Abstract
In Japan, the prevalence of extended-spectrum β-lactamase (ESBL) producers in hospital settings has shown an increasing trend. In the present study, we investigated the prevalence of the fecal carriage of ESBL-producing Enterobacteriaceae among healthy adults. Stool samples were collected for the phenotypic and genotypic identification of ESBL-producing Enterobacteriaceae. We found the prevalence of ESBL producers to be 6.4% by phenotypic identification, and 92.9% of them possessed the bla CTX-M gene. Among the CTX-M ESBL-producing Enterobacteriaceae, we identified 11 Escherichia coli and 2 Klebsiella pneumonia strains. The findings suggest that the fecal carriage of CTX-M-type ESBL producers by healthy people is rapidly increasing in Japan. This may be one of the causes of the increased spread of ESBL-producing bacteria in hospitals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The genes for extended-spectrum β-lactamases (ESBLs), which hydrolyse extended-spectrum cephalosporins and are inhibited by clavulanic acid, are spreading among Enterobacteriaceae [1]. In particular, Escherichia coli strains that produce CTX-M-type β-lactamases have become widely prevalent in the general population and are probably being imported into the hospital community [2].
In Japan, the prevalence of ESBL producers among clinical isolates has shown an increasing trend. In 2000, Komatsu et al. [3] reported a 0.5% prevalence of ESBL producers in the feces of diarrhea patients. Another review reported prevalences of 0.6, 7.2, 10.0, and 14% in clinical isolates of E. coli and Klebsiella pneumoniae in Japan in 1990, 1995, 2000, and 2003, respectively [4]. A hospital study in southwestern Japan showed that the prevalence of ESBL-producing bacteria increased from 3.6 to 15.2% between 2003 and 2009 [5]. However, little is known about the frequency of ESBL-producing bacteria in healthy Japanese people. Therefore, the aim of this study was to investigate the prevalence of the fecal carriage of ESBL-producing Enterobacteriaceae among asymptomatic Japanese adults.
Stool samples from adult volunteers in Osaka, Japan, were collected from July 2009 to June 2010. Written informed consent was obtained from each individual who volunteered to participate in the study. We analyzed a total of 218 samples, 1 from each participant.
All participants were asked to fill out a standardized questionnaire including general demographic circumstances, antibiotic usage within 3 months prior to sample collection, and lifetime history of hospitalization.
Stool samples were assessed for the presence of ESBL-producing Enterobacteriaceae by phenotypic and genotypic methods. The phenotypic methods included plating the stool samples on MacConkey agar supplemented with 2 μg/ml cefotaxime (CTX-MacConkey). The results were confirmed using cefotaxime and ceftazidime with and without clavulanic acid, as recommended by the Clinical and Laboratory Standards Institute [6]. Isolates were identified using conventional biochemical tests. Tests for susceptibility to imipenem, meropenem, amikacin, gentamicin, ciprofloxacin, norfloxacin, and tetracycline were conducted by the disc diffusion method using an SN Disc (Nissui Pharmaceutical, Tokyo, Japan).
Bacterial DNA was extracted from the isolates by boiling the bacterial suspensions. DNA samples with a concentration of 0.1 ng/μl were used as the templates for polymerase chain reaction (PCR) analysis. The universal primers CTX-M-U1 (5′-ATG TGC AGY ACC AGT AAR GTK ATG GC-3′) and CTX-M-U2 (5′-TGG GTR AAR TAR GTS ACC AGA AYC AGC GG-3′) were used to detect the bla CTX-M gene, and the primers bla-SHV.SE (5′-ATG CGT TAT ATT CGC CTG TG-3′) and bla-SHV.AS (5′-TGC TTT GTT ATT CGG GCC AA-3′) were used to detect the bla SHV gene, as described previously [7]. DNA from the reference E. coli bla CTX-M-positive strain was used as the positive control. The PCR products were visualized by 2% agarose gel electrophoresis, followed by staining with GelRed Nucleic Acid Gel Stain (Biotium, Hayward, CA, USA). DNA sequencing was used for genotyping the bla CTX-M genes. The nucleotide sequences were analyzed with CLUSTALW and BLAST software (http://clustalw.ddbj.nig.ac.jp/top-j.html).
