Abstract
Endoplasmic reticulum (ER) stress is involved in a wide range of pathological circumstances including neurodegenerative disorders, diabetes mellitus, ischemic injury, cancers, atherosclerosis, inflammation, infection, toxicity of chemicals and metals, and psychotic diseases. ER stress is also involved in some physiological events including development of particular cell types. A number of pathophysiological triggers cause accumulation of unfolded proteins in the ER, i.e., ER stress. In response to accumulation of unfolded/misfolded proteins, cells adapt themselves to the stress conditions via a coordinated adaptive program, the unfolded protein response (UPR). UPR is a double-edged sword. It induces both prosurvival and proapoptotic signaling. It also triggers both proinflammatory and anti-inflammatory signals. In this review, I summarize current knowledge on putative, pathophysiological roles of ER stress in the kidney.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The endoplasmic reticulum (ER) is the site of biosynthesis for steroids, cholesterol and other lipids. It is also the subcellular entrance for a number of secretory and structural proteins and provides a unique environment for appropriate protein folding and assembly to produce functional, mature proteins. Homeostasis in the ER is maintained by a coordinated adaptive program, so-called the unfolded protein response (UPR). A number of microenvironmental, developmental and pathophysiological insults as well as a wide range of chemical substances cause accumulation of unfolded proteins in the ER, i.e., ER stress [1] (Table 1). In response to accumulation of unfolded proteins, cells adapt themselves to the stress conditions via UPR; attenuation of general translation, induction of ER chaperones and activation of ER-associated degradation (ERAD) to eliminate immature proteins. If the stress is beyond the capacity of the adaptive machinery, cells undergo apoptosis (Fig. 1). Accumulating evidence suggests that ER stress and UPR are involved in a diverse range of pathological situations, including ischemia, diabetes mellitus, neurodegenerative disorders, infection and chemical-induced tissue injury [1, 2] (Table 1). ER stress is also implicated in some physiological events, e.g., development of particular cell types, including plasma cells, pancreatic β-cells, hepatocytes and osteoblasts [3]. However, information is limited regarding roles of ER stress and UPR in the renal pathophysiology. This article summarizes current knowledge on putative, pathophysiological roles of ER stress in the kidney.
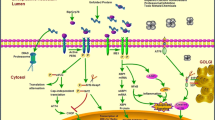
Unfolded protein response (UPR). In response to accumulation of unfolded protein in the endoplasmic reticulum (ER), cells adapt themselves to the stress condition via UPR, i.e., translational suppression, induction of ER chaperones and ERAD factors to eliminate unfolded proteins via productive folding and enhanced degradation by the proteasome pathway. If the stress is beyond the capacity of the adaptive machinery, cells undergo apoptosis
UPR and cell function
Cell fates
Three major transducers for sensing ER stress have been identified on the membrane of the ER. These include RNA-dependent protein kinase-like ER kinase (PERK), activating transcription factor 6 (ATF6) and inositol-requiring ER-to-nucleus signal kinase 1 (IRE1). Activation of PERK causes phosphorylation of eukaryotic translation initiation factor 2α (eIF2α), which leads to general inhibition of protein synthesis. In response to ER stress, 90 kDa ATF6 (p90ATF6) transits to the Golgi where it is cleaved by site-1 protease (S1P) and site-2 protease (S2P), yielding an active transcription factor 50 kDa ATF6 (p50ATF6). Similarly, activated IRE1 catalyzes removal of a small intron from the mRNA of X-box binding protein 1 (XBP1). This splicing event creates a translational frame-shift in XBP1 mRNA to produce an active transcription factor. Active p50ATF6 and XBP1 subsequently bind to the ER stress response element (ERSE) and the UPR element (UPRE), leading to expression of target genes, including an ER chaperone 78 kDa glucose-regulated protein (GRP78) and ERAD factors involved in degradation of unfolded proteins. These pathways are generally considered as prosurvival UPR [4] (Fig. 2).
