of creatinine that was calculated in subjects with serum creatinine higher than 1.5 mg/dl intersected near the origin. Intercepts (95% CI) of the regression lines in males and females were −0.9 (−3.8, 2.0) ml/min/1.73 m2 and −2.2 (−6.5, 2.1) ml/min/1.73 m2, respectively. The values were not significantly different from zero.
Serum concentrations of creatinine and cystatin C in subjects with GFR under 15 ml/min/1.73 m2. Unlike serum creatinine (open circle), serum cystatin C (closed circle) did not increase as much in association with reduction of GFR in subjects with GFR under 15 ml/min/1.73 m2. Smooth lines show the fit of the data
Discussion
Serum creatinine and cystatin C are well-known markers of GFR. However, few studies have investigated their comparative performance relative to GFR measured by inulin renal clearance. In present study, we observed a significant difference in performance of creatinine and cystatin C as GFR markers. Gender and age effects were more prominent in creatinine. Serum creatinine was 25.2% lower in females, and declined by 5.2% for every 20 years of age, while serum cystatin C was 8.2% lower in females, and was not significantly associated with age. These results are almost consistent with the reports of Stevens et al. and Knight et al. [5, 6]. Stevens et al. reported that older age was associated with lower serum cystatin C level after adjustment of GFR measured by iothalamate clearance [5]. On the other hand, Knight et al. reported that older age was associated with higher serum cystatin C level after adjusting for creatinine clearance [6]. The backgrounds of the study population such as ethnicity, renal function and physique were different between the studies. The variable factors and methods of GFR measurement may influence the results of the studies.
Generation of creatinine is affected by muscle mass, which is dependent on physique such as weight and height. Higher serum creatinine was associated with higher body weight, height, and BMI after adjustment for GFR, gender and age (Table 2). The association of these parameters with serum cystatin C level was much smaller than with creatinine. These results are consistent with the report of Stevens et al. [5] except for BMI. They reported that the percent change of cystatin C was higher than the value of creatinine according to the change in BMI, and speculated an association between fat mass and cystatin C levels. The mean and interquartile range of BMI in the study by Stevens et al. were 27.7 and 7.3 kg/m2, while the values in our study were much lower (22.9 and 4.8 kg/m2, respectively). The lower prevalence of obesity in our subjects may contribute to the inconsistent results regarding the association with BMI.
The relationship between GFR and 1/cystatin C was almost linear, evaluated by the smoothed line fit to the data (Figs. 2, 3). On the other hand, the relationship between GFR and 1/creatinine was slightly upwardly curving. This could be one of the reasons that reduces the performance of creatinine in the correlation analysis. Creatinine is secreted from tubules as well as filtered from glomeruli. Therefore, the creatinine clearance (CCR) exceeds the GFR. About 20% of creatinine is secreted from the tubule in normal subjects [1], and the tubular secretion increases up to about 50% with reduction in GFR [1]. The change in tubular secretion may contribute to the upward curve of the reciprocal plot of creatinine. We made a simulation model in which the CCR/GFR ratio was increased from 1.2 to 1.7 according to the reduction in GFR with constant generation of creatinine (Table 5; Fig. 6, model A). The simulation model confirmed the effect of tubular secretion on the upward curving of the reciprocal plot.
