Abstract
Healthcare-associated pneumonia (HCAP) is a new category that is essential in the present aging society. Knowing the different characteristics and outcomes between patients with HCAP and community-acquired pneumonia (CAP) would help physicians manage and treat HCAP patients. Although HCAP is thought to be heterogeneous in regions, there are no reports from a metropolitan area in Japan. We retrospectively reviewed the clinical findings of all consecutive pneumonia patients who required hospitalized care in our hospital between April 2006 and March 2010. There were 184 (35.0%) patients with HCAP and 342 (65.0%) patients with CAP. Previous hospitalization within 90 days of the infection was the most common criterion for HCAP (63.0%). HCAP patients were significantly older than CAP patients (82.5 vs. 70.0 years, P < 0.001). The percentage of patients with poor functional status was higher in HCAP than CAP (64.0% vs. 26.6%, P < 0.001). Hospital mortality was significantly higher in HCAP patients than in CAP patients (15.8% vs. 5.0%, P < 0.001). Low levels of serum albumin (odds ratio, 0.126; 95% CI, 0.025–0.640; P = 0.012) and high scores in the ADROP (age, dehydration, respiratory failure, orientation, and blood pressure) system (odds ratio, 2.846; 95% CI, 1.449–5.587; P = 0.002) were the risk factors for HCAP mortality. In conclusion, patients with HCAP have different epidemiological characteristics compared with those with CAP in a metropolitan area of Japan. Outcomes and risk factors for mortality of patients with HCAP included poor nutritional status and high severity scores on the pneumonia severity scoring system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pneumonias have traditionally been classified as community-acquired pneumonia (CAP) and hospital-acquired pneumonia (HAP) [1]. This distinction is important to guide the diagnosis and treatment of patients with pneumonia.
Healthcare-associated pneumonia (HCAP) is a new category that has been documented in the 2005 American Thoracic Society (ATS) and Infectious Disease Society of America (IDSA) guidelines [2]. However, reported characteristics of patients with HCAP were controversial among the reports. The ATS/IDSA guidelines state that the epidemiology of HCAP is similar to that of HAP. They also state that the patients with HCAP should receive empirical therapy directed at multidrug-resistant (MDR) pathogens [2]. Other reports from the United States have documented very similar results about the epidemiology and the treatment strategy of HCAP [3–5]. The British Thoracic Society guidelines state there is no difference in the distribution of causative pathogens between patients with HCAP and elderly patients with CAP. They also state that patients with HCAP should be treated by using the same classes of antibiotics as those with CAP, although the definitions of HCAP are somewhat different from reports from the United States [6, 7]. These differences of opinion are thought to result from the heterogeneity of HCAP, including regional differences. Further data on HCAP are required.
There are no guidelines for HCAP in Japan, although the Japanese Respiratory Society (JRS) is preparing a new category named nursing- and healthcare-associated pneumonia. Guidelines from the United States may not be relevant to the Japanese population and healthcare system. The aim of this study was to clarify the differences in clinical characteristics between patients with HCAP and CAP and to investigate the prognostic factors of HCAP in a metropolitan area in Japan.
Materials and methods
Study design
With the agreement of the Committee for Ethics of Tokyo Metropolitan Hiroo General Hospital, we retrospectively reviewed the clinical findings of patients with pneumonia who had been hospitalized at the Department of Respiratory Medicine at Tokyo Metropolitan Hiroo General Hospital between April 2006 and March 2010. Our hospital is a 476-bed community hospital with an emergency room and is located in a central urban region of Japan. All the patients or their next of kin were informed at the time of hospitalization that the medical chart might be used for later statistical analysis and gave their consent.
Definitions
Pneumonia was defined as the presence of a new infiltrate on chest radiograph plus one or more of the following: fever (temperature ≥38.0°C) or hypothermia (temperature <35.0°C); new-onset cough with or without sputum production; pleuritic chest pain; dyspnea; and altered breath sounds on auscultation [8]. HCAP and CAP were defined according to ATS/IDSA guidelines [2]. HCAP included patients with any of the following: (1) previous hospitalization for a minimum of 2 days in the past 90 days; (2) residence in a nursing home or long-term care facility; (3) received intravenous chemotherapy or home wound care in the past 30 days; (4) receiving outpatient hemodialysis or peritoneal dialysis; or (5) had a family member with an MDR pathogen.