Induction of UPR by ER stress through three major transducers; RNA-dependent protein kinase-like ER kinase (PERK), activating transcription factor 6 (ATF6) and inositol-requiring ER-to-nucleus signal kinase 1 (IRE1). Activation of PERK leads to phosphorylation of eukaryotic translation initiation factor 2α (eIF2α), which causes general inhibition of protein synthesis. In response to ER stress, 90-kDa ATF6 (p90ATF6) transits to the Golgi where it is cleaved by site-1 protease (S1P) and site-2 protease (S2P), yielding an active transcription factor, 50 kDa ATF6 (p50ATF6). Similarly, activated IRE1 catalyzes removal of a small intron from the mRNA of X-box binding protein 1 (XBP1). This splicing event creates a translational frameshift in XBP1 mRNA to produce an active transcription factor. Active p50ATF6 and XBP1 subsequently bind to the ER stress response element (ERSE) and the UPR element (UPRE), leading to expression of target genes encoding ER chaperones and ERAD factors involved in degradation of unfolded proteins
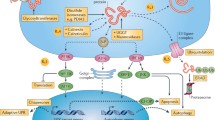
During the UPR, however, death signals, as well as survival signals, are also transduced [5]. For example, expression of a proapoptotic gene CCAAT/enhancer-binding protein-homologous protein (CHOP) is triggered by a transcription factor ATF4 that is induced by the PERK–eIF2α pathway. The ATF6 pathway and the IRE1 pathway may also induce expression of CHOP [6]. ER stress also activates caspase-12 (or caspase-4 in humans) localized at the ER membrane through an interaction with IRE1 and tumor necrosis factor receptor-associated factor 2 (TRAF2), leading cells to undergo apoptosis. ER stress causes conformational changes and/or oligomerization of proapoptotic Bak and Bax at the ER membrane [7], leading to release of Ca2+ from the ER. It activates calpain in the cytosol, which cleaves procaspase-12 to caspase-12 in the ER [8]. Down-regulation of Bcl-2 transcription by CHOP may also be involved in the induction of apoptosis by ER stress [9]. Another important pathway involved in the ER stress-initiated apoptosis is the IRE1–apoptosis signal-regulating kinase 1 (ASK1)–c-Jun N-terminal kinase (JNK) pathway. The cytoplasmic part of IRE1 binds TRAF2, an adaptor protein that couples plasma membrane receptors to JNK activation [10]. ER stress activates ASK1 through formation of an IRE1–TRAF2–ASK1 complex, and this molecular event is essential for activation of JNK by ER stress [11]. These pathways are considered as proapoptotic UPR (Fig. 3).
Induction of proapoptotic UPR by ER stress. Activation of the PERK–eIF2α pathway induces a transcription factor ATF4. Consequently, ATF4 induces expression of proapoptotic CCAAT/enhancer-binding protein-homologous protein (CHOP) through activation of the amino acid response element (AARE). The ATF6 pathway (and the IRE1 pathway) may also induce expression of CHOP via activation of ERSE. ER stress causes conformational changes and/or oligomerization of proapoptotic Bak and Bax at the ER membrane, leading to release of Ca2+ from the ER. It activates calpain in the cytosol, which cleaves procaspase-12 to caspase-12. ER stress also activates caspase-12 localized at the ER membrane through an interaction with IRE1 and tumor necrosis factor receptor-associated factor 2 (TRAF2), leading cells to undergo apoptosis. The IRE1–TRAF2 interaction also allows for recruitment and activation of apoptosis signal-regulating kinase 1 (ASK1) and downstream c-Jun N-terminal kinase (JNK), both of which are involved in a variety of proapoptotic signaling
Mitogen-activated protein (MAP) kinases and nuclear factor-κB (NF-κB)
In general, ER stress activates MAP kinases and NF-κB and thereby causes cellular activation. UPR has the ability to activate stress kinases, including JNK and p38 MAP kinase, via the IRE1–ASK1 pathway [12]. Similarly, UPR activates NF-κB through multiple mechanisms, possibly, via the IRE1 pathway [13, 14] and/or the PERK–eIF2α pathway [15, 16]. In response to ER stress, IκB kinase (IKK) forms a complex with IRE1α through the adapter protein TRAF2 [13, 14]. Other reports also showed that phosphorylation of eIF2α is necessary and sufficient to activate NF-κB [15, 16], but mechanisms involved have not been fully elucidated.
In contrast to those previous reports, we recently reported that preceding ER stress may blunt subsequent activation of NF-κB. We found that, in glomerular cells, expression of monocyte chemoattractant protein 1 and inducible nitric oxide synthase in response to tumor necrosis factor-α (TNF-α) is abrogated by some agents, K-7174 and geranylgeranylacetone, that induce UPR [17–19]. The suppression of gene expression was associated with attenuated NF-κB activation. Induction of UPR by other ER stress inducers also reproduces the suppressive effects of K-7174 and geranylgeranylacetone on NF-κB and NF-κB-dependent gene expression in cytokine-treated cells [17, 18]. These results raise a possibility that, although ER stress activates NF-κB in the early phase, consequent UPR suppresses cellular responses to subsequent inflammatory stimuli in the later phase.
Molecular mechanisms involved in the anti-inflammatory potential of UPR are largely unknown. However, we found that A20, one of the major negative regulators for NF-κB, is induced by ER stress, suggesting a possible involvement of this molecule in the blunted responses to inflammatory stimuli under ER stress conditions [20]. Another possibility is involvement of TRAF2. In the TNF-α signaling, TNF receptor 1 (TNFR1), TNFR1-associated death domain (TRADD), TNFR-interacting protein (RIP) and TRAF2 are essential for NF-κB activation [21]. Recently, Hu et al. reported that, in thapsigargin (inhibitor of sarco/endoplasmic reticulum Ca2+-ATPase)- or tunicamycin (inhibitor of protein glycosylation)-treated cells, TNFR1, TRADD and RIP proteins are maintained at the same levels as those in untreated cells, whereas the level of TRAF2 protein is selectively down-regulated [13]. The blunted responses of NF-κB to TNF-α under ER stress may be caused by down-regulation of TRAF2.
