Abstract
Background
Treatment of complex anal fistulas remains difficult. However, treatment with stem cells has had an encouraging success rate when applied to complex perianal fistulas. We systematically reviewed the current evidence through meta-analysis.
Methods
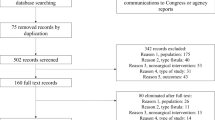
We performed an electronic literature search on PubMed, Embase, and the Cochrane Library and identified studies (published between January 1946 and August 2017) that used stem cells to treat patients with complex perianal fistula. Each paper was evaluated for treatment success rate, target patients, types of stem cells used, number of cells used, and criteria for complete healing. Potential publication bias was assessed via visual inspection of a funnel plot and Orwin’s fail-safe N. Out of 171 papers, 16 were included in the meta-analysis.
Results
The overall healing rate of stem cell injection therapy for patients with complex perianal fistulas was 62.8% (95% CI 53.5–71.2, I2 = 54.05%), whereas those for patients with Crohn’s perianal fistulas alone and complex anal fistulas not associated with Crohn’s disease were 64.1% and 61.5% (p = 0.840), respectively. Healing rates for autologous and allogenic stem cell treatment were 69.4% and 50.7% (p = 0.020), respectively. Four comparative studies out of 16 studies were analyzed separately. Stem cell therapy increased the healing rate compared to the control groups (OR 0.379, 95% CI 0.152–0.947).
Conclusions
Stem cell therapy is a good treatment option for complex perianal fistulas, which cannot be healed by conventional operative procedures. However, further research for additional supportive evidence, such as a large-scale randomized controlled trial, is required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Perianal fistula is a common anal disease that can be easily overlooked. However, recurrence is common, and it is often difficult to treat with conventional methods. Complex perianal fistula (CPF), which is difficult to treat with existing surgical methods, sometimes appears in patients with Crohn’s disease (CD) in cases of multiple perianal fistulas not related to CD or due to radiotherapy-related complications [1,2,3,4]. Although the prevalence of CPF in South Korea has not been identified, the prevalence of CD is steadily increasing [5]. Consequently, the prevalence of CPF is expected to increase.
Surgery is the best treatment option for a perianal fistula. The two important treatment principles are (1) preventing recurrence by removing the lesion and (2) minimizing the deterioration of anal sphincter function to avoid complications such as fecal incontinence. Simple perianal fistulas can be fully treated without serious complications or recurrence by lay-open fistulotomy. However, treating a CPF is not easy, and various surgical methods (e.g., cutting or non-cutting seton drain, mucosal advancement flap, fibrin glue, collagen plugs) have been employed for this purpose. Loungnarath et al. [6] reported that using fibrin glue to treat CPF resulted in recurrence within 3 months in most cases and a low successful treatment rate of 31%, while patients who had previously undergone other treatments had a successful treatment rate of only 22%. Balciscueta et al. [7] used an endorectal advancement flap procedure for CPF and reported a recurrence rate of only 21%; however, when full-thickness advancement flap was performed, fecal incontinence was observed in 20.4% of the cases. Chuang-Wei et al. [8], who treated CPF with cutting seton, also reported a high rate of fecal incontinence (24.1%). Thus, treatment outcomes for CPF have been inconsistent, while high recurrence rates and a somewhat high rate of fecal incontinence are reported [4, 6, 9, 10].
Therefore, the therapeutic efficacy of stem cells for various intractable diseases is being studied. Since adipose-derived stem cells (ADSCs) that are easy to harvest can differentiate into various cell types [11,12,13], there is an increasing interest in studies applying ADSCs for treating intractable diseases that are difficult to treat conventionally [14,15,16,17]. Favorable outcomes have been achieved using stem cell injection for treating CPF [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. However, individual studies used small population sizes and different research methods. Thus, the verifiability and precision of stem cell therapy for treating CPF need improvement.
Our study addresses the need for a meta-analysis that can provide a comprehensive overview of the overall therapeutic effect and clinical efficacy of stem cell therapy for treating CPF.
Materials and methods
This study was conducted in accordance with the reporting guidelines for systematic literature reviews as recommended by preferred reporting items for systematic reviews and meta-analyses (PRISMA) [33].
