Abstract
The community structure of caprellids inhabiting two species of seagrass (Cymodocea nodosa and Zostera marina) was investigated on the Andalusian coast, southern Spain, using uni and multivariate analyses. Three meadows were selected (Almería, AL; Málaga, MA; Cádiz, CA), and changes in seagrass cover and biomass were measured from 2004 to 2005. Four caprellid species were found; the density of Caprella acanthifera, Phtisica marina and Pseudoprotella phasma was correlated to seagrass biomass. No such correlation was found for Pariambus typicus, probably because this species inhabits sediments and does not cling to the seagrass leaves. We recorded a significant decrease in seagrass cover and biomass in MA due to illegal bottom trawling fisheries. Phtisica marina and P. typicus were favoured by this perturbation and increased their densities after the trawling activities. A survey of reports on caprellids in seagrass meadows around the world showed no clear latitudinal patterns in caprellid densities (ranging from 6 to 1,000 ind/m2 per meadow) and species diversity. While caprellid abundances in seagrass meadows are often very high, the number of species per meadow is low (range 1–5).
Similar content being viewed by others
Introduction
Caprellids are small marine peracaridean crustaceans, which inhabit algae, hydroids, ascidians, anthozoans, bryozoans, sponges and seagrasses (McCain 1968; Guerra-García 2001). They feed on suspended materials, prey on other organisms, or graze on epibiotic fauna and flora (Caine 1974; Guerra-García et al. 2002; Thiel et al. 2003), and they are important prey for many coastal fish species (Caine 1987, 1989, 1991). Caprellids are morphologically well adapted to cling to the substrata; with their pereopods they can firmly hold onto branches of algae, seagrass, bryozoans and hydrozoans. The pleopods, which are used for swimming in other amphipod crustaceans, are very reduced in caprellids; therefore, although caprellids can swim (Caine 1979a) there are not very efficient swimmers. However, caprellids can be distributed passively by clinging to artificial (buoys, ropes, litter) and natural (macroalgae) floating materials, so the cosmopolitan distribution of many littoral caprellid species might be facilitated by the fact that they are often associated with fouling communities on floating objects (Thiel et al. 2003). Recently, caprellids have also been found to be useful bioindicators of marine pollution and environmental stress (Guerra-García and García-Gómez 2001; Ohji et al. 2002; Takeuchi et al. 2004; Guerra-García and Koonjul 2005). Although amphipods (gammarids and caprellids) are regular inhabitants of seagrass meadows, there is a lack of ecological and behavioural studies on the caprellid communities associated to seagrasses.
Seagrasses are distributed worldwide (600,000 km2 of the marine bottoms are covered by these spermatophytes) and play an important role in the general coastal dynamics and biology (Larkum et al. 1989; Templado 2004). When compared with neighbouring areas, the meadows reveal higher abundances and species richness (Edgar et al. 1994). The main factors contributing to this improvement in biodiversity are availability of microhabitat, protection from predators, trophic resources, sediment settling, hydrodynamic force reduction (see Pranovi et al. 2000). Seagrass beds of the temperate zone support large numbers of invertebrate species and individuals, thereby providing abundant food for fishes, compared to adjacent unvegetated areas (Nakamura and Sano 2005). At European coasts, four native seagrass species are known, which are all distributed along the littoral of Andalusia, southern Spain: Zostera marina Linnaeus, Cymodocea nodosa (Ucria) Ascherson, Posidonia oceanica (Linnaeus) Delile and Zostera noltii Hornemann. In spite of the abundance of these seagrass meadows in southern Spain, caprellid communities associated to these plants have been scarcely studied, and the only records of caprellids from these habitats come from general faunistic or ecological studies (Edgar 1990; Sánchez-Jerez and Ramos Esplá 1996; Rodríguez-Ruiz et al. 2001; Luque et al. 2004; Ballesteros et al. 2004). This lack of information is also applicable to other areas around the world (Takeuchi and Hino 1997).