Cell differentiation
UPR has specialized roles in developmental processes. For example, UPR is required during differentiation of some professional cells, including plasma cells, pancreatic β cells, hepatocytes and osteoblasts [4].
Plasma cell
During differentiation of B lymphocytes into plasma cells, UPR drives biogenesis of the ER in response to high-levels of secretory protein synthesis and takes an essential part in the differentiation of B cells [22]. In particular, the IRE1–XBP1 branch of UPR plays a crucial role. It is based on the facts that (1) IRE1-deficient B cells do not differentiate into plasma cells in vitro [23], (2) XBP1-deficient B cells fail to differentiate into plasma cells in vivo [24] and (3) ectopic expression of the spliced form of XBP1 restores immunoglobulin production in XBP1-deficient B cells in vitro [25].
Pancreatic β cell
PERK is an eIF2 protein kinase highly expressed in the pancreas. PERK-deficient mice show defect in β cells and develop diabetes [26]. In humans, mutations in the PERK gene cause Wolcott–Rallison syndrome, which manifests as an infantile-onset, insulin-requiring diabetes [27]. Furthermore, eIF2-knock-in mice in which all eIF2 kinases are affected develop severe β cell dysfunction prior to birth [28], suggesting the role of the PERK–eIF2 pathway.
Hepatocyte
Both IRE1- and XBP1-deficient mice exhibit hypoplastic fetal livers. In XBP1-null hepatocytes, cell growth is severely affected, and apoptosis is accelerated [29]. PERK-deficient mice also show a defect in hepatocytes [26], indicating that multiple branches of UPR are required for normal development of hepatocytes.
Osteoblast
ATF4 induced by the PERK–eIF2α pathway regulates osteoblast-specific gene expression and differentiation of osteoblasts [30]. Mice and humans deficient in the PERK gene have the same abnormality of the bone trabeculae as that in ATF4-deficient mice [27, 31], suggesting a crucial role of the PERK–eIF2α–ATF4 pathway in osteogenesis.
ER stress and the kidney
Glomerular disease
Glomerular diseases are developed by various causes, but little is known about involvement of ER stress in glomerular injury. However, recent investigations suggested roles of ER stress in some types of glomerular diseases, especially proteinuric diseases caused by podocyte injury.
Congenital nephrotic syndrome
Congenital nephrotic syndrome of the Finnish type (CNF) is an autosomal-recessive disorder characterized by massive proteinuria. The gene responsible for this disease encodes a podocyte-specific protein, nephrin, the crucial component of the slit diaphragm [32]. More than 60 different mutations in the nephrin gene have been identified in patients with CNF. The most common mutations are missense mutations, resulting in single amino acid substitutions. A previous study showed that the majority of the missense mutations lead to protein misfolding and consequent retention of the mutants in the ER [33]. Liu et al. reported that a chemical chaperone that corrects the cellular trafficking of misfolded mutant proteins rescues the mutant nephrin to be transported to the plasma membrane to function similarly to the wild-type nephrin [34].
Mutation in podocin, a prohibitin homology-domain protein that is also localized at the slit diaphragm, is another common cause of hereditary nephrotic syndrome in humans. The NPHS2 gene encoding podocin is linked to the autosomal recessive-type of steroid-resistant nephrotic syndrome. Ohashi et al. reported that the R138Q mutant of podocin, one of the most common missense mutations in the NPHS2 gene, is retained in the ER, suggesting that trafficking of the mutant podocin is disturbed [35]. Treatment of the cells with chemical chaperones elicits normal cellular redistribution of R138Q podocin to the plasma membrane [35].
These results suggest a role of ER stress in some congenital nephrotic syndromes. Interestingly, Fujii et al. reported that glucocorticoid, the most popular therapeutic agent for nephrosis, may exert an anti-proteinuric effect via facilitation of intracellular trafficking of nephrin under an ER stress condition. They found that glucose starvation evokes ER stress and formation of hypoglycosylated nephrin that is retained in the ER. Dexamethasone rescues the impaired trafficking and promotes synthesis of fully glycosylated nephrin [36].
Membranous nephropathy
Passive Heymann nephritis in rodents is a model of membranous nephropathy in humans. In this model, complement C5b-9 induces injury of glomerular podocytes, resulting in proteinuria. Cybulsky et al. reported that exposure of cultured podocytes to C5b-9 increases GRP78 and GRP94 at both mRNA and protein levels. Knockdown of GRP78 via antisense GRP78 enhances complement-dependent injury of cultured podocytes [37]. In vivo, glomerular GRP78 and GRP94 proteins are up-regulated in proteinuric rats with passive Heymann nephritis, and pretreatment of the rats with an inducer of GRPs reduces proteinuria [37]. The same group also showed that complements induce phosphorylation of PERK and eIF2α in cultured podocytes and that PERK and eIF2α phosphorylation is enhanced in glomeruli of rats with Heymann nephritis. Fibroblasts from PERK-deficient mice are more susceptible to complement-mediated cytotoxicity, suggesting a prosurvival role of the PERK–eIF2α branch of UPR [38]. These results suggested that complement-induced podocyte injury in vitro and in vivo is associated, possibly mediated by ER stress, and that ER stress may be involved in the pathogenesis of membranous nephropathy. Using immunohistochemical staining, Bek et al. recently reported up-regulation of CHOP in podocytes of proteinuric human kidneys (membranous nephropathy, focal segmental glomerulosclerosis and minimal change nephropathy) as well as kidneys of rats with puromycin nephrosis, a model of minimal change nephropathy [39].