Statistical analysis was performed using SPSS 16.0 (SPSS, Chicago, IL, USA). Variables were analyzed by univariate analysis using χ 2 or Fisher’s exact test, as appropriate. Statistical significance was set at p < 0.05.
The characteristics of the study participants are shown in Table 1. The participants were aged between 20 and 70 years, with a median age of 41.9 years. Most had not used antibiotics within the 3 months prior to sample collection and most did not have a history of hospitalization.
Of the 218 samples, 65 (29.8%) showed bacterial growth on CTX-MacConkey agar and 14 (6.4%) showed ESBL production (Table 2). All the 14 isolates were identified from different participants. On PCR analysis, 92.9% (13 of 14) of the ESBL-positive isolates were positive for the bla CTX-M gene, and 1 sample carried the bla SHV gene. By genotyping of bla CTX-M genes, CTX-M-14 (38.5%) and CTX-M-2 (30.8%) ESBL types were predominantly identified. The other samples harbored the CTX-M-8, CTX-M-3, and CTX-M-15 types (15.4, 7.7, and 7.7%, respectively).
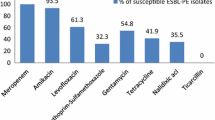
Among the CTX-M ESBL-producing Enterobacteriaceae, 84.6% (11 of 13) were identified as E. coli and 15.4% (2 of 13) as K. pneumoniae. The SHV-type ESBL producer was identified as E. coli. We did not identify any participants who harbored both ESBL-producing K. pneumoniae and E. coli as well as different CTX-M genotypes. Antibiotic susceptibility tests showed that 92.9% (13 of 14) of the ESBL-positive bacteria were susceptible to all the tested antibiotics, except for tetracycline. Five of the 14 ESBL producers were resistant to only tetracycline, and 1 was resistant to gentamicin and quinolones in addition to tetracycline.
Statistical analysis did not reveal any significant association between ESBL production and antibiotic usage or hospitalization experience, although these findings may be attributed to the small number of ESBL-positive cases identified in this study.
To the best of our knowledge, this is the first published data regarding the prevalence of ESBL-producing Enterobacteriaceae among healthy Japanese adults. Other countries have reported a 2.4–13.1% prevalence of fecal carriage of ESBL-producing Enterobacteriaceae in healthy people [8–12]. This value reached 58.2% in developing countries, such as Thailand, where antibiotics are used without prescription [12]. However, in Japan, where antibiotic usage is strictly regulated, the 6.4% prevalence of ESBL-producing Enterobateriaceae among healthy individuals is relatively high.
The high prevalence (92.9%) of CTX-M-type ESBLs identified in the present study is consistent with the global trend of CTX-M dominance. The prevalence of the CTX-M-14 enzyme belonging to the CTX-M-9 group and the CTX-M-2 enzyme belonging to the CTX-M-2 group was compatible with the findings of previous studies carried out in hospital settings, which have reported that the CTX-M-9, CTX-M-2, and CTX-M-1 groups are the predominant CTX-M-type ESBLs in E. coli in Japan [4, 5, 13, 14]. This similarity between the CTX-M-type ESBL groups identified in community and hospital settings may indicate that people who are already carriers of ESBL producers are visiting hospitals. In previous studies of clinical isolates in Japan, ESBL-producing bacteria showed coresistance to fluoroquinolones [5, 15]. However, our data showed that most of the ESBL producers (92.9%) identified from healthy individuals were susceptible to all antibiotics, except tetracycline. Furthermore, our study did not show any significant association between the fecal carriage of ESBL-positive Enterobacteriaceae and recent antibiotic use or hospitalization, which indicates the existence of other risk factors. Because the study enrolled only a limited number of participants, possible factors that contribute to the prevalence of ESBL-producing bacteria in healthy people in Japan should be investigated in a larger number of participants.