Because of the ecology and economic importance of seagrass meadows, their protection has been proposed in recent legislation at local, national and international level. Seagrass meadows at the Andalusian coast have been decreasing steadily. In addition to natural processes, anthropogenic factors are likely to have influenced this decline (Sánchez-Jerez and Ramos-Esplá 1996). Besides the increasing of urban and industrial areas in the littoral zone, there is an important effect of the bottom trawling fisheries. Large numbers of trawlers usually work illegally over seagrass meadows, causing physical degradation and critical regression of the meadows. In fact, although variation in structural complexity in seagrass may well be produced by other environmental factors, human activities such as trawling play a very important role in the SE of the Iberian Peninsula (Sánchez-Lizaso et al. 1990). For all these reasons, a research programme on seagrass meadows of the coast of Andalusia, southern Spain was initiated in 2004. The project, supported by the Environmental Agency of the Andalusian Government, is intended to control and detect temporal changes in seagrass biomass, density, cover and associated fauna. To properly assess changes in the extent of seagrass meadows throughout time, sampling effort was mainly focused on meadows edges. As a part of this general project, we studied of the community structure of the caprellid amphipods associated to three seagrass meadows of the Andalusian coast during the years 2004 and 2005, using uni and multivariate approaches. Furthermore, as one of the studied seagrass meadow was severely affected during the study by trawling fisheries, we also tested the responses of caprellids to seagrass regression produced by trawlers.
Methods
Study sites
The three seagrass meadows selected for the present study are distributed along the oriental coast of Andalusia (Fig. 1). At the occidental coast, water transparency is significantly lower and no seagrass can be found. The main characteristics of the three selected meadows are given in Table 1.
Sampling procedure
Estimation of changes in seagrass cover
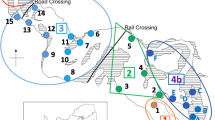
Once the exact location and dimensions of the three seagrass meadows had been checked using SCUBA, the four edges (north, south, east and west) were located. On each edge, 6 fixed quadrats of 1 × 1 m2 (Fig. 2) were marked with sheaves for monitoring the seagrass cover through time. Each quadrat (24 for each meadow, 72 in total) was photographed and, using image analysis, divided into 64 subsquares. In each subsquare the seagrass presence-absence was checked and the cover of the quadrat was express as the percentage of presence numbers from the total (64 subsquares). Cover was measured in summer 2004 and 2005.
Estimation of seagrass biomass and caprellid composition
On each edge five random samples of 15 × 15 cm2 (Fig. 2) were collected in summer 2004 and 2005. Samples were sieved (mesh size of 0.5 mm), fixed in ethanol 85% and stained with bengal rose. In the laboratory, caprellid amphipods were sorted and identified to species level under a binocular microscope. Seagrass of each sample was separated and the biomass (dry weight) measured.
Sediment characteristics
Three samples of sediment were collected from each seagrass meadow in summer 2004 and 2005. Sediment samples were stored at −20°C in pre-cleaned glass jars until analysis. Granulometry was determined by Buchanan and Kain’s method (Buchanan and Kain 1984). Organic contents were analysed by ashing samples of sediment to 500°C for 6 h and re-weighing (Estacio et al. 1997).
Statistical analysis
Variations in sediment granulometry and organic matter between 2004 and 2005 were tested using one-way ANOVA after verifying the normality of the data (Kolmogorov–Smirnov test) and the homogeneity of variances (Levene test).
The influence of location and time (year 2004 vs. 2005) on cover and biomass of the seagrass was analyzed using two-way ANOVA. Variations of the seagrass biomass and cover between 2004 and 2005 and changes of caprellid densities for each seagrass meadow were tested using Kruskal–Wallis test.
To explore the relationship between caprellid density and seagrass biomass, correlation analyses were conducted. The affinities among seagrass based on caprellid species were established by cluster analysis using UPGMA method. Multivariate analyses were carried out using the PRIMER (Plymouth Routines in Multivariate Ecological Research) package (Clarke and Gorley 2001). For univariate analyses, the BMDP (BioMedical Data Programs) was used (Dixon 1983).
Results
Sediment characteristics
The seagrass meadows of Almería (AL) and Cádiz (CA) were dominated by fine sands while in the seagrass of Málaga (MA) very fine sands predominated (Fig. 3). The seagrass of MA was affected by trawling activities from 2004 to 2005. As a result, a significant change in granulometry was measured (an increase in fine sand, F = 21.7, P < 0.05, and a decrease in very fine sand, F = 39.3, P < 0.01). As to organic matter (Fig. 4), there were no significant differences between 2004 and 2005 for the three studied meadows.