Ischemic injury
Ischemia causes ER stress through hypoxia and nutritional deprivation and thereby induces tissue injury. Using an in vitro model of ischemia reperfusion (2-deoxyglucose plus antimycin A followed by glucose re-exposure), Cybulsky et al. reported that podocytes subjected to ischemia-reperfusion exhibit phosphorylation of PERK and eIF2α. They also found that PERK-deficient fibroblasts are more susceptible to ischemia-reperfusion-triggered cellular death, indicating an anti-apoptotic role of the PERK–eIF2α branch of UPR in ischemic injury of podocytes [38].
Endotoxin produces profound declines in glomerular blood flow and causes acute renal failure even when systemic pressures are preserved [40]. This effect is mediated by locally generated vasodilators, including nitric oxide. Using transgenic sensor mice for ER stress [41], we recently reported that intraperitoneal administration with lipopolysaccharide causes rapid, transient induction of systemic ER stress, and it was associated with dramatic up-regulation of GRP78 in particular organs including the kidney [42]. As described, ER stress activates NF-κB in the early phase, and expression of inducible nitric oxide synthase is regulated by NF-κB. Induction of ER stress in the kidney may, in part, underlie sepsis-induced acute renal failure.
Tubular disease
Acute renal failure is caused by a variety of triggers, including hypoxia/ischemia, heavy metal intoxication and nephrotoxic agents, such as antibiotics, anti-cancer agents, immunosuppressants and non-steroidal anti-inflammatory drugs (NSAIDs). In general, acute renal failure is characterized by damage of tubular cells; apoptosis and/or necrosis. In particular, apoptosis is thought to play a crucial role in the development of tubular injury during acute renal failure [43].
Ischemic injury
Montie et al. reported that cardiac arrest-induced ischemia and subsequent reperfusion cause phosphorylation of PERK and eIF2α in the rat kidney, especially in tubular epithelial cells [44]. This observation indicates that renal ischemia-reperfusion induces ER stress, activates UPR and causes cellular damage in the renal tubules. Bando et al. provided more evidence for the crucial role of ER stress in renal ischemic injury [45]. They found that renal tissues from patients with acute renal failure display strong induction of 150-kDa oxygen-regulated protein (ORP150), an ER chaperone. In a rodent model of renal ischemia-reperfusion injury, ORP150 is induced in the kidney, principally in the renal tubules [45]. Cultured tubular cells exposed to hypoxia display induction of ORP150. Tubular cells transfected with ORP150 exhibit resistance to hypoxic stress, whereas knockdown of ORP150 by antisense ORP150 renders the cells susceptible to hypoxic cell death [45]. Furthermore, transgenic mice overexpressing ORP150 are resistant to renal ischemia-reperfusion injury. In contrast, mice with lower levels of ORP150 show enhanced ischemic injury [45]. These data suggest a key role for ER stress in the ischemic renal tubular injury.
Anticancer agent
The chemotherapeutic agent cisplatin is used for the treatment of various solid tumors. Despite its therapeutic effectiveness, its nephrotoxic side effects significantly limit the clinical use. Cisplatin causes generation of reactive oxygen species (ROS), activation of MAP kinases and induction of inflammation and fibrogenesis via generation of cytokines [46]. In particular, ROS have been considered as important mediators for cisplatin-induced tubular injury. Liu et al. showed that, in cisplatin-treated tubular cells, cleavage of procaspase-12 precedes that of procaspases-3 and -9. They also showed that overexpression of anti-caspase-12 antibody significantly attenuates cisplatin-induced apoptosis [47]. Furthermore, ER stress preconditioning to induce ER chaperones is effective for attenuation of cisplatin cytotoxicity in several tubular cell lines [48]. These results suggest that ER stress and consequent activation of caspase-12 play a pivotal role in cisplatin-induced nephrotoxicity. Indeed, Peyrou et al. recently provided in vivo evidence for the involvement of ER stress in cisplatin-induced renal injury. They showed that, after administration with cisplatin in rats, activation of the XBP1 pathway and cleavage of procaspase-12 are observed in the kidney [49].
NSAID
A previous report showed that certain NSAIDs cause ER stress in gastric mucosal cells [50]. Recently, we also found that indomethacin, but not other NSAIDs tested, induces ER stress in murine podocytes [51]. It is known that NSAIDs exert nephrotoxicity [52], and ER stress may underlie the nephrotoxic effects of NSAIDs. Indeed, Lorz et al. reported that paracetamol (also known as acetaminophen) induces apoptosis of tubular epithelial cells, which is correlated with up-regulation of CHOP and cleavage of procaspase-12 [53].