In summary, our results suggest that the prevalence of the fecal carriage of CTX-M-type ESBL producers, mostly E. coli, by healthy Japanese people is rapidly increasing. This may be one of the causes of the dissemination of ESBL-producing bacteria in hospitals. A broader and more detailed study of the prevalence of ESBL producers in the community is needed to prevent potential treatment failures associated with serious infections caused by these bacteria.
References
Falagas ME, Karageorgopoulos DE. Extended-spectrum beta-lactamase-producing organisms. J Hosp Infect. 2009;73(4):345–54.
Pitout JD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8(3):159–66.
Komatsu M, Aihara M, Shimakawa K, Yamanaka T, Matsuo S. Detection of extended spectrum beta-lactamases producing Enterobacteriaceae in feces. Kansenshogaku Zasshi. 2000;74(3):250–8.
Hawkey PM. Prevalence and clonality of extended-spectrum beta-lactamases in Asia. Clin Microbiol Infect. 2008;14(Suppl 1):159–65.
Chong Y, Yakushiji H, Ito Y, Kamimura T. Clinical and molecular epidemiology of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a long-term study from Japan. Eur J Clin Microbiol Infect Dis. 2010. doi:10.1007/S10096-010-1057-1.
National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. Document M100-S14. Wayne, PA: NCCLS; 2004.
Monstein HJ, Ostholm-Balkhed A, Nilsson MV, Nilsson M, Dornbusch K, Nilsson LE. Multiplex PCR amplification assay for the detection of bla SHV, bla TEM and bla CTX-M genes in Enterobacteriaceae. APMIS. 2007;115(12):1400–8.
Kader AA, Kumar A, Kamath KA. Fecal carriage of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in patients and asymptomatic healthy individuals. Infect Control Hosp Epidemiol. 2007;28(9):1114–6.
Moubareck C, Daoud Z, Hakime NI, Hamze M, Mangeney N, Matta H, et al. Countrywide spread of community- and hospital-acquired extended-spectrum beta-lactamase (CTX-M-15)-producing Enterobacteriaceae in Lebanon. J Clin Microbiol. 2005;43(7):3309–13.
Tian SF, Chen BY, Chu YZ, Wang S. Prevalence of rectal carriage of extended-spectrum beta-lactamase-producing Escherichia coli among elderly people in community settings in China. Can J Microbiol. 2008;54(9):781–5.
Valverde A, Coque TM, Sanchez-Moreno MP, Rollan A, Baquero F, Canton R. Dramatic increase in prevalence of fecal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae during nonoutbreak situations in Spain. J Clin Microbiol. 2004;42(10):4769–75.
Sasaki T, Hirai I, Niki M, Nakamura T, Komalamisra C, Maipanich W, et al. High prevalence of CTX-M beta-lactamase-producing Enterobacteriaceae in stool specimens obtained from healthy individuals in Thailand. J Antimicrob Chemother. 2010;65(4):666–8.
Shibata N, Kurokawa H, Doi Y, Yagi T, Yamane K, Wachino J, et al. PCR classification of CTX-M-type beta-lactamase genes identified in clinically isolated gram-negative bacilli in Japan. Antimicrob Agents Chemother. 2006;50(2):791–5.
Suzuki S, Shibata N, Yamane K, Wachino J, Ito K, Arakawa Y. Change in the prevalence of extended-spectrum-beta-lactamase-producing Escherichia coli in Japan by clonal spread. J Antimicrob Chemother. 2009;63(1):72–9.
Hirakata Y, Matsuda J, Miyazaki Y, Kamihira S, Kawakami S, Miyazawa Y, et al. Regional variation in the prevalence of extended-spectrum beta-lactamase-producing clinical isolates in the Asia-Pacific region (SENTRY 1998–2002). Diagn Microbiol Infect Dis. 2005;52(4):323–9.
Acknowledgments
This work was supported by KAKENHI (Grant-in-Aid for Exploratory Research), the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Conflict of interest
There is no conflict to declare.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Luvsansharav, UO., Hirai, I., Niki, M. et al. Prevalence of fecal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae among healthy adult people in Japan. J Infect Chemother 17, 722–725 (2011). https://doi.org/10.1007/s10156-011-0225-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10156-011-0225-2