Seagrass cover and biomass
Using the fixed squares of 1 × 1 m2 to estimate changes in cover, we measured a significant decrease of seagrass cover (from 57 to 17%, Kruskal–Wallis (K) = 32.4, P < 0.001) in Málaga (MA), probably due to the effect of fishing trawlers in the area. The effect was also significant for seagrass biomass in MA (from 55 to 23 g/m2; K = 18.2, P < 0.001) (Fig. 5). When pooled data for all seagrass meadows were analysed using two-way ANOVA, significant differences in cover and biomass were measured for both location (AL, MA or CA) and year (2004 or 2005) (Table 2). However, a significant interaction was also measured between the two factors, due to the divergent behaviour of the Málaga meadow.
Caprellid community
Four caprellid species were found during the present study: Caprella acanthifera (Leach, 1814), Pariambus typicus (Kröyer, 1844), Phtisica marina Slabber, 1769, and Pseudoprotella phasma (Montagu, 1804). Species abundances for the three studied seagrass meadows are given in Fig. 6. Phtisica marina and P. typicus were the dominant species, while P. phasma was largely restricted to Almería and Caprella acanthifera was only found in Cádiz.
In Almería (AL) and Cádiz (CA) there were no significant differences in caprellid densities between 2004 and 2005. However, a significant increase in number of specimens were measured for P. marina and P. typicus in Málaga (MA), probably as a result of the perturbation associated with trawling activities (P.marina, from 5.9 to 15.7 ind/225 cm2, K = 9.8, P < 0.05; P. typicus, from 0.6 to 3.1 ind/225 cm2, K = 4.4, P < 0.05).
For both 2004 and 2005 the caprellid community, although consisting only of four species, allowed for discriminating among the three meadows, according to the cluster analysis (Fig. 7). Each meadow was characterised by a specific composition and/or abundance of the caprellid community.
The density of P. marina, P. phasma and C. acanthifera was significantly correlated to the seagrass biomass (r = 0.51, 0.40 and 0.50, respectively; P < 0.05) while there was no correlation for P. typicus.
Discussion
Caprellid community on Andalusian seagrasses
The four caprellid species found in the studied seagrass meadows, C. acanthifera, P. marina, P. phasma and P. typicus, are common species on many different substrata (see Guerra-García 2001). Phtisica marina is a cosmopolitan species that lives on algae, hydroids, ascidians, anthozoans, sponges, bryozoans and sediments (Guerra-García 2001). It can bear stressed areas of low hydrodynamics and high rates of sedimentation and organic matter (Guerra-García and García-Gómez 2001). Caprella acanthifera is also widely distributed in the Mediterranean, being especially abundant on algae, but also associated with a variety of substrata. Pseudoprotella phasma is usually associated with hydroids, but can be found also on algae, anthozoans and sediment. Pariambus typicus has been found mainly on sediments and also associated with echinoderms (Guerra-García 2001). Consequently, no specific associations could be established between these caprellids and seagrass species; however, specific associations between caprellids and seagrass species have been reported in other areas (e.g. Caprella japonica living on the seagrass Phyllospadix iwatensis in Japan, Takeuchi and Hino 1997). Although there is a lack of studies dealing with caprellids on seagrass around the Iberian Peninsula, the caprellid composition is very similar in the four seagrass species (Table 3).
Caprellids may play an important role in seagrass meadows. Caprellids living on seagrass are important prey for many fishes (Caine 1991; Horinouchi et al. 1998; Rodríguez-Ruiz et al. 2001; Sánchez-Jerez et al. 2000). Some species of fishes associated with seagrass meadows consume primarily caprellids, especially during juvenile stages (see Kwak et al. 2005). In fact, in shallow water ecosystems, caprellidean and gammaridean amphipods are considered to be one of the most important prey items for fishes, especially for those less than 10 cm in body length (Takeuchi and Hino 1997). Furthermore, the amphipod and gastropod grazers are very important in controlling periphyton and ephiphytes of seagrass (Jernakoff and Nielsen, 1997). For example, Caine (1980) reported that in the absence of Caprella laeviuscula, periphyton biomass increased by 411% in Z. marina beds.