Clinical, microbiological, and severity evaluation
We compared HCAP and CAP in terms of demographic information, comorbidities, results of laboratory findings, disease severity, and outcomes. Additionally, the results of microbiological studies, such as sputum culture, tracheal aspiration culture, or bronchoalveolar lavage fluid culture, within 48 h of hospitalization, were compared between the two groups. These samples were cultured semiquantitatively, using Micro Scan Walk Away 40 Plus (Siemens Healthcare Diagnostic, USA). The length of hospital stay, initial treatment failure, and survival were also evaluated. Initial treatment failure was defined when the patient died during the treatment, or when the antibiotics had no effect and were switched to other antibiotics, or broader-spectrum antibiotics were added. To stratify patients into risk class, we used the prediction rule calculated according to the ADROP (age, dehydration, respiratory failure, orientation disturbance, and low blood pressure) scoring system for CAP and the IROAD (immunodeficiency, respiratory failure, orientation disturbance, age, and dehydration) scoring system for HAP proposed by the Japanese Respiratory Society [9, 10].
Data analysis
The statistical significance of differences between groups was examined using the Chi-square test or Mann–Whitney’s U test. Multiple logistic regression analysis was used to assess the role of several variables as risk factors for mortality. Statistical significance at a P value less than 0.05 was used for all analyses (SPSS 2001; SPSS, Chicago, IL, USA).
Results
Patient characteristics
Among the 526 patients who underwent evaluation during the study period, there were 184 patients with HCAP (35.0%) and 342 patients with CAP (65.0%). The background information of patients with HCAP is shown in Table 1. Of the HCAP patients, 116 (63.0%) had been hospitalized for at least 2 days within the past 90 days; 81 (44.0%) had resided in a nursing home or long-term care facility; 43 had resided in a special nursing home for the elderly, 17 in a paid home for the aged, 14 in a geriatric health services facility, 4 in a group home for elderly patients with dementia, 1 in sanctuary facilities for women, 1 in support facilities for handicapped persons, and 1 in facilities for patients with mental disorders. Seventy-nine (42.9%) had received home infusion therapy including antibiotics or home wound care in the past 30 days. No patients received outpatient hemodialysis or peritoneal dialysis, and none had a family history of MDR pathogen infection.
The demographic and clinical data of patients with HCAP and CAP are presented in Table 2. The mean age was 74.4 years (range, 18–100 years). Patients with HCAP were significantly older than those with CAP [82.5 (range, 18–100) vs. 70.0 (range, 36–99) years; P < 0.001]. Gender difference was not statistically significant between the two groups. Body height, body weight, and body mass index were higher in CAP.
Patients with poor functional status were defined as being bedridden or those who used a wheelchair and had difficulty walking. Percentage of patients with poor functional status was higher in HCAP than CAP (64.0% vs. 26.6%, P < 0.001), although no data were available about the activities of daily living for 49 patients. More patients with HCAP were receiving enteral feeding (14.7% vs. 4.7%, P < 0.001), and aspiration pneumonia was significantly more common in HCAP patients (50.5% vs. 17.5%, P < 0.001).
Of 184 patients, 166 patients (90.2%) with HCAP had comorbidities; the most frequently encountered comorbid conditions were respiratory diseases (44.0%), dementia (48.9%), and cerebrovascular disease (40.2%). A significantly higher percentage of patients with HCAP had cerebrovascular disease and dementia compared to those with CAP (40.2% vs. 16.1%, P < 0.001; 48.9% vs. 15.2%, P < 0.001, respectively).
Previous treatment of antibiotics were performed more frequently in 95 (27.7%) patients with CAP than in 27 (14.6%) patients with HCAP (P = 0.001). In both groups, however, previous treatment with antibiotics had no effect on the clinical outcome.
Laboratory findings at admission are presented in Table 3. Blood urea nitrogen was higher in HCAP patients than in CAP patients (24.1 ± 16.7 vs. 19.4 ± 12.1 mg/dl, P < 0.001). Hemoglobin (11.6 ± 1.9 vs. 12.6 ± 2.3, P < 0.001), hematocrit (35.1 ± 6.0 vs. 37.4 ± 5.4, P < 0.001), serum C-reactive protein (CRP) (10.7 ± 8.9 vs. 14.2 ± 10.8, P < 0.001), and serum albumin (3.1 ± 0.5 vs. 3.5 ± 0.6, P < 0.001) were significantly lower in HCAP patients than in CAP patients.
