Abstract
Background/aim
No accepted therapy has been established for progressive IgA nephropathy (IgAN). The purpose of the present study was to assess low-dose steroid therapy in the treatment of patients with IgAN.
Methods
A prospective trial of low-dose steroid therapy was performed in patients with IgAN with mild histological activities. Twenty-four patients in the steroid group and 24 patients in the control group were included in this study. The initial dose of prednisolone was 0.4 mg/kgBW/day (20–30 mg/day), gradually tapered to 5–10 mg/day over 24 months. The patients with mild active inflammatory lesions were treated with prednisolone. The patients assigned to the control group were treated with dipyridamole or zilazep hydrochloride in a dose of 150 or 300 mg/day.
Results
In all of the patients studied, serum creatinine levels did not significantly change over 24 months. However, daily proteinuria significantly reduced after 24 months of steroid therapy (0.97 ± 0.75 vs. 0.31 ± 0.51 g/day, P = 0.0012), even if did not change after 24 months of anti-platelet drugs (0.89 ± 0.49 vs. 0.68 ± 0.69 g/day, P = 0.2289), respectively. In addition, the grade of hematuria significantly reduced after 24 months of steroid therapy (35.6 ± 36.3 RBC/HPF vs. 13.7 ± 28.4 RBC/HPF, P = 0.0249) and 24 months of anti-platelet drugs (30.1 ± 37.1 RBC/HPF vs. 12.4 ± 20.3 RBC/HPF, P = 0.0465), respectively. Systolic and diastolic blood pressures did not significantly change during treatment with steroid or anti-platelet drugs. Vascular changes (0.63 ± 0.73) in the steroid group were lower than those (1.08 ± 0.88) in the control group (P = 0.008).
Conclusion
Our data suggested that low-dose steroid therapy for IgAN patients with mild inflammatory lesions could reduce the amount of urinary protein excretion and prevent deterioration of renal function, provided the histological findings in the renal biopsies showed mild vascular lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since IgA nephropathy (IgAN) was first described by Berger and Hinglasis in 1968 [1], it has now been recognized as one of the most common types of primary glomerulonephritis worldwide [2]. Although the prognosis of IgAN was considered as relatively good previously, long-term observation revealed that 20–40% of IgAN patients could progress to end-stage renal disease [3]. It is of interest to confirm adequate therapeutic strategies for IgAN.
Kobayashi et al. [4] recently reported that steroid therapy has a long-term beneficial effect for stabilization of renal function in progressive IgAN with massive proteinuria of 1.0 g/day or more when patients have a preserved renal function with a creatinine clearance (Ccr) of 70 ml/min or more, although the effectiveness of steroid therapy in IgAN is controversial.
In contrast, in patients with heavy proteinuria and preserved renal function, steroid therapy was recommended [5]. In a prospective randomized multicenter study, Pozzi et al. [6] reported that steroid therapy reduced proteinuria and protected against deterioration in renal function in patients with IgAN. In addition, significant amelioration of histopathological changes was reported in IgAN patients who were receiving steroid therapy [7]. However, the ideal steroid therapy dosage for IgAN has not been determined, and it is essential to establish a gold standard steroid therapy protocol that has definite effects in preventing the progression of IgAN and has minimal toxicity.
We therefore designed a prospective, controlled trial of low-dose prednisolone therapy (0.4 mg/kgBW/day) for IgAN patients with mild inflammatory activities of renal histological characteristics and ascertained the clinical parameters that influence the effectiveness of steroid therapy.
Subjects and methods
Subjects
The diagnosis of IgAN was based on the light microscopic findings showing mesangioproliferative changes, mesangial IgA and C3 deposition in an immunofluorescence study and the presence of electron-dense deposits in the mesangial area with electron microscopy. Patients with systemic diseases, such as diabetes mellitus, collagen disease, abnormal hyper gamma globulinemia and chronic liver disease, were excluded from this study. Forty-eight patients satisfying the criteria were enrolled in the study. The present study was an open-labeled, randomized, two-group study with a parallel-group design. The institutional review board approved anonymous use of data for study. Written informed consent was obtained from all subjects. The study was conducted in compliance with the Declaration of Helsinki and Committees on Human Research. After informed consent was obtained, two doctors who did not know the histological scores randomly assigned the patients to either the steroid or control group. The doctors used two envelopes consisting of A (steroid group) or B (control group) and containing study instructions. The doctors were not involved in the care of the study patients. Twenty-four patients were assigned to the steroid group and 24 patients to the control group. Clinical data were evaluated with age at the time of renal biopsy and blood pressure, and laboratory data, including serum creatinine (S-Cr mg/dl), urinary protein excretion (U-P g/day) and urinary red blood cell counts (U-RBC/HPF) at the time of renal biopsy were also evaluated.