Antibiotic
Aminoglycosides are major nephrotoxic antibiotics that cause tubular injury. The toxicity of aminoglycosides is related to their uptake by proximal tubules and disruption of metabolism of anionic phospholipids, especially phosphoinositides [54]. Jin et al. reported that geneticin causes cleavage of m-calpain and procaspase-12 in NRK cells, indicating involvement of ER stress [55]. Peyrou et al. reported that ER stress preconditioning is effective for decreasing the toxicity of gentamicin in LLC-PK1 cells [48]. They also showed activation of the XBP1 pathway and cleavage of procaspase-12 in rat kidneys after administration with gentamicin [49].
Immunosuppressant
Calcineurin inhibitors, cyclosporine A (CsA) and tacrolimus (FK506), improve allograft survival in organ transplantation. However, chronic allograft dysfunction is the major hindrance to long-term graft survival, and nephrotoxicity of calcineurin inhibitors is one of major factors responsible for chronic allograft dysfunction [56]. Justo et al. reported that tubular cell apoptosis induced by CsA is associated with induction of CHOP [57]. We found that, in renal tubular cells, CsA and FK506 cause up-regulation of endogenous and exogenous indicators for ER stress [58]. The induction of ER stress by these drugs is reversible and observed similarly in several other non-immune cells. Systemic administration with CsA into mice also causes rapid, significant induction of ER stress in the kidney [58]. Furthermore, Peyrou et al. reported that ER stress preconditioning is effective for decreasing the toxicity of CsA in several tubular cell lines [48]. These results suggest a role of ER stress in the nephrotoxicity of calcineurin inhibitors.
Heavy metal intoxication
Several heavy metals induce renal tubular injury, and cadmium is one of the most famous nephrotoxic metals. Environmental exposure of humans to cadmium via drinking water, foods and cigarette smoke cause accumulation of this metal in a variety of organs, especially in the kidney. A typical example is Itai-itai disease in Japan, in which cadmium poisoning was caused via prolonged ingestion of industrially polluted water and rice. A characteristic clinical feature of this disease is renal insufficiency manifested by tubular injury and dysfunction. Previous investigations suggested that toxic effects of cadmium on the tubules are caused through several mechanisms, e.g., structural alterations in junctional complexes (disruption of tight and gap junctions) and cellular death caused by oxidative stress [59, 60]. In addition to these mechanisms, a pathogenic role of ER stress has been implicated in cadmium-induced apoptosis of tubular cells [61, 62]. We found that cadmium elicits ER stress in tubular cells in vitro and in vivo and consequently causes apoptosis [62]. Overexpression of ER chaperone GRP78 or ORP150 suppresses cadmium-triggered apoptosis. In response to cadmium, activation of the PERK–eIF2α pathway, the ATF6 pathway and the IRE1–XBP1 pathway is induced. In cadmium-exposed LLC-PK1 cells, the ATF6 pathway and the IRE1 pathway are proapoptotic UPR via induction of CHOP, activation of XBP1 and consequent phosphorylation of JNK. In contrast, the PERK–eIF2α pathway is anti-apoptotic and counteracts the effects of proapoptotic UPR [62]. Interestingly, ER stress is triggered not only by cadmium, but also by several other nephrotoxic metals [62], indicating a possibility that a similar mechanism is generally involved in metal-induced renal injury.
Previous studies indicated involvement of ROS in cadmium-induced renal tubular injury. For example, exposure of LLC-PK1 cells to cadmium causes generation of ROS, which is associated with a decrease in glutathione levels and consequent cellular death [63, 64]. Another report showed that cadmium-triggered apoptosis of tubular cells is inhibited by an antioxidant [65]. Recently, we demonstrated that cadmium-induced ER stress is inhibited by antioxidants. In contrast, suppression of ER stress does not attenuate cadmium-triggered oxidative stress, suggesting that ER stress is downstream of oxidative stress [66]. We also found that O2 − is selectively involved in cadmium-triggered, ER stress-mediated apoptosis through activation of the ATF6–CHOP and IRE1–XBP1–JNK pathways [66].
Renal development
As described, UPR plays important roles in the development of some professional cells. Currently, information is limited regarding whether and how ER stress and UPR are involved in the renal development, but a previous report showed that expression of GRP78 is induced in developing metanephric kidneys [67]. Further investigation will be required to clarify developmental significance of this observation and to identify roles of UPR in the kidney development.
Conclusion and perspective
Accumulating evidence indicates roles of ER stress and UPR in a wide range of renal pathophysiologies. The current knowledge on the relationship between ER stress and the kidney has been summarized in Table 2. However, the majority of previous studies provided only phenomenological evidence, e.g., induction of ER stress markers under particular pathological situations. Extensive investigation will be required to examine how ER stress and UPR contribute to individual renal pathologies. In particular, ER stress/UPR is a double-edged sword for cell survival and inflammatory responses. It is essential to disclose not only the dark side (pathological significance), but also its light side (developmental and physiological significance) of ER stress in the kidney.