Clinical parameters for severity index and scores of the ADROP system and the IROAD system are shown in Table 4. Compared with CAP patients, HCAP patients had more severe conditions than CAP patients according to the ADROP system and the IROAD system (average ADROP score, 2.4 ± 1.2 in HCAP vs. 1.6 ± 1.1, P < 0.001; average IROAD score, 1.3 ± 1.1 in HCAP vs. 2.0 ± 1.2 in CAP, P < 0.001).
Clinical outcomes
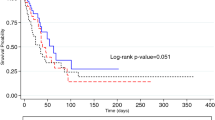
Selection, duration, and administration of antibiotic treatment were decided by the medical team in charge and were carried out according to the JRS guideline for CAP [9]. Table 5 shows the initial antibiotic treatments and clinical outcomes. Patients with HCAP received monotherapy with beta-lactams, including carbapenem, more frequently than did patients with CAP (Table 5). The failure rates of initial treatment of patients with HCAP were higher and the mean length of hospital stay of patients with HCAP was significantly longer than those of CAP patients (35.% vs. 23.4%, P = 0.002; 26.0 ± 25.9 vs. 17.2 ± 22.8 days, P < 0.001, respectively). Duration of intravenous antibiotic use in patients with HCAP was longer than that in patients with CAP (12.5 ± 16.6 vs. 9.1 ± 7.9 days, P = 0.002). Moreover, after the finish of intravenous antibiotics treatment, patients with HCAP stayed in the hospital longer than did patients with CAP (13.6 ± 20.3 days vs. 8.1 ± 18.9 days, P = 0.002). Hospital mortality was significantly higher in patients with HCAP than in those with CAP (15.8% vs. 5.0%, P < 0.001). The percentage of readmission following 30 or 90 days after leaving the hospital was higher in patients with HCAP (16.1% vs. 4.3%, P < 0.001; 25.2% vs. 9.2%, P < 0.001, respectively).
Bacteriological findings
Samples obtained from respiratory tracts were investigated. Microbiological evaluation was performed in 454 patients [294 (86.8%) in CAP vs. 160 (87.0%) in HCAP]. The distribution of isolated microorganisms varied among the two groups (Table 6). Staphylococcus aureus was the dominant pathogen (31.0% in HCAP, 20.4% in CAP), and its subtypes methicillin-sensitive S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA) were isolated from patients with HCAP and CAP. The isolation rate of MRSA in HCAP patients was significantly higher than that in CAP patients. The isolation rate of MRSA in patients from whom S. aureus were isolated was higher in patients with HCAP than that in patients with CAP (64.9% in HCAP vs. 24.2% in CAP). The isolation rate of Streptococcus pneumoniae in patients with HCAP was lower than that in patients with CAP (4.9% in HCAP vs. 14.3% in CAP, P = 0.001). Additionally, the isolation rate of Corynebacterium sp. was significant higher in patients with HCAP.
Risk factors for mortality in patients with HCAP
We evaluated the differences between survivors and nonsurvivors in HCAP patients (Table 7). Compared to survivors, nonsurvivors showed lower body mass index (18.7 ± 4.0 in survivors vs. 16.0 ± 2.9 in nonsurvivors, P = 0.017), higher levels of blood urea nitrogen (21.5 ± 10.6 vs. 37.9 ± 30.9 mg/dl, P < 0.001), higher levels of CRP (10.0 ± 8.2 vs. 14.4 ± 11.8 mg/dl, P = 0.016) lower levels of serum albumin (3.2 ± 0.5 vs. 2.7 ± 0.5 g/dl, P < 0.001), and higher scores for ADROP and IROAD (2.2 ± 1.0 vs. 3.3 ± 1.1, P < 0.001; 1.8 ± 1.0 vs. 3.1 ± 1.1, P < 0.001, respectively).
To detect the risk factors for mortality in HCAP patients, we examined the odds ratio (OR) using multiple logistic regression analysis. Low levels of serum albumin [OR, 0.126, 95% confidence interval (CI), 0.025–0.640; P = 0.012] and high scores for ADROP (OR, 2.846, 95% CI, 1.449–5.587; P = 0.002) were associated with increased mortality in patients with HCAP.
Discussion
This retrospective study showed that characteristics of patients with HCAP were different from those of patients with CAP including epidemiology, microbiology, and outcomes and that the risk factors for mortality of patients with HCAP included high severity scores and poor nutritional status.