Patients were examined at baseline and then every 3 months. At each visit, patients were asked about clinical symptoms, possible treatment complications and drug consumption. Blood pressure was measured, and urinalysis and serum biochemistry were performed. The amount of urinary protein excretion per day was estimated at each visit. Blood pressure was usually maintained at under 140 mmHg in the systole and 90 mmHg in the diastole with a calcium channel blocker, an angiotensin-converting enzyme inhibitor (ACE-I).
Histological examination of renal biopsy specimens
All specimens were obtained with the percutaneous needle biopsy method. The specimens were fixed with 10% phosphate-buffered formalin (pH 7.2), embedded in paraffin and cut into 2-μm sections. Hematoxylin and eosin, periodic acid Schiff (PAS), silver methenamine and Masson trichrome stainings were performed for light microscopy.
Each specimen was evaluated for glomerular, interstitial and vascular changes as previously described [8]. The changes were scored semiquantitatively by two independent observers without any knowledge of the clinical data. The percentages of glomeruli exhibiting glomerular obsolescence, crescent formation and glomerular tuft adhesion to Bowman’s capsule were estimated. Mesangial cell proliferation in each patient was semiquantitatively graded into four degrees of severity: grade 0, no abnormality; grade 1, mild; grade 2, moderate; and grade 3, severe. The extent of interstitial fibrosis was semiquantitatively graded into four categories according to the proportion of fibrotic lesions to the total cortical area: grade 0, less than 5% of total cortical area; grade 1, 5–20%; grade 2, 20–40%; grade 3, more than 40%. Arterio-arteriolosclerotic changes were also evaluated using a similar grading scale: grade 0, no abnormality; grade 1, mild; grade 2, moderate; grade 3, severe.
Protocol of the treatment
This study was not an open-labeled, but randomized, two-group study with a parallel-group design. The study was prospective, controlled and performed in a single center. After informed consent was obtained, patients were assigned to either the steroid or control group. Treatment with prednisolone or anti-platelet drugs (dipyridamole or zilazep hydrochloride) was indicated in the patients who met the following histological criteria in both groups: mild inflammatory activities, presence of cellular and/or fibrocellular crescents, mesangial interposition with mononuclear cell infiltration and interstitial inflammatory cell infiltration. The patients assigned to the steroid group were initially treated with 0.4 mg/kgBW/day of prednisolone (20–30 mg/day) for the first 4 weeks, and the dose was gradually reduced to 10–20 mg on alternate days for the next 12 months, and then 5–10 mg on alternate days for a subsequent year. When the treatment was effective, alternate-day prednisolone 5–10 mg administration was continued during the next follow-up period. When the treatment was not effective, the dose was further reduced to discontinuation. The patients assigned to the control group were treated with dipyridamole or zilazep in a dose of 150 or 300 mg/day. An anti-platelet agent (dipyridamole or zilazep hydrochloride) was administrated in combination with prednisolone in all the patients in the steroid group. An angiotensin-converting enzyme inhibitor (ACE-I) was administered for the hypertensive patients (four patients in the steroid group, 20%).
Statistical analysis
Data are expressed as means ± SD. The significance of differences between groups was examined with the Student’s t test for nonpaired samples and by the χ2-test. For comparison of stratified data, χ2 test was used. Changes in serum Cr, U-P and U-RBC from baseline to last follow-up were compared between the steroid and control groups by means of t test. The evolution of clinical parameters was analyzed in both groups by repeated-measurement analysis of variance (ANOVA). When differences could be demonstrated, values were compared with the baseline using the paired-sample t test. A P value of less than 0.05 was considered statistically significant.
Results
Baseline characteristics of the 48 patients in the steroid and control groups are summarized in Table 1. The subjects in the steroid group consisted of 7 males and 17 females with a mean age of 37.9 ± 10.1 years. Prednisolone was started at an initial dose of 30 mg/day in 5 cases and 20 mg/day in 19 cases. The duration of the steroid therapy was 24 months in all patients. Daily proteinuria was more than 1.0 g/day in nine cases, of which no patients had nephrotic-range proteinuria. The remaining 15 cases showed mild proteinuria of less than 1.0 g/day. The mean of proteinuria was 0.97 ± 0.75 g/day. Four cases showed mild elevation of serum creatinine ranging from 1.21 to 1.58 mg/dl (0.92 ± 0.26 mg/dl).