Simulation models for reciprocal plots of creatinine and cystatin C. Detailed values for simulation models are described in Table 5. Model A is a simulation model for the reciprocal plot of creatinine. CCR/GFR ratio increases according to reduction of GFR. This leads to the upward curve of the reciprocal plot of creatinine. Model B is a simulation model for the reciprocal plot of cystatin C with constant non-renal clearance. This model shows the linear relationship between GFR and 1/cystatin C. Model C is a simulation model for the reciprocal plot of cystatin C without non-renal clearance. Controlled change in the generation of cystatin C is required to keep the linear relationship between GFR and 1/cystatin C
The reciprocal plot of serum cystatin C had a significantly negative intercept, suggesting an apparent non-renal elimination of the marker. Assuming that the generation of cystatin C is independent of GFR, non-renal elimination of cystatin C is estimated as the intercept of the reciprocal plot. We obtained an apparent non-renal clearance of cystatin C of about 8 ml/min/1.73 m2. Sjostrom et al. [7, 8] reported similar results using the reciprocal plot of cystatin C. They measured GFR by plasma clearance of iohexol and reported the apparent non-renal elimination of cystatin C as 22.3 ml/min/1.73 m2 in a preliminary study and 14.1 ml/min/1.73 m2 in a second study[7, 8]. We made two simulation models to explain the apparent non-renal elimination of cystatin C. The first is a simulation model with constant non-renal clearance set at 8 ml/min. The generation of cystatin C was set as the value at which serum cystatin C was 0.8 mg/L when GFR was 100 ml/min. The simulation model with constant non-renal clearance shows a linear relationship between GFR and reciprocal cystatin C with negative intercept (Table 5; Fig. 6, model B). In the second simulation model, the generation of cystatin C was decreased according to reduction in GFR. The same straight line as seen in model B could be drawn. In this case, generation of cystatin C decreases at an accelerated pace according to reduction of GFR (Table 5; Fig. 6, model C). This suggests that non-renal elimination of cystatin C is a more plausible mechanism than rapid reduction of cystatin C generation. Tenstad et al. [9] investigated renal handling of radiolabeled human cystatin C in rat. A considerable amount of extra-renal plasma clearance of labeled cystatin C was observed in nephrectomized rats, that contributed about 15% of the total plasma clearance of cystatin C. A relatively high uptake of radioactivity was recorded in the spleen. They speculated about the removal of cystatin C in the reticuloendothelial system.
Unlike serum creatinine, serum cystatin C did not increase in association with reduction of GFR in subjects with GFR below 15 ml/min/1.73 m2. The performance of serum cystatin C was not good in these subjects, and therefore,serum cystatin C should not be used for evaluating GFR in subjects with end-stage renal disease. Apparent non-renal elimination of cystatin C contributed to the low performance of the marker. On the contrary, even if non-renal elimination of cystatin C is present at a comparable level, percentage of non-renal elimination in the total clearance would be small in subjects with normal and mildly reduced GFR, suggesting that the effect of non-renal elimination on serum levels of cystatin C is small and difficult to detect. Although the difference between the correlation coefficients of reciprocal cystatin C and reciprocal creatinine was small, cystatin C had a better correlation with GFR than creatinine in total subjects, suggesting that cystatin C concentration may be better than creatinine at predicting GFR in subjects with normal and mildly reduced GFR.
Eriksen et al. [10] showed that cystatin C is not a better estimator of GFR than creatinine, based on the testing of several GFR equations. They showed a large bias of GFR equations from cystatin C compared with GFR equations from creatinine, such as the CKD–EPI equation in the general population. The authors mentioned that the most important factor of the large bias of the cystatin C equations was probably that GFR equations from cystatin C were all developed in populations with CKD and low GFR. The influence of non-GFR factors on plasma cystatin C may differ between these patients and the general population. Also, standardization between assays and laboratories is lacking for cystatin C.
We agree with the comments of Eriksen et al. When the equation from cystatin C was developed from subjects with lower GFR, non-GFR elimination of cystatin C may influence the performance of the equation in subjects with normal or mildly reduced GFR. We have to study the model for GFR estimation including factors such as non-renal clearance. The measurement of cystatin C has not been standardized. There is up to 20% difference in the cystatin C values among various reagent companies [11, 12]. When the cystatin C value has a 20% positive bias compared with the value that was used for development of a GFR estimation equation, the measurement bias leads to about 20% underestimation of GFR and lower performance of the equation. The standardization of the measurement is a fundamental problem of the accuracy of the GFR equations derived from cystatin C. Standardization of the measurement of cystatin C is nowin progress worldwide [13], after which, development and validation of GFR equations from cystatin C will be required.
There are several limitations. The study subjects were almost all patients with native kidney disease, so it may not be possible to generalize the results to healthy subjects. It has been reported that cystatin C levels were influenced by factors other than GFR, such as thyroid function [14, 15], inflammation [5, 6], smoking [6, 8] and immunosuppressive therapy [16]. Multivariate analysis was not adjusted for the above factors in the present study.
