Abstract
Acinetobacter baumannii is an important cause of postneurosurgical meningitis. The emergence of carbapenem-resistant strains in this setting has caused a therapeutic challenge. We investigated the clinical implications of postneurosurgical meningitis caused by carbapenem-resistant A. baumannii. In this study, we retrospectively reviewed the medical records of patients more than 16 years of age with A. baumannii meningitis that developed after a neurosurgical procedure at five university-affiliated hospitals between January 2005 and May 2011. Of 40 cases identified, 22 (55.0 %) were caused by carbapenem-resistant strains. Of those evaluable 36 patients with A. baumannii meningitis, 14 (38.9 %) died of meningitis. Meningitis-related mortality was significantly related to carbapenem resistance (59.1 % versus 7.1 %; P = 0.002). In patients with meningitis caused by carbapenem-resistant A. baumannii, colistimethate-containing regimens (4/13 versus 7/9; P = 0.040), intrathecal or intraventricular (IT/IVR) administration of antibiotics (2/13 versus 8/9; P = 0.001), and combined intravenous and IT/IVR therapy (2/13 versus 6/9; P = 0.026) were significantly associated with cure. This study shows that use of colistimethate and combined systemic and local administration of antibiotics should be considered for the treatment of meningitis caused by carbapenem-resistant A. baumannii.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acinetobacter baumannii is often responsible for a wide spectrum of nosocomial infections and is usually multidrug resistant [1]. Recent studies have documented Acinetobacter species as the leading gram-negative cause of nosocomial meningitis, especially in neurosurgical patients [2, 3]. Nosocomial meningitis caused by A. baumannii is difficult to treat because the choice of antibiotics is restricted by multidrug resistance and limited penetration through the blood–brain barrier [4]. Although carbapenems were major therapeutic options in the treatment of A. baumannii meningitis, the increasing prevalence of carbapenem-resistant A. baumannii (CRAB) has caused a serious therapeutic challenge [3, 5, 6]. The aim of this study was to assess clinical implications of postneurosurgical meningitis caused by CRAB.
Patients and methods
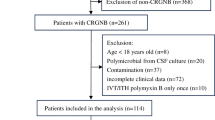
To identify patients with a positive cerebrospinal fluid (CSF) culture for A. baumannii, we reviewed CSF culture results in the microbiology laboratory database at five university-affiliated hospitals in Republic of Korea. Patients more than 16 years of age with a diagnosis of nosocomial A. baumannii meningitis occurring after undergoing a neurosurgical procedure between January 2005 and May 2011 were enrolled. Data on demographic and clinical characteristics, CSF findings, antimicrobial susceptibilities, antibiotic treatment, and outcomes were abstracted from the medical record. This study was approved by the institutional review board at each hospital.
Antimicrobial susceptibility testing was performed using the automated Vitek-2 system (bioMérieux, France) at each hospital. Susceptibility testing to rifampicin and tigecycline was not done. Antimicrobial susceptibility of A. baumannii was evaluated according to the CLSI guidelines [7]. Carbapenem resistance was defined as in vitro resistance to imipenem. Isolates showing intermediate resistance were considered to be resistant in this study.
A. baumannii meningitis was defined according to the CDC criteria: the isolation of A. baumannii from CSF culture and fever >38 °C in the absence of another cause and any of the following: increased white cells (>10/mm3 with >50 % polymorphonuclear leukocytes), increased protein (>45 mg/dl), and/or decreased glucose levels (<40 mg/dl) in the CSF [8]. Parenteral antimicrobial therapy was considered as appropriate if it included at least one antimicrobial agent that was effective in vitro according to drug susceptibility testing and was administrated by the dosage recommended in the IDSA guideline [9] or the product information [10]. Intravenous (IV) monotherapy of an aminoglycoside was considered inappropriate because of the low penetration into the CSF. Intrathecal (IT) or intraventricular (IVR) treatment with an aminoglycoside or colistimethate was classified as appropriate if the isolate was susceptible to the drugs in vitro [11].
To assess the outcome, patients were followed up until discharge or death in the hospital.
Mortality was classified as meningitis related if death was caused by meningitis or its complications, but not if it was caused by a preexisting serious illness after bacteriological cure and clinical recovery from meningitis [12]. Student’s t test or the Mann–Whitney U test was used to compare continuous variables and χ 2 or Fisher’s exact test to compare categorical variables. A P value less than 0.05 was considered statistically significant.