The subjects in the control group consisted of 5 males and 19 females with a mean age of 38.3 ± 12.7 years. The duration of the anti-platelet therapy was 24 months in all patients. Daily proteinuria was more than 1.0 g/day in nine cases, of which no patients had nephrotic-range proteinuria. The remaining 15 cases showed mild proteinuria of less than 1.0 g/day. The mean of proteinuria was 0.90 ± 0.50 g/day. Eight cases showed mild elevation of serum creatinine ranging from 1.21 to 1.58 mg/dl (1.15 ± 0.35 mg/dl). At the time of renal biopsy, the steroid and control groups were similar in distribution of age, sex, initial clinical presentation such as U-P, U-RBC, blood pressure and treatment with ACE-I. Serum Cr levels (1.15 ± 0.35 mg/dl) in the control group were significantly higher than those (0.92 ± 0.26 mg/dl) in the steroid group (P = 0.015).
The comparison of the pathological findings of the patients in each group at the time of renal biopsy is summarized in Table 2. The percentage of glomerular obsolescence, adhesion and crescent formation was similar in both groups. No significant difference was observed in the grade of mesangial proliferation and interstitial fibrosis in both groups. Vascular changes (0.63 ± 0.73) in the steroid group was lower than those (1.08 ± 0.88) in the control group (P = 0.008).
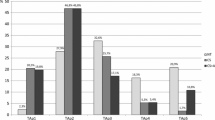
Changes in renal function, daily proteinuria, grade of hematuria and blood pressure in the 48 cases were observed for 24 months. As shown in Fig. 1, serum creatinine levels did not significantly change during treatment with steroid or anti-platelet drugs. However, daily proteinuria significantly reduced after 24 months of steroid therapy (0.97 ± 0.75 vs. 0.31 ± 0.51 g/day, P = 0.0012), even if did not change after 24 months of anti-platelet drugs (0.89 ± 0.49 vs. 0.68 ± 0.69 g/day, P = 0.2289), respectively (Fig. 2). In addition, the grade of hematuria significantly reduced after 24 months of steroid therapy (35.6 ± 36.3 RBC/HPF vs. 13.7 ± 28.4 RBC/HPF, P = 0.0249) and 24 months of anti-platelet drugs (30.1 ± 37.1 RBC/HPF vs. 12.4 ± 20.3 RBC/HPF, P = 0.0465), respectively (Fig. 3). As shown in Fig. 4, systolic and diastolic blood pressures did not significantly change during treatment with steroid or anti-platelet drugs.
In an attempt to investigate if the vascular lesions are associated with the effectiveness of steroid therapy in IgAN patients, we divided the patients who had been treated with steroids into two groups according to the time course of serum creatinine levels at the end of observation period. The definition of improvement was assessed as less than serum creatinine levels at the start of steroid therapy. The grade of arteriosclerosis was lower (0.52 ± 0.48) in the improved group (n = 16) than those (0.71 ± 0.56) in the unimproved group (n = 8) (P < 0.01).
Discussion
The main purpose of this study was to evaluate the effectiveness of low-dose steroid therapy for IgAN patients with mild inflammatory activities. The present study demonstrated that low-dose steroid therapy significantly reduced proteinuria and hematuria in association with no changes in serum creatinine levels in the IgAN patients studied. Moreover, the patients in whom renal function improved or showed no significant changes were the cases who had minor vascular lesions, such as arteriosclerosis. In the present study, the primary outcome was a change in urinary protein excretion from the base-line because it has been proven that the degree of proteinuria is one of the most important prognostic indicators in IgAN [9–11].
Pozzi et al. [6] reported that the steroid therapy for IgAN was effective when the patient met the following clinical criteria: the serum creatinine level was under 1.5 mg/dl, and the amount of urinary protein excretion was 1.0–3.5 g/day. The patients treated with steroid therapy (40–50 mg/day as an initial dose) showed a significant reduction in the amount of urinary protein excretion (2.0 ± 0.6–0.67 ± 0.5 g/day) and a higher percentage of renal survival after steroid therapy. They suggested that the effect of steroid treatment was associated with a decrease in urinary protein excretion. Our regimen using low-dose prednisolone (20–30 mg/day as an initial dose) had an antiproteinuric effect, but its effect was not sufficient to improve renal function. An insufficient dose of prednisolone in our protocol may be the reason for the discrepancy between the effects on proteinuria and the serum creatinine levels.