To our knowledge, several major studies have investigated the differences in the characteristics of patients with HCAP and CAP: four from Japan [11–14], two from the United States [3–5], and one each from the UK [7], Spain [15], Italy [16], and Korea [17]. To discuss the characteristics of patients with HCAP, it is important to compare the patient selection in each report, because HCAP is thought to be heterogeneous in regions where there are different proportions of elderly patients and differences in the healthcare system. Based on world population prospects by the United Nations [18], the proportion of population aged over 65 years in 2010 varied worldwide: 13.0% in United States, 16.6% in UK, 17.2% in Spain, 20.4% in Italy, and 22.6% in Japan. In addition, in Japan all citizens have the same health insurance and can receive health care impartially, a distinctive characteristic of the reports from Japan. In the present study, the ratio of HCAP is 35.0%. Although this ratio is slightly lower than those of the previous reports from Japan [11, 12], those studies were conducted in relatively rural regions. The present report is the first study conducted in an urban region of Japan where the population over 65 years of age represents only 20.2% of the population [19], as compared to 23.1% of the total Japanese population in 2011 [20].
Patients with HCAP are heterogeneous for being defined as having HCAP. The present study shows that previous hospitalization within 90 days of the infection was the most common criterion for HCAP (63.0%); residence in a nursing home or long-term care facility was observed in 44.0% of the present patients. The components of patients with HCAP differ from region to region in one nation (Table 8) [11, 12, 14]. The present results might reflect the features of an urban area in Japan, except that our data included no patients receiving hemodialysis or peritoneal dialysis because our hospital has no dialysis equipment.
In the present study, patients with HCAP were older, often had poor functional status and comorbidities, and frequently received enteral feedings, compared to patients with CAP. Moreover, patients with HCAP more frequently had aspiration pneumonia. These characteristics of HCAP are consistent with previous reports from Japan [11–14] and other countries [3, 5, 7, 15, 16, 21].
The major goals of evidence-based guidelines for the management of any kind of pneumonia emphasize early administration of appropriate antibiotics at adequate doses. It was reported that HCAP is included in the spectrum of HAP and VAP, and that patients with HCAP need therapy for MDR pathogens [2]. Brito and Niederman [4] proposed an algorithm for antibiotic therapy of HCAP that divided patients into four groups based on assessment of severity of illness and the presence of risk factors for MDR pathogens. Ewig et al. [22] emphasized that the concept of HCAP contributed to confusion and potentially led to overtreatment. In our study, S. aureus was the most frequently isolated microorganism in both HCAP and CAP, and the isolation rate of MRSA in HCAP patients was significantly higher than in CAP patients. The frequently isolation of S. aureus, especially MRSA, shows that many of these may not be the causative organisms, but rather the colonized organisms. Therefore, an association between the isolation of MDR pathogens and mortality of HCAP patients was not found in the present study. However, the failure rates of initial treatment of HCAP patients were higher than those of CAP patients. These results may indicate that physicians should pay particular attentions to the initial treatment of patients with HCAP.
In the present study, low levels of serum albumin were also associated with HCAP mortality. In previous reports [23, 24], hypoalbuminemia was a predictive factor for poor prognosis in several comorbid conditions and the older patient population. This simple marker may be useful for clinical care and risk adjustment.
Several important limitations of this investigation should be noted. First, the data were retrospectively collected from a single institution. Second, many CAP patients were treated as outpatients. Also, the present study included only a part of the CAP patients. This is an important limitation that reduced the number of patients with CAP and affected the characteristics of CAP patients.
JRS is preparing the category of nursing and healthcare-associated pneumonia. However, we think studies from Japan using the HCAP category are still necessary for international coordination. We used the HCAP category intentionally.
In summary, HCAP was different from CAP in epidemiology, microbiology, and outcome. The risk factors of mortality for patients with HCAP included high severity score and poor nutritional status. To improve mortality in patients with HCAP, physicians may need to accurately evaluate severity and nutritional status. We believe that our results reflect the clinical features of patients with HCAP in urban areas of Japan.
References
Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell D, Dean NC, et al. Infectious Disease Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27–72.
American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416.
Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005;128:3854–62.
Brito V, Niederman MS. Health-care-associated pneumonia is a heterogeneous disease, and all patients do not need the same broad-spectrum antibiotic therapy as complex nosocomial pneumonia. Curr Opin Infect Dis. 2009;22:316–25.
Micek ST, Kollef KE, Reichley RM, Roubinian N, Kollef MH. Health care-associated pneumonia and community-acquired pneumonia: a single-center experience. Antimicrob Agents Chemother. 2007;51:3568–73.