Caprellids and seagrass biomass. Vertical distribution of caprellids in the meadow
In tropical seagrass meadows, seagrass biomass has usually been found correlated with both number and abundance of invertebrate species. A thick vegetation provides better protection from predators and a larger plant surface to cling on (Heck and Wetstone 1977). In the present study, the abundance of P. marina, P. phasma and C. acanthifera correlated with seagrass biomass while the abundance of P. typicus did not. This may be explained by the vertical distribution of the caprellid species in the seagrass meadow. Phtisica marina is distributed on both blades and sediment; P. phasma and C. acanthifera live mainly on leaves while P. typicus can be found within sediments, among sand grains (personal observation; Fig. 8). This species seems to live in sediments regardless of the local seagrass biomass, i.e. even in plain sediments. In contrast, the abundances of the other species were highly correlated with seagrass biomass, since they depend on seagrass blades to cling on. Curiously, in a study conducted in a seagrass meadow of Z. marina in the Salcombe Estuary, UK, P. typicus was more abundant in fragmented than in continuous areas (Frost et al. 1999).
According to Virnstein et al. (1984), most free-living gammarid amphipods tend to hide between seagrass blades while caprellids live more exposed on the surface and tips of the blades. Perhaps counteracting their vulnerable position on the leaf surfaces, caprellids have a skeleton-like morphology, which may make them less conspicuous to visual predators (Virnstein et al. 1984). In fact, although more behavioural studies are necessary for most species, Caprella japonica and C. tsugarensis have been observed to hold seagrasses in a “parallel” posture (Takeuchi and Hino 1997). Although caprellids are reported to be more “camouflaged” in seagrass meadows than in other habitats, there are other substrates, such as hydroids, where caprellids can remain unnoticed by predators. Caine (1979b) compared populations of Caprella laeviuscula living on Zostera and on the hydroid Obelia, and found that predation was higher on Zostera than on Obelia, especially of juveniles and females. Juvenile and female selectivity by predators are related to visual discernibility; juveniles lack a protective coloration on Zostera, and the brood pouch of ovigerous females is white. On Obelia, the lighter colour blends with the background and juveniles resemble polyps.
Trawling effect on caprellid community
The present study showed that P. marina and P. typicus were clearly favoured by the perturbation associated with trawling activities. Sánchez-Jerez and Ramos-Esplá (1996) also found higher densities of some caprellid species in areas impacted by trawlers in comparison with control areas along C. nodosa meadows. Frost et al. (1999) found higher densities of P. typicus in fragmented areas of Z. marina beds than in continuous zones. Phtisica marina and P. typicus have been reported to be very good colonizers of sediments after dredging in harbours (Guerra-García et al. 2003), and excellent recolonizers of defaunated sands in experimental trays (Guerra-García and García-Gómez 2006). These two species can be considered as “opportunistic”, profitting from perturbations of soft bottoms, such as dredging or trawling.
Latitudinal patterns of caprellid distribution on seagrass meadows
Although the biogeographical distribution of caprellid species on a global scale is not well known so far, it has been reported that the highest caprellid species diversity is found in temperature waters (around 30° latitude), and that species diversity decreases both towards colder waters of higher latitudes and towards the equator (Laubitz 1970; Abele 1982; Thiel et al. 2003). This pattern has been also measured for gammaridean amphipods. However, the typical pattern for many other groups of crustaceans such as ostracods, copepods, stomatopods or decapods is different: a decrease of species richness from the equator towards higher latitudes (Abele 1982). If only seagrass meadows are considered, the amphipod pattern changes as diversity increases significantly with decreasing latitude, being maximal near the equator (Virnstein et al. 1984). These authors summarised the available information to determine whether any latitudinal pattern exists for the seagrass-associated epifauna, and to examine hypotheses, which might explain the observed patterns. They found that the diversity of decapods and amphipods in seagrass meadows increases with decreasing latitude, while the diversity of isopods and fishes showed nonsignificant trends with latitude. Interestingly, density of amphipods showed no pattern with latitude, which is apparently inconsistent with either the tropical decrease or increase predicted by the predation and primary production hypotheses. Consequently, Virnstein et al. (1984) pointed out that, contrary to evidence from other biological systems, it appears that latitude is, in general, an inconsistent predictor of differences in the epifauna of seagrass communities. If we consider only the caprellidean amphipods, species richness and abundances obtained in the present study are similar to those measured in other seagrass meadows around the world, and data of Table 4 support the idea that there is no clear latitudinal pattern for density values (in fact, the correlation between latitude values of localities in Table 4 and caprellid densities is not significant, r = 0.19, NS). Data of total caprellid abundance range from 6 to 1,000 individuals/m2, but the methods differed among studies, which makes comparisons difficult. Furthermore, there is a lack of data for areas near the equator and further studies are necessary.