Ballardie and Roberts reported a controlled trial of prednisolone. They included only patients with impaired renal function (serum creatinine >1.5 mg/dl) in the study [12]. The study showed a significant effect on improving renal outcome by treating patients with prednisolone (40 mg/day as an initial dosage) and cyclophosphamide for an initial 3 months, and then azathioprine, continued for a minimum of 2 years. One patient in the treatment group developed pulmonary tuberculosis and was successfully treated. There was no evidence of other adverse effects. It was concluded that their therapy was beneficial in improving the clinical course of progressive IgAN with an acceptably low risk of side effects.
Nolin and Courteau [5] showed in their review that steroid therapy is only beneficial in selected IgAN patients with nephrotic syndrome and minor grade histological characteristics. However, patients with minor histological characteristics and IgA deposition and who clinically demonstrate nephrotic syndrome and an excellent response to steroids are supposed to have an overlapping syndrome of IgAN and lipoid nephrosis [13]. When considering the effects of steroid therapy in IgAN, we should distinguish between such patients and those with massive proteinuria caused by IgAN.
Recently, clinical studies in Japan reported that steroid therapy (40–50 mg/day as an initial dose) was beneficial for IgAN even if the disease was in the advanced stage. Tamura et al. [14] observed eight patients with impaired renal function and showed that the urinary protein excretion significantly decreased (2.57 ± 1.12–1.12 ± 0.84 g/day, P < 0.005), and the serum creatinine levels did not change (2.76 ± 1.32–2.56 ± 1.10 mg/dl) after treatment. They suggested that steroid therapy could delay the progression to end-stage renal failure. Tomiyoshi et al. [15] suggested that steroid therapy (40–50 mg/day as an initial dose) was effective for IgAN patients who showed a high percentage of cellular and fibrocellular crescents and/or segmental glomerular sclerosis, although their Ccr was under 70 ml/min. We also reported the effectiveness of steroid therapy for advanced IgAN patients with reduced renal function [16]. However, the effectiveness of low-dose steroid therapy for IgAN remains unclear.
Previously, we have reported the histological indices that predict the effectiveness and ineffectiveness of steroid therapy (40–50 mg/day as an initial dose) in IgAN [17]. According to that study, the severity of acute glomerular extracapillary lesions (cellular and/or fibrocellular crescents) predicted the possibility of proteinuria reduction, while the severity of interstitial inflammation suggested a high risk of renal dysfunction even after steroid therapy. However, the efficacy of the indication of steroid therapy for IgAN patients with mild inflammatory activities, such as crescent formation and interstitial change, has not been elucidated. To reduce the side effects of steroid therapy, we used low-dose prednisolone for IgAN patients with mild inflammatory lesions. In the present study, no significant differences were observed in the percentage of glomerular obsolescence, percent of glomerular crescents, grades of mesangial cell proliferation and the extent of interstitial fibrosis in two groups divided by the serum creatinine levels. However, the grade of arteriosclerosis was lower in the improved group than in the unimproved group, suggesting that the extent of vascular lesions, such as hyalinosis and arteriosclerosis in the renal vasculature, may be a marker for the effectiveness of low-dose steroid therapy in patients with mild inflammatory activities.
Recently, Katafuchi et al. [18] have reported a prospective, randomized, controlled trial of low-dose prednisolone therapy for IgAN patients compared with those using anti-platelet drugs. Considering changes in proteinuria from baseline, the degree of reduction in proteinuria at last follow-up was significantly lower in the steroid group than in anti-platelet group. However, kidney survival was similar in both groups. They have suggested that an insufficient dose of prednisolone may be the reason for the discrepancy between the effect on proteinuria and kidney survival. Compared with our patients studied, histological characteristics seemed to be moderate in their patients.
In conclusion, we suggest that low-dose steroid therapy for IgAN patients with mild inflammatory activities could reduce the amount of urinary protein excretion and may restore the residual renal function, if the histological findings in renal biopsies showed mild vascular lesions. Careful histological interpretation of renal biopsy specimens is necessary to determine the indication for low-dose steroid therapy in combination with the clinical evaluation of the disease activity in IgAN patients. We consider that low-dose steroid therapy is one of the beneficial strategies for IgAN patients with mild inflammatory activities during every clinical stage of IgAN. However, a randomized controlled trial is absolutely necessary to evaluate the efficacy and safety of low-dose steroid therapy for patients with IgAN with mild histological activities.