Results
Forty cases of postneurosurgical A. baumannii meningitis from 40 different patients were identified. Twenty-two isolates (55.0 %) were resistant to imipenem (including 6 with intermediate resistance). These CRAB isolates were resistant to many antibiotics: 100 % to ceftazidime and cefepime, 95.5 % (21/22) to ciprofloxacin and gentamicin, 90.0 % (9/10) to ampicillin/sulbactam, and 35.7 % (5/14) to amikacin. Three CRAB isolates were multiresistant to imipenem, amikacin, and ciprofloxacin. All 18 CRAB isolates tested were susceptible to colistin.
All patients had undergone one or more neurosurgical procedures. Twenty-two patients (55.0 %) had intracranial devices, including an external ventricular drain (15 patients), a lumbar drain (4), and a ventriculoperitoneal shunt (4), before the diagnosis of this infection. Demographic and clinical characteristics including primary neurosurgical diagnosis, nonneurosurgical condition, prior surgical procedure, interval between the last neurosurgery and meningitis, CSF profile, and Glasgow Coma Scale at the diagnosis of meningitis were not significantly different between patients with meningitis caused by carbapenem-susceptible A. baumannii (CSAB) and those with CRAB meningitis. Excluding 4 patients with CSAB meningitis who were transferred to other hospitals, 22 (61.1 %) died during follow-up. Of those, 14 (38.9 %) deaths were directly attributable to meningitis, and the remaining 8 died of other conditions, including nosocomial pneumonia (5 patients), intracranial hemorrhage (2), and acute myocardial infarction (1), despite microbiological eradication in CSF. Meningitis-related mortality was higher in patients with CRAB meningitis than in those with CSAB meningitis (59.1 % versus 7.1 %; P = 0.002).
Of 22 patients with CRAB meningitis, 11 received IV or IT/IVR colistimethate as follows: 5 patients with IV + IT/IVR colistimethate, 2 with IV followed by IT/IVR colistimethate because of the development of nephrotoxicity, 2 only with IV colistimethate, 1 with oral rifampicin and IV + IT/IVR colistimethate, and 1 with oral rifampicin, IV colistimethate, and IT/IVR amikacin. Ten patients were treated with IT/IVR administration of antibiotics as follows: 8 patients with colistimethate and 2 with an aminoglycoside (amikacin and gentamicin) in combination with other parenteral antibiotics (colistimethate and sulbactam). IV doses of administered colistimethate ranged between 240 and 300 mg (colistin base activity) per day, and IT/IVR doses were uniformly 10 mg per course in our study patients. Toxicity related to local administration was not reported in patients treated with IT/IVR antibiotics.
Of those patients with CRAB meningitis, no significant differences in clinical characteristics were found between patients who died of meningitis and those who were cured (Table 1). Mortality was significantly lower in patients who received colistimethate-containing regimens (4/13 versus 7/9; P = 0.040), IT/IVR administration of antibiotics (2/13 versus 8/9; P = 0.001), and combined IV and IT/IVR therapy (2/13 versus 6/9; P = 0.026).
Discussion
Acinetobacter baumannii meningitis had a high mortality in this study. Meningitis-related mortality in patients with A. baumannii meningitis was reported at 20 % to 33.3 % in previous studies [5, 13], a similar rate to that found in our study (38.9 %). Higher mortality rates, from 71.4 % to 72.7 %, although these figures were overall rates, have been observed in hospitals where large numbers of CRAB were isolated [6, 14]. In this study mortality was higher in patients with CRAB meningitis than in those with CSAB meningitis. A previous study revealed that all 9 patients with CRAB meningitis died whereas 11 of 19 patients with CSAB meningitis did [6]. These findings suggest that mortality in patients with A. baumannii meningitis is associated with carbapenem resistance.
Our study has the following limitations. First, this is a retrospective study and does not have a sufficient number of patients to provide adequate statistical power. Second, we did not determine whether the mortality was related to the inappropriate antimicrobial therapy. We could not categorize study patients according to the appropriateness of antimicrobial therapy because there was a high variability in the prescribed antimicrobial regimens, duration of therapy, and even start time of appropriate therapy. Similar findings were also observed in another study [5]. Antibiotic regimens were often not described in detail in previous studies [6, 14]. Nevertheless, inappropriate antimicrobial therapy for A. baumannii meningitis was associated with mortality [6, 14]. One study demonstrated that all patients who survived A. baumannii meningitis had received appropriate therapy, in contrast to only 69.2 % of those who did not survive [14]. Other risk factors for mortality in A. baumannii meningitis included the presence of intracranial devices [5].