As to the number of species, the very low species richness of caprellids in seagrass meadows (one to five species) does not allow clear conclusion about any latitudinal pattern.
References
Abele LG (1982) Biogeography. In: Abele LG (ed) The biology of Crustacea. Systematics, the Fossil Record, and Biogeography. Academic Press, New York, pp 241–304
Ballesteros E, García-Raso JE, Salas C, Gofás S, Moreno D, Templado J (2004) La comunidad de Cymodocea nodosa: flora y fauna. In: Luque AA, Templado J (eds) Praderas y bosques marinos de Andalucía. Consejería de Medio Ambiente, Junta de Andalucía Sevilla, pp 146–152
Bologna PAX, Heck KL (1999) Macrofaunal associations with seagrass epiphytes. Relative importance of trophic and structural characteristics. J Exp Mar Biol Ecol 242:21–39
Buchanan JD, Kain JM (1984) Measurement of the physical and chemical environment. In: Holme HL, McIntre AD (eds) Methods for the study of marine benthos. Blackwell, Oxford, pp 30–50
Caine EA (1974) Comparative functional morphology of feeding in three species of caprellids (Crustacea, Amphipoda) from the northwestern Florida gulf coast. J Exp Mar Biol Ecol 15:81–96
Caine EA (1979a) Functions of swimming setae within caprellid amphipods (Crustacea). Biol Bull 156:169–178
Caine EA (1979b) Population structures of two species of caprellid amphipods (Crustacea). J Exp Mar Biol Ecol 40:103–104
Caine EA (1980) Ecology of two littoral species of caprellid amphipods (Crustacea) from Washington, USA. Mar Biol 56:327–335
Caine EA (1987) Potential effect of floating dock communities on a South Carolina estuary. J Exp Mar Biol Ecol 108:83–91
Caine EA (1989) Caprellid amphipod behaviour and predatory strikes by fish. J Exp Mar Biol Ecol 126:173–180
Caine EA (1991) Caprellid amphiods: fast food for the reproductively active. J Exp Mar Biol Ecol 148:27–33
Clarke KR, Gorley RN (2001) PRIMER (Plymouth routines in multivariate ecological research) v5: user manual/tutorial. PRIMER-E Ltd, Plymouth
Currás A (1990) Estudio de la fauna bentónica del la Ría del Eo (Lugo). PhD Thesis, Univeristy of Santiago, Spain
Dixon WJ (1983) BMDP statistical software. University of California Press, Berkely
Edgar GJ (1990) Population regulation, population dynamics and competition amongst mobile epifauna associated with seagrass. J Exp Mar Biol Ecol 144:205–234
Edgar GJ, Shaw C, Watson GF, Hammond LS (1994) Comparisons of species richness, size-structure and production of benthos in vegetated and unvegetated habitats in Western-Port, Victoria. J Exp Mar Biol Ecol 176:201–226
Estacio FJ, García-Adiego EM, Fa D, García-Gómez JC, Daza JL, Hortas F, Gómez-ARiza JL (1997) Ecological analysis in a polluted area of Algeciras Bay (Southern Spain): external “versus” internal outfalls and environmental implications. Mar Pollut Bull 34:780–793
Fredriksen S, Christie H, Saethre BA (2005) Species richness in macroalgae and macrofauna assemblages on Fucus serratus L. (Phaeophyceae) and Zostera marina L. (Angiospermae) in Skagerrak, Norway. Mar Biol Res 1:2–19
Frost MT, Rowden AA, Attril MJ (1999) Effect of habitat fragmentation on the macroinvertebrate infaunal communities associated with the seagrass Zostera marina L. Aquat Cons 9:255–263
Guerra-García JM (2001) Habitat use of the Caprellidea (Crustacea: Amphipoda) from Ceuta, North Africa. Ophelia 55:27–38