In addition to elucidating the pathological relevance of ER stress and UPR to renal diseases, it should also be important to evaluate renoprotective potential of agents that modulate ER stress. Several previous reports showed that in vivo administration with 4-phenylbutyrate (4-PBA), a chemical chaperone that stabilizes protein conformation and improves the folding capacity of the ER, results in attenuation of ER stress and amelioration of ER stress-related pathologies [68, 69]. In the kidney, the therapeutic usefulness of 4-PBA has been indicated in CNF [33]. We recently reported that in vivo administration with 4-PBA significantly reduced ER stress-triggered apoptosis in the urinary bladder subjected to outlet obstruction [70]. Takizawa et al. reported that TM2002, an inhibitor of advanced glycation end products, attenuates ER stress-triggered cell death in vitro [71]. They also showed that in vivo administration with this agent significantly ameliorates ischemia-reperfusion injury in the kidney [72]. Seeking for chemical modulators of ER stress should be our next step of investigation and may open a window towards novel therapeutic approaches to kidney diseases.
References
Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci. 2001;26:504–10.
Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110:1389–98.
Yoshida H. ER stress and diseases. FEBS J. 2007;274:630–58.
Wu J, Kaufman RJ. From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ. 2006;13:374–84.
Kim R, Emi M, Tanabe K, Murakami S. Role of the unfolded protein response in cell death. Apoptosis. 2006;11:5–13.
Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol. 2002;318:1351–65.
Zong WX, Li C, Hatzivassiliou G, Lindsten T, Yu QC, Yuan J, et al. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J Cell Biol. 2003;162:59–69.
Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–65.
McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–59.
Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–6.
Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, et al. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–55.
Sekine Y, Takeda K, Ichijo H. The ASK1-MAP kinase signaling in ER stress and neurodegenerative diseases. Curr Mol Med. 2006;6:87–97.
Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor-α links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1α-mediated NF-κB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071–84.
Kaneko M, Niinuma Y, Nomura Y. Activation signal of nuclear factor-κB in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol Pharm Bull. 2003;26:931–5.
Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, et al. Translational repression mediates activation of nuclear factor-κB by phosphorylated translation initiation factor 2. Mol Cell Biol. 2004;24:10161–8.
Jiang HY, Wek SA, McGrath BC, Scheuner D, Kaufman RJ, Cavener DR, et al. Phosphorylation of the α subunit of eukaryotic initiation factor 2 is required for activation of NF-κB in response to diverse cellular stresses. Mol Cell Biol. 2003;23:5651–63.
Hayakawa K, Hiramatsu N, Okamura M, Yao J, Paton AW, Paton JC, et al. Blunted activation of NF-κB and NF-κB-dependent gene expression by geranylgeranylacetone: involvement of unfolded protein response. Biochem Biophys Res Commun. 2008;365:47–53.
Takano Y, Hiramatsu N, Okamura M, Hayakawa K, Shimada T, Kasai A, et al. Suppression of cytokine response by GATA inhibitor K-7174 via unfolded protein response. Biochem Biophys Res Commun. 2007;360:470–5.
Endo S, Hiramatsu N, Hayakawa K, Okamura M, Kasai A, Tagawa Y, et al. Geranylgeranylacetone, an inducer of the 70-kDa heat shock protein (HSP70), elicits unfolded protein response and coordinates cellular fate independently of HSP70. Mol Pharmacol. 2007;72:1337–48.
Hayakawa K, Hiramatsu N, Okamura M, Yamazaki H, Yao J, Paton AW, Paton JC, Kitamura M. Acquisition of anergy to proinflammatory cytokines in non-immune cells through endoplasmic reticulum stress response: a mechanism for subsidence of inflammation. J Immunol (in press).
Devin A, Lin Y, Yamaoka S, Li Z, Karin M, Liu Z. The α and β subunits of IκB kinase (IKK) mediate TRAF2-dependent IKK recruitment to tumor necrosis factor (TNF) receptor 1 in response to TNF. Mol Cell Biol. 2001;21:3986–94.
Rush JS, Sweitzer T, Kent C, Decker GL, Waechter CJ. Biogenesis of the endoplasmic reticulum in activated B lymphocytes: temporal relationships between the induction of protein N-glycosylation activity and the biosynthesis of membrane protein and phospholipid. Arch Biochem Biophys. 1991;284:63–70.
Zhang K, Wong HN, Song B, Miller CN, Scheuner D, Kaufman RJ. The unfolded protein response sensor IRE1α is required at 2 distinct steps in B cell lymphopoiesis. J Clin Invest. 2005;115:268–81.
Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, et al. Plasma cell differentiation requires the transcription factor XBP1. Nature. 2001;412:300–7.
Iwakoshi NN, Lee AH, Vallabhajosyula P, Otipoby KL, Rajewsky K, Glimcher LH. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP1. Nat Immunol. 2003;4:321–9.
Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–63.
Delepine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C. EIF2AK3, encoding translation initiation factor 2-α kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet. 2000;25:406–9.
Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7:1165–76.
Reimold AM, Etkin A, Clauss I, Perkins A, Friend DS, Zhang J, et al. An essential role in liver development for transcription factor XBP-1. Genes Dev. 2000;14:152–7.
Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin–Lowry syndrome. Cell. 2004;117:387–98.
Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, et al. The PERK eukaryotic initiation factor 2 α kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol. 2002;22:3864–74.
Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, et al. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–82.
Liu L, Done SC, Khoshnoodi J, Bertorello A, Wartiovaara J, Berggren PO, et al. Defective nephrin trafficking caused by missense mutations in the NPHS1 gene: insight into the mechanisms of congenital nephrotic syndrome. Hum Mol Genet. 2001;10:2637–44.
Liu XL, Doné SC, Yan K, Kilpeläinen P, Pikkarainen T, Tryggvason K. Defective trafficking of nephrin missense mutants rescued by a chemical chaperone. J Am Soc Nephrol. 2004;15:1731–8.
Ohashi T, Uchida K, Uchida S, Sasaki S, Nihei H. Intracellular mislocalization of mutant podocin and correction by chemical chaperones. Histochem Cell Biol. 2003;119:257–64.
Fujii Y, Khoshnoodi J, Takenaka H, Hosoyamada M, Nakajo A, Bessho F, et al. The effect of dexamethasone on defective nephrin transport caused by ER stress: a potential mechanism for the therapeutic action of glucocorticoids in the acquired glomerular diseases. Kidney Int. 2006;69:1350–9.
Cybulsky AV, Takano T, Papillon J, Khadir A, Liu J, Peng H. Complement C5b-9 membrane attack complex increases expression of endoplasmic reticulum stress proteins in glomerular epithelial cells. J Biol Chem. 2002;277:41342–51.
Cybulsky AV, Takano T, Papillon J, Bijian K. Role of the endoplasmic reticulum unfolded protein response in glomerular epithelial cell injury. J Biol Chem. 2005;280:24396–403.
Bek MF, Bayer M, Müller B, Greiber S, Lang D, Schwab A, et al. Expression and function of C/EBP homology protein (GADD153) in podocytes. Am J Pathol. 2006;168:20–32.
Johnson JP, Rokaw MD. Sepsis or ischemia in experimental acute renal failure: what have we learned? New Horiz. 1995;3:608–14.
Hiramatsu N, Kasai A, Du S, Takeda M, Hayakawa K, Okamura M, et al. Rapid, transient induction of ER stress in the liver and kidney after acute exposure to heavy metal: evidence from transgenic sensor mice. FEBS Lett. 2007;581:2055–9.
Hiramatsu N, Kasai A, Hayakawa K, Yao J, Kitamura M. Real-time detection and continuous monitoring of ER stress in vitro and in vivo by ES-TRAP: evidence for systemic, transient ER stress during endotoxemia. Nucleic Acids Res. 2006;34:e93.
Ueda N, Kaushal GP, Shah SV. Apoptotic mechanisms in acute renal failure. Am J Med. 2000;108:403–15.
Montie HL, Kayali F, Haezebrouck AJ, Rossi NF, Degracia DJ. Renal ischemia and reperfusion activates the eIF2α kinase PERK. Biochim Biophys Acta. 2005;1741:314–24.
Bando Y, Tsukamoto Y, Katayama T, Ozawa K, Kitao Y, Hori O, et al. ORP150/HSP12A protects renal tubular epithelium from ischemia-induced cell death. FASEB J. 2004;18:1401–3.
Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci. 2007;334:115–24.
Liu H, Bowes R. Endoplasmic reticulum stress-associated caspase-12 mediates cisplatin-induced LLC-PK1 cell apoptosis. J Am Soc Nephrol. 2005;16:1985–92.
Peyrou M, Cribb AE. Effect of endoplasmic reticulum stress preconditioning on cytotoxicity of clinically relevant nephrotoxins in renal cell lines. Toxicol In Vitro. 2007;21:878–86.
Peyrou M, Hanna PE, Cribb AE. Cisplatin, gentamicin, and p-aminophenol induce markers of endoplasmic reticulum stress in the rat kidneys. Toxicol Sci. 2007;99:346–53.
Tsutsumi S, Gotoh T, Tomisato W, Mima S, Hoshino T, Hwang HJ, et al. Endoplasmic reticulum stress response is involved in nonsteroidal anti-inflammatory drug-induced apoptosis. Cell Death Differ. 2004;11:1009–16.
Okamura M, Takano Y, Hiramatsu N, Hayakawa K, Kasai A, Yao J, Kitamura M. Suppression of cytokine response in podocytes by indomethacin: involvement of unfolded protein response (abstract). Proceedings of BMB 2007. 2007;4P-0584.