Lim WS, Baudouin SV, George RC, Hill AT, Jamieson C, Le Jeune I, et al. Pneumonia Guidelines Committee of the BTS Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64(suppl III):iii1–55.
Lim WS, Macfarlane JT. A prospective comparison of nursing home acquired pneumonia with community acquired pneumonia. Eur Respir J. 2001;18:362–8.
Carratala J, Fernandez-Sabe N, Ortega L, Castellsague X, Roson B, Dorca J, et al. Outpatient care compared with hospitalization for community-acquired pneumonia: a randomized trial in low risk patients. Ann Intern Med. 2005;142:165–72.
The Committee for the Japanese Respiratory Society Guidelines in the Management of Respiratory Infections. The Japanese Respiratory Society guidelines for the management of community-acquired pneumonia in adults. Respirology 2006;11:S1–S133.
The Committee for the Japanese Respiratory Society Guidelines in the Management of Respiratory Infections. The Japanese Respiratory Society guidelines for the management of hospital-acquired pneumonia in adults. Respirology 2009;14:S1–S71.
Shindo Y, Sato S, Maruyama E, Ohashi T, Ogawa M, Hashimoto N, et al. Health-care-associated pneumonia among hospitalized patients in a Japanese community hospital. Chest. 2009;135:633–40.
Seki M, Hashiguchi K, Tanaka A, Kosai K, Kakugawa T, Awaya Y, et al. Characteristics and disease severity of healthcare-associated pneumonia among patients in a hospital in Kitakyushu, Japan. J Infect Chemother 2010. doi:10.1007/s10156-010-0127-8.
Maruyama T, Niederman MA, Kobayashi T, Kobayashi H, Takagi T, D’Alessandro-Gabazza CN, et al. A prospective comparison of nursing home-acquired pneumonia with hospital-acquired pneumonia in non-intubated elderly. Respir Med. 2008;102:1287–95.
Yamagishi Y, Mikamo H. A retrospective study of health care-associated pneumonia patients at Aichi Medical University hospital. J Infect Chemother 2011. doi:10.1007/s10156-011-0252-z.
Carratala J, Mykietiuk A, Fernandez-Sabe N, Suarez C, Dorca J, Verdaguer R, et al. Health care-associated pneumonia requiring hospital admission. Arch Intern Med. 2007;167:1393–9.
Venditti M, Falcone M, Corrao S, Licata G, Serra P, and the Study Group of Italian Society of Internal Medicine. Outcomes of patients hospitalized with community-acquired, health care-associated, and hospital-acquired pneumonia. Ann Intern Med 2009;150:19–26.
Park HK, Song JU, Um SW, Koh WJ, Suh GY, Chung MP, et al. Clinical characteristics of health care-associated pneumonia in a Korean teaching hospital. Respir Med. 2010;104:1729–35.
United Nations Population Division, World Population Prospects: The 2008 Revision Populations Database, 11 March 2009 Updated. http://esa.un.org/unpp/index.asp?panel=2. Accessed 6 February 2011.
Statistics Division Bureau of General Affairs, 31 January 2011 Updated. Statistics of Tokyo. http://www.toukei.metro.tokyo.jp/index.htm. Accessed 6 February 2011.
Ministry of Internal Affairs and Communications, Statistics Bureau, Director-General for Policy Planning(Statistical Standards)and Statistical Research and Training Institute, 10 September 2009 Updated. http://www.stat.go.jp/data/topics/topi411.htm. Accessed 6 February 2011.
Rello J, Luján M, Gallego M, Valles J, Belmonte Y, Fontanals D, et al. Why mortality is increased in health-care-associated pneumonia. Lessons from pneumococcal bacteremic pneumonia. Chest. 2010;137:1138–44.
Ewig S, Welte T, Chastre J, Torres A. Rethinking the concepts of community-acquired and health-care-associated pneumonia. Lancet Infect Dis. 2010;10:279–87.
Corti MC, Guralnik JM, Salive ME, Sorkin JD. Serum albumin level and physical disability as predictors of mortality in older persons. JAMA. 1994;272:1036–42.
Walter LC, Brand RJ, Counsell SR, Palmer RM, Landefeld CS, Fortinsky RH. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001;285:2987–94.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sugisaki, M., Enomoto, T., Shibuya, Y. et al. Clinical characteristics of healthcare-associated pneumonia in a public hospital in a metropolitan area of Japan. J Infect Chemother 18, 352–360 (2012). https://doi.org/10.1007/s10156-011-0344-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10156-011-0344-9