Guerra-García JM, García-Gómez JC (2001) The spatial distribution of Caprellidea (Crustacea: Amphipoda): a stress bioindicator in Ceuta (North Africa, Gibraltar area). PSZNI Mar Ecol 22:357–367
Guerra-García JM, Corzo J, García-Gómez JC (2002) Clinging behaviour of the Caprellidea (Amphipoda) from the Strait of Gibraltar. Crustaceana 75:41–50
Guerra-García JM, Corzo J, García-Gómez JC (2003) Short-term benthic recolonization after dredging in the harbour of Ceuta, North Africa. PSZNI Mar Ecol 24:217–229
Guerra-García JM, Koonjul MS (2005) Metaprotella sandalensis (Crustacea: Amphipoda: Caprellidae): a bioindicator of nutrient enrichment on coral reefs? A preliminary study at Mauritius Island. Environ Monit Assess 104:353–367
Guerra-García JM, García-Gómez JC (2006) Recolonization of defaunated sediments: fine versus gross sand and dredging versus experimental trays. Est Coast Shelf Sci 68:328–342
Heck F, Wetstone GS (1977) Habitat complexity and invertebrate species richness and abundance in tropical seagrass meadows. J Biogeogr 4:135–142
Horinouchi M, Sano N, Taniuchi T, Shimizu M (1998) Food and microhabitat resource use by Rudarius ercodes and Ditrema temmincki coexisting in a Zostera bed at Aburatsubo, central Japan. Fisheries Sci 64:563–568
Jernakoff P, Nielsen J (1997) The relative importance of amphipod and gastropod grazers in Posidonia sinuosa meadows. Aquat Bot 56:183–202
Jernakoff P, Nielsen J (1998) Plant-animal associations in two species of seagrasses in Western Australia. Aquat Bot 60:359–376
Kwak SN, Baeck GW, Klumpp DW (2005) Comparative feeding ecology of two sympatric greenling species, Hexagrammos otakii and Hexagrammos agrammus in eelgrass Zostera marina beds. Environ Biol Fish 74:129–140
Larkum AWD, McComb AJ, Shepherd SA (1989) Biology of seagrasses: a treatise on the biology of segrasses with special reference to the Australian region. Elsevier, Amsterdam
Laubitz DR (1970) Studies on the Caprellidae of the American North Pacific. Publ Biol Oceanogr 1:1–88
Luque AA, Templado J, Barrajón A, Cuesta S, González M, Larrad A, López E, Ortiz M, López de la Cuadra CM, López PJ, Remón JM, Moreno D (2004) La diversidad faunística de las praderas de Posidonia oceanica de Almería. In: Luque AA, Templado J (eds) Praderas y bosques marinos de Andalucía. Consejería de Medio Ambiente, Junta de Andalucía Sevilla, pp 273–282
McCain JC (1968) The Caprellidea (Crustacea: Amphipoda) of the Western North Atlantic. Bull US Nat Museum 278:1–116
Moreira J (2003) La fauna bentónica de la Ensenada de Baiona (Galicia, NO Península Ibérica): Diversidad, Análisis de las comunidades, dinámica de poblaciones y distribución vertical. PhD Thesis, University of Vigo, Spain
Nakamura Y, Sano M (2005) Comparison of invertebrate abundance in a seagrass bed and adjacent coral and sand areas at Amitori Bay, Iriomote Island, Japan. Fish Sci 71:543–550
Nelson WG (1979) An analysis of structural pattern in an eelgrass (Zostera marina L.) amphipod community. J Exp Mar Biol Ecol 39:231–264
Ohji M, Takeuchi I, Takahashi S, Tanabe S, Miyazaki N (2002) Differences in the acute toxicities of tributyltin between the Caprellidea and the Gammaridea (Crustacea: Amphipoda). Mar Pollut Bull 44:16–24
Pranovi F, Curiel D,Rismondo A, Marzocchi M, Scattolin M (2000) Variations of the macrobenthic community in a seagrass transplanted area of the Lagoon of Venice. Sci Mar 64:303–310