Conclusion
The performance of serum cystatin C was not good in subjects with very low GFR; non-renal elimination of cystatin C may contribute to the result. Reciprocal cystatin C had a better correlation with GFR than creatinine in total subjects. Age, gender, body weight, height and BMI had a much smaller effect on cystatin C level than creatinine. These results suggested the superiority of cystatin C over creatinine in predicting GFR in subjects with normal and mildly reduced GFR.
References
Levey AS, Perrone RD, Madias NE. Serum creatinine and renal function. Annu Rev Med. 1988;39:465–90.
Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40:221–6.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, on behalf of the collaborators developing the Japanese equation for estimating GFR, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S. Modification of the CKD epidemiology collaboration (CKD-EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis. 2010;56:32–8.
Stevens LA, Schmid CH, Greene T, Li L, Beck GJ, Joffe MM, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75:652–60.
Knight EL, Verhave JC, Spiegelman D, Hillege HL, Zeeuw DD, Curhan GC, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65:1416–21.
Sjostrom PA, Tidman MA, Jones IL. Determination of the production rate and non-renal clearance of cystatin C and estimation of the glomerular filtration rate from the serum concentration of cystatin C in humans. Scand J Clin Lab Invest. 2005;65:111–24.
Sjostrom PA, Jones IL, Tidman MA. Cystatin C as a filtration marker—haemodialysis patients expose its strengths and limitations. Scand J Clin Lab Invest. 2009;69:65–72.
Tenstad O, Roald AB, Grubb A, Aukland K. Renal handling of radiolabelled human cystatin C in the rat. Scand J Clin Lab Invest. 1996;56:409–14.
Eriksen BO, Mathisen UD, Melsom T, Ingebretsen OC, Jenssen TG, Njolstad I, et al. Cystatin C is not a better estimator of GFR than plasma creatinine in the general population. Kidney Int. 2010;78:1305–11.
Levinson S. Calibration of the Siemens cystatin C immunoassay has changed over time. Clin Chem. 2011;57:777–8.
Voskoboev NV, Larson TS, Rule AD, Lieske C. Importance of cystatin C assay standardization. Clin Chem. 2011;57:1209–11.
Grubb A, Blirup-Jensen S, Lindstrom V, Schmidt C, Althaus H, Zegers I. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med. 2010;48:1619–21.
Kotajima N, Yanagawa Y, Aoki T, Tsunekawa K, Morimura T, Ogiwara T, et al. Influence of thyroid hormones and transforming growth factor-β1 on cystatin C concentrations. J Int Med Res. 2010;38:1365–73.
Fricker M, Wiesli P, Brandle M, Schwegler B, Schmid C. Impact of thyroid dysfunction on serum cystatin C. Kidney Int. 2003;63:1944–7.
Rule AD, Bergstralh EJ, Slezak JM, Bergert J, Larson TS. Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int. 2006;69:399–405.
Acknowledgments
This study was supported in part by a grant from the Japanese Society of Nephrology and Health and Labor Sciences Research Grant from the Ministry of Health, Labor and Welfare, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the collaborators for developing Japanese equation for estimating GFR.