This study found that use of colistimethate-containing regimens and IT/IVR administration of antibiotics were significantly associated with cure in patients with CRAB meningitis, which was consistent with a recent retrospective study [5]. In that study, none of eight patients with CRAB meningitis treated with combined IV and IT colistimethate died [5]. Colistimethate is the drug of choice for the treatment of CRAB infections. Available data indicate that the penetration of colistimethate and colistin into CSF is poor, even in patients with inflamed meninges [15]. Therefore, IT or IVR administration of colistimethate is usually combined with the systemic colistimethate therapy for CRAB meningitis [15, 16]. Toxicity potentially related to local administration of polymyxins was often noted, but no irreversible toxicity was reported [16]. These findings suggest that combination of IV and IT/IVR colistimethate is a useful option in the treatment of A. baumannii meningitis [5, 17]. A recent review recommended combination therapy with IV colistimethate plus IT/IVR aminoglycoside for the treatment of CRAB meningitis [4].
In summary, CRAB meningitis showed higher mortality compared with CSAB meningitis. Use of colistimethate and combined systemic and local administration of antibiotics should be considered for the treatment of this infection.
References
Cai Y, Chai D, Wang R, Liang B, Bai N. Colistin resistance of Acinetobacter baumannii: clinical reports, mechanisms and antimicrobial strategies. J Antimicrob Chemother. 2012;67:1607–15.
van de Beek D, Drake JM, Tunkel AR. Nosocomial bacterial meningitis. N Engl J Med. 2010;362:146–54.
Kim HI, Kim SW, Park GY, Kwon EG, Kim HH, Jeong JY, et al. The causes and treatment outcomes of 91 patients with adult nosocomial meningitis. Korean J Intern Med. 2012;27:171–9.
Kim BN, Peleg AY, Lodise TP, Lipman J, Li J, Nation R, et al. Management of meningitis due to antibiotic-resistant Acinetobacter species. Lancet Infect Dis. 2009;9:245–55.
Rodriguez Guardado A, Blanco A, Asensi V, Perez F, Rial JC, Pintado V, et al. Multidrug-resistant Acinetobacter meningitis in neurosurgical patients with intraventricular catheters: assessment of different treatments. J Antimicrob Chemother. 2008;61:908–13.
Metan G, Alp E, Aygen B, Sumerkan B. Acinetobacter baumannii meningitis in post-neurosurgical patients: clinical outcome and impact of carbapenem resistance. J Antimicrob Chemother. 2007;60:197–9.
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 21st informational supplement M100–S21. Wayne: CLSI; 2011.
Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–40.
Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267–84.
Coly-Mycin parenteral package insert. Bristol, TN: Monarch Pharmaceuticals; 2005.
Tangden T, Enblad P, Ullberg M, Sjolin J. Neurosurgical gram-negative bacillary ventriculitis and meningitis: a retrospective study evaluating the efficacy of intraventricular gentamicin therapy in 31 consecutive cases. Clin Infect Dis. 2011;52:1310–6.
Durand ML, Calderwood SB, Weber DJ, Miller SI, Southwick FS, Caviness VS Jr, et al. Acute bacterial meningitis in adults: a review of 493 episodes. N Engl J Med. 1993;328:21–8.
Siegman-Igra Y, Bar-Yosef S, Gorea A, Avram J. Nosocomial Acinetobacter meningitis secondary to invasive procedures: report of 25 cases and review. Clin Infect Dis. 1993;17:843–9.
Tuon FF, Penteado-Filho SR, Amarante D, Andrade MA, Borba LA. Mortality rate in patients with nosocomial Acinetobacter meningitis from a Brazilian hospital. Braz J Infect Dis. 2010;14:437–40.
Imberti R, Cusato M, Accetta G, Marino V, Procaccio F, Del Gaudio A, et al. Pharmacokinetics of colistin in cerebrospinal fluid after intraventricular administration of colistin methanesulfonate. Antimicrob Agents Chemother. 2012;56:4416–21.
Falagas ME, Bliziotis IA, Tam VH. Intraventricular or intrathecal use of polymyxins in patients with gram-negative meningitis: a systematic review of the available evidence. Int J Antimicrob Agents. 2007;29:9–25.
Khawcharoenporn T, Apisarnthanarak A, Mundy LM. Intrathecal colistin for drug-resistant Acinetobacter baumannii central nervous system infection: a case series and systematic review. Clin Microbiol Infect. 2010;16:888–94.
Acknowledgments
This work was supported by the 2010 Inje University research grant.
Conflict of interest
We have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Moon, C., Kwak, Y.G., Kim, BN. et al. Implications of postneurosurgical meningitis caused by carbapenem-resistant Acinetobacter baumannii . J Infect Chemother 19, 916–919 (2013). https://doi.org/10.1007/s10156-013-0608-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10156-013-0608-7