References
Berger J, Hinglais N. Les deposits intercapillarired’IgA-IgG. J Urol Nephrol. 1968;74:694–5.
D’Amico G, Minetti L, Ponticelli C, Fellin G, Ferrario F, Barbiano di Belgioioso G, et al. Prognostic indicators in idiopathic IgA mesangial nephropathy. Q J Med. 1986;59:363–78.
Koyama A, Igarashi M, Kobayashi M. Natural history and risk factors for immunoglobulin A nephropathy in Japan. Am J Kidney Dis. 1997;29:526–32.
Kobayashi Y, Hiki Y, Kokubo T, Horii A, Tateno S. Steroid therapy during the early stage of progressive IgA nephropathy. A 10-year follow-up study. Nephron. 1996;72:237–42.
Nolin L, Courteau M. Management of IgA nephropathy: evidence-based recommendations. Kidney Int Suppl. 1999;70:S56–S62.
Pozzi C, Bolasco P, Fogazzi GB, Andrulli S, Altieri P, Ponticelli C, et al. Corticosteroids in IgA nephropathy: a randomized controlled trial. Lancet. 1999;353:883–7.
Shoji T, Nakanishi I, Suzuki A, Hayashi T, Togawa M, Okada N, et al. Early treatment with corticosteroids ameliorates proteinuria, proliferative lesions, and mesangial phenotypic modulation in adult diffuse proliferative IgA nephropathy. Am J Kidney Dis. 2000;35:194–201.
Taneda S, Honda K, Nitta K, Kobayashi H, Uchida K, Yumura W, et al. Clinicopathological study of IgA nephropathy in adults: the influence of onset age on clinical and renal histological findings and on the effect of steroid therapy. Clin Exp Nephrol. 1999;3:96–103.
D’Amico G, Colasanti G, Barbiano di Belgioioso G, Fellin G, Ragni A, Egidi F, et al. Long-term follow-up of IgA mesangial nephropathy: clinico-histological study in 374 patients. Semin Nephrol. 1987;4:355–8.
Alamartine E, Sabatier JC, Guerin C, Berliet JM, Berthoux F. Prognostic factors in mesangial IgA glomerulonephritis: an extensive study with univariate and multivariate analysis. Am J Kidney Dis. 1991;1:12–19.
Donadio JV, Bergstralh EJ, Offord KP, et al. Clinical and histopathological associations with impaired renal function in IgA nephropathy. Clin Nephrol. 1994;41:65–71.
Ballardie FW, Roberts IS. Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. J Am Soc Nephrol. 2002;13:142–8.
Lai KN, Lai FM, Ho CP, Chan KW. Corticosteroid therapy in IgA nephropathy with nephritic syndrome: a long-term controlled trial. Clin Nephrol. 1986;26:174–180.
Tamura S, Ueki K, Ideura H, Tsukada Y, Maezawa A, Kawai H, et al. Corticosteroid therapy in patients with IgA nephropathy with impaired renal function. Clin Nephrol. 2001;55:192–5.
Tomiyoshi Y, Sakemi T, Ikeda Y, Ohtsuka Y, Nakamura M, Fujisaki T. Cellular crescents and segmental glomerular necrosis in IgA nephropathy are indicative of the beneficial effects of corticosteroid therapy. Internal Med. 2001;40:862–6.
Moriyama T, Honda K, Nitta K, Yumura W, Nihei H. The effectiveness of steroid therapy for patients with advanced IgA nephropathy and impaired renal function. Clin Exp Nephrol. 2004;237–42.
Honda K, Nitta K, Kobayashi H, Uchida K, Horita S, Kawashima A, et al. Clinical significance of histological grading and staging for predicting the effectiveness of steroid therapy in IgA nephropathy. Clin Exp Nephrol. 2000;4:241–50.
Katafuchi R, Ikeda K, Mizumasa T, Tanaka H, Ando T, Yanase T, et al. Controlled, prospective trial of steroid treatment in IgA nephropathy: a limitation of low-dose prednisolone therapy. Am J Kidney Dis. 2003;41:972–83.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Koike, M., Takei, T., Uchida, K. et al. Clinical assessment of low-dose steroid therapy for patients with IgA nephropathy: a prospective study in a single center. Clin Exp Nephrol 12, 250–255 (2008). https://doi.org/10.1007/s10157-008-0036-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-008-0036-7