Appendix
Appendix
The following investigators participated in the project for developing the Japanese equation for estimating GFR: Asahikawa Medical University; Kenjiro Kikuchi, Masakazu Haneda: Hokkaido University Hospital; Seiji Hashimoto: Sapporo Medical University; Nobuyuki Ura: Tohoku University Hospital; Sadayoshi Ito, Hiroshi Sato: Fukushima Medical University; Tsuyoshi Watanabe: Jichi Medical University; Yasuhiro Ando, Eiji Kusano: Gunma University; Yoshihisa Nojima: University of Tsukuba; Kunihiro Yamagata, Chie Saitoh: Toride Kyodo General Hospital; Tatsuo Shiigai, Yoshitaka Maeda: Saitama Medical University; Hiromichi Suzuki, Yusuke Watanabe: Saitama Medical University; Tetsuya Mitarai, Hajime Hasegawa: The University of Tokyo; Toshiro Fujita: Jikei University; Tatsuo Hosoya: Nihon University; Koichi Matsumoto, Takayuki Fujita: Tokyo Women’s Medical University; Kousaku Nitta: Tokyo Women’s Medical University Medical Center East; Tsutomu Sanaka: Tokyo Medical and Dental University; Eiichiro Kanda, Sei Sasaki: Juntendo University; Yasuhiko Tomino: Tokyo Medical University Hospital; Toshiyuki Nakano: Showa University; Tadao Akizawa, Hirokazu Honda: Showa University Fujigaoka Hospital; Yoshihiko Inoue, Ashio Yoshimura: Toranomon Hospital; Hiroshi Tsuji: Toho University; Yasushi Ohashi: Teikyo University; Hideaki Nakajima Shunya Uchida: Kyorin University; Akira Yamada: Yokohama City University; Satoshi Umemura, Mai Yanagi: St. Marianna University; Hiro Yamakawa, Kenjiro Kimura: Niigata University; Shinichi Nishi, Fumitake Gejyo: Hamamatsu University; Akira Hishida, Hideo Yasuda, Tomoyuki Fujikura: Nagoya University; Seiichi Matsuo: Aichi Medical University; Hirokazu Imai, Wataru Kitagawa: Fujita Health University Hospital; Satoshi Sugiyama: Mie University; Shinsuke Nomura, Eiji Ishikawa: Toyama Prefectural Central Hospital; Masahiko Kawabata: Hiroyuki Iida: Kanazawa University Hospital; Takashi Wada: Kanazawa Medical University; Hitoshi Yokoyama: University of Fukui Hospital; Haruyoshi Yoshida: Shiga University of Medical Science; Takashi Uzu, Masayoshi Sakaguchi: Kyoto University Hospital, Atsushi Fukatsu: Osaka University; Yasuyuki Nagasawa, Shiro Takahara: Osaka City University; Eiji Ishimura, Yoshiki Nishizawa: Kobe University; Masafumi Fukagawa, Michio Umezu: Hyogo College of Medicine; Nakanishi Takeshi, Izumi Masaaki: Okayama University, Hitoshi Sugiyama, Hirofumi Makino: Kawasaki Medical School; Naoki Kashihara, Tamaki Sasaki: Hiroshima University; Noriaki Yorioka: University of Tokushima; Toshio Doi: Kagawa University; Masakazu Kono: Ehime University; Takafumi Okura: Kyushu University Hospital; Kazuhiko Tsuruya, Akiko Ono: Fukuoka University; Takao Saito, Yasuhiro Abe: Nagasaki University; Akira Furusu: Kurume University; Seiya Okuda: Kumamoto University; Kimio Tomita: University of Miyazaki; Shouichi Fujimoto: University Hospital of The Ryukyus; Iseki Kunitoshi, Schuichi Takishita: Koto Hospital; Isao Ebihara, Yuko Shima: Nara Medical University; Koji Harada, Yoshihiko Saito: Dokkyo Medical University; Atsushi Numabe, Toshihiko Ishimitsu: Kitano Hospital; Eri Muso, Toshiyuki Komiya: Musashino Red Cross Hospital; Ryoichi Ando: Toyonaka Municipal Hospital; Megumu Fukunaga: Ohmihachiman Community Medical Center; Tsuguru Hatta: Sendai Shakaihoken Hospital; Osamu Hota: Tosei General Hospital; Inaguma Daijo: Matsuyama Clinic; Kazuhiro Matsuyama: Rokko Island Hospital; Naoyuki Nakano: Shuwa General Hospital; Masashi Inoshita: Kanagawa Rehabilitation Hospital; Masahisa Kusaka, Masato Mizuguchi.
About this article
Cite this article
Horio, M., Imai, E., Yasuda, Y. et al. Performance of serum cystatin C versus serum creatinine as a marker of glomerular filtration rate as measured by inulin renal clearance. Clin Exp Nephrol 15, 868–876 (2011). https://doi.org/10.1007/s10157-011-0525-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-011-0525-y