Maniglia R, Schwartz AB, Moriber-Katz S. Non-steroidal anti-inflammatory nephrotoxicity. Ann Clin Lab Sci. 1988;18:240–52.
Lorz C, Justo P, Sanz A, Subirá D, Egido J, Ortiz A. Paracetamol-induced renal tubular injury: a role for ER stress. J Am Soc Nephrol. 2004;15:380–9.
Kaloyanides GJ. Antibiotic-related nephrotoxicity. Nephrol Dial Transplant. 1994;9(Suppl 4):130–4.
Jin QH, Zhao B, Zhang XJ. Cytochrome c release and endoplasmic reticulum stress are involved in caspase-dependent apoptosis induced by G418. Cell Mol Life Sci. 2004;61:1816–25.
Williams D, Haragsim L. Calcineurin nephrotoxicity. Adv Chronic Kidney Dis. 2006;13:47–55.
Justo P, Lorz C, Sanz A, Egido J, Ortiz A. Intracellular mechanisms of cyclosporin A-induced tubular cell apoptosis. J Am Soc Nephrol. 2003;14:3072–80.
Du S, Hiramatsu N, Hayakawa K, Kasai A, Okamura M, Shimada T, et al. Novel, anti-inflammatory potential of cyclosporine A and tacrolimus (FK506) via induction of unfolded protein response (abstract). J Am Soc Nephrol. 2007;18:F-PO587.
Jeong SH, Habeebu SS, Klaassen CD. Cadmium decreases gap junctional intercellular communication in mouse liver. Toxicol Sci. 2000;57:156–66.
Thevenod F. Nephrotoxicity and the proximal tubule. Insights from cadmium. Nephron Physiol. 2003;93:87–93.
Liu F, Inageda K, Nishitai G, Matsuoka M. Cadmium induces the expression of Grp78, an endoplasmic reticulum molecular chaperone, in LLC-PK1 renal epithelial cells. Environ Health Perspect. 2006;114:859–64.
Yokouchi M, Hiramatsu N, Hayakawa K, Kasai A, Takano Y, Yao J, et al. Atypical, bidirectional regulation of cadmium-induced apoptosis via distinct signaling of unfolded protein response. Cell Death Differ. 2007;14:1467–74.
Gennari A, Cortese E, Boveri M, Casado J, Prieto P. Sensitive endpoints for evaluating cadmium-induced acute toxicity in LLC-PK1 cells. Toxicology. 2003;183:211–20.
Prozialeck WC, Lamar PC. Effects of glutathione depletion on the cytotoxic actions of cadmium in LLC-PK1 cells. Toxicol Appl Pharm. 2005;134:285–95.
Thevenod F, Friedmann JM, Katsen AD, Hauser IA. Up-regulation of multidrug resistance P-glycoprotein via NF-κB activation protects kidney proximal tubule cells from cadmium- and reactive oxygen species-induced apoptosis. J Biol Chem. 2000;275:1887–96.
Yokouchi M, Hiramatsu N, Hayakawa K, Okamura M, Du S, Kasai A, et al. Involvement of selective reactive oxygen species upstream ofproapoptotic branches of unfolded protein response. J Biol Chem. 2008;283:4252–60.
Plisov SY, Ivanov SV, Yoshino K, Dove LF, Plisova TM, Higinbotham KG, et al. Mesenchymal-epithelial transition in the developing metanephric kidney: gene expression study by differential display. Genesis. 2000;27:22–31.
Kubota K, Niinuma Y, Kaneko M, Okuma Y, Sugai M, Omura T, et al. Suppressive effects of 4-phenylbutyrate on the aggregation of Pael receptors and endoplasmic reticulum stress. J Neurochem. 2006;97:1259–68.
Qi X, Hosoi T, Okuma Y, Kaneko M, Nomura Y. Sodium 4-phenylbutyrate protects against cerebral ischemic injury. Mol Pharmacol. 2004;66:899–908.
Sawada N, Yao J, Hiramatsu N, Hayakawa K, Araki I, Takeda M, Kitamura M. Involvement of hypoxia-triggered endoplasmic reticulum stress in outlet obstruction-induced apoptosis in the urinary bladder. Lab Invest. 2008;88:553–63
Takizawa S, Izuhara Y, Kitao Y, Hori O, Ogawa S, Morita Y, et al. A novel inhibitor of advanced glycation and endoplasmic reticulum stress reduces infarct volume in rat focal cerebral ischemia. Brain Res. 2007;1183:124–37.
Izuhara Y, Nangaku M, Takizawa S, Takahashi S, Shao J, Oishi H, et al. A novel class of advanced glycation inhibitors ameliorates renal and cardiovascular damage in experimental rat models. Nephrol Dial Transplant. 2008;23:497–509.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented at the 37th Eastern Regional Meeting of the Japanese Society of Nephrology.
About this article
Cite this article
Kitamura, M. Endoplasmic reticulum stress in the kidney. Clin Exp Nephrol 12, 317–325 (2008). https://doi.org/10.1007/s10157-008-0060-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-008-0060-7