Riera R, Guerra-García JM, Brito MC, Núñez J (2003) Estudio de los caprélidos de Lanzarote, Islas Canarias (Crustacea: Amphipoda: Caprellidea). Vieraea 31:157–166
Rodríguez-Ruiz S, Sánchez-Lizaso JL, Ramos-Esplá AA (2001) Cambios estacionales en la dieta de Diplodus annularis (L., 1758) en el sudeste ibérico. Boletín IEO 17:87–95
Sánchez-Lizaso JL, Guillén JE, Ramos-Esplá AA (1990) The regression of Posidonia oceanica meadows in El Campello (Spain). Rapp Comm Int Mer Medit 32:7
Sánchez-Jerez P, Ramos-Esplá AA (1996) Detection of environmental impacts by bottom trawling on Posidonia oceanica (L.) Delile meadows: sensitivity of fish and macroinvertebrate communities. J Aquat Ecosys Health 5:239–253
Sánchez-Jerez P, Barberá-Cebrian C, Ramos-Esplá AA (1999) Comparison of the epifauna spatial distribution in Posidonia oceanica, Cymodocea nodosa and unvegetated bottoms: importance of meadow edges. Acta Oecol 20:391–405
Sánchez-Jerez P, Barberá-Cebrian C, Ramos-Esplá AA (2000) Influence of the structure of Posidonia oceanica meadows modified by bottom trawling on crustacean assemblages: comparison of amphipods and decapods. Sci Mar 64:319–326
Sfriso A, Birkemeyer T, Ghetti PF (2001) Benthic macrofauna changes in area of Venice lagoon populated by seagrasses or seaweeds. Mar Environ Res 52:323–349
Stoner AW (1983) Distributional ecology of amphipods and tanaidaceans associated with three seagrass species. J Crust Biol 3:505–518
Takeuchi I, Hino A (1997) Community structure of caprellid amphipods (Crustacea) on seagrasses in Otsuchi Bay, Northeastern Japan, with reference to the association of Caprella japonica (Schurin) and Phyllospadix iwatensis Makino. Fisheries Sci 63:327–331
Takeuchi I, Takahashi S, Tanabe S, Miyazaki N (2004) Butylin concentrations along the Japanese coast from 1997 to 1999 monitored by Caprella spp. (Crustacea: Amphipoda). Mar Environ Res 57:397–414
Templado J (2004) Las praderas de fanerógamas marinas. Introducción. In: Luque AA, Templado J (eds) Praderas y bosques marinos de Andalucía. Consejería de Medio Ambiente, Junta de Andalucía Sevilla, pp 57–59
Thiel M, Guerra-García JM, Lancellotti DA, Vásquez N (2003) The distribution of littoral caprellids (Crustacea: Amphipoda: Caprellidea) along the Pacific coast of continental Chile. Rev Chil Hist Nat 76:297–312
Thom R, Miller B, Kennedy M (1995) Temporal patterns of grazers and vegetation in a temperate seagrass system. Aquat Bot 50:201–205
Virnstein RW, Nelson WG, Lewis FG, Howard RK (1984) Latitudinal patterns in seagrass epifauna: do patterns exist, and can they be explained? Estuaries 7:310–330
Acknowledgments
Support of this work was provided by the Ministerio de Educación y Ciencia (Project CGL2007-60044/BOS) co-financed by FEDER funds, and by the Consejería de Medio Ambiente and Consejería de Innovación, Ciencia y Empresa, Junta de Andalucía (project P07-RNM-02524).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H.-D. Franke.
Rights and permissions
About this article
Cite this article
González, A.R., Guerra-García, J.M., Maestre, M.J. et al. Community structure of caprellids (Crustacea: Amphipoda: Caprellidae) on seagrasses from southern Spain. Helgol Mar Res 62, 189–199 (2008). https://doi.org/10.1007/s10152-008-0107-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10152-008-0107-x