Research topic and selection criteria
We investigated the complete healing rates in patients with CPF who received stem cell injection. The selection criteria used in the study were established based on participants, intervention, comparator, outcomes, timing of outcome, setting, and study design (PICOTS-SD). Here, participants represent patients with CPF that could not be treated with conventional treatment; intervention represents local stem cell injection; comparator represents conventional surgical methods for perianal fistula; outcome represents complete healing rate; timing of outcome represents the entire follow-up period from stem cell therapy to complete healing; setting includes both outpatients and inpatients; and study design represents randomized control trials (RCTs) and non-RCTs, including observational and case–control studies. The search was limited to articles pertaining to human studies; simple case reports and studies involving pediatric patients and patients with active inflammatory bowel disease who required systematic treatment were excluded.
Literature search
Pubmed, Embase, and Cochrane Library were searched using “anal fistulas” and “stem cells” as the keywords. All articles published before August 22, 2017 were searched. The search was limited to human studies and articles published in English. The search terms for Pubmed are defined below:
((“rectal fistula”[MeSH Terms] OR (“rectal”[All Fields] AND “fistula”[All Fields]) OR “rectal fistula”[All Fields] OR (“anal”[All Fields] AND “fistulas”[All Fields]) OR “anal fistulas”[All Fields]) OR (“rectal fistula”[MeSH Terms] OR (“rectal”[All Fields] AND “fistula”[All Fields]) OR “rectal fistula”[All Fields] OR (“fistula”[All Fields] AND “ano”[All Fields]) OR “fistula in ano”[All Fields]) OR (perianal[All Fields] AND (“fistula”[MeSH Terms] OR “fistula”[All Fields] OR “fistulas”[All Fields]))) AND ((“stem cells”[MeSH Terms] OR (“stem”[All Fields] AND “cells”[All Fields]) OR “stem cells”[All Fields]) OR (precursor[All Fields] AND (“cells”[MeSH Terms] OR “cells”[All Fields])) OR (“stem cells”[MeSH Terms] OR (“stem”[All Fields] AND “cells”[All Fields]) OR “stem cells”[All Fields] OR (“progenitor”[All Fields] AND “cells”[All Fields]) OR “progenitor cells”[All Fields]) OR (“stromal cells”[MeSH Terms] OR (“stromal”[All Fields] AND “cells”[All Fields]) OR “stromal cells”[All Fields]) OR (“stem cells”[MeSH Terms] OR (“stem”[All Fields] AND “cells”[All Fields]) OR “stem cells”[All Fields] OR (“stem”[All Fields] AND “cell”[All Fields]) OR “stem cell”[All Fields])) AND (Humans[Mesh]).
Data selection and extraction
After excluding duplicate articles, preliminary screening was performed by reviewing the titles and abstracts. Of the 62 articles screened, 15 articles for conference presentations, without access to the full-text, were excluded. The full text of 47 articles was assessed for eligibility; case reports, animal studies, and review articles were excluded. Additionally, simple report-type articles containing only the results with no details about patient selection/exclusion criteria, healing criteria for perianal fistula, type of stem cells, and extraction methods were excluded. 19 articles were selected for qualitative synthesis; three of these were excluded: two that reported long-term follow-up outcomes from the same original study and one that examined only patients with rectovaginal fistula. Two independent investigators (S. C and B.G. J) participated in the literature selection, and any disagreement about article selection was resolved after reaching an agreement based on sufficient discussion.
Finally, 155 articles did not meet the selection criteria and 16 were selected for the final meta-analysis (Fig. 1).
Study selection and data collection
Selected articles were reviewed to investigate publication year and country, study design, study population size and complete healing rate, study selection criteria, presence or absence of Crohn’s perianal fistulas, number of stem cells injected, type of stem cells, methods and time points for determining complete healing of anal fistula, and disclosure of research funding source.
Assessment of risk of bias in individual studies
Each study was assessed for risk of bias based on the eight items (12 if a control group was included) presented in the methodological index for non-randomized studies (MINORS):
-
1.
Is the objective of the study clearly stated?
-
2.
Are the subject selection criteria clearly listed?
-
3.
Were the data collected prospectively?
-
4.
Does the endpoint correspond with the study objective?
-
5.
Was there an unbiased endpoint assessment?
-
6.
Was the length of the follow-up period sufficient to show the primary outcomes?
-
7.
Did the follow-up loss exceed 5%?
-
8.
Was the sample size calculated prospectively and was information about the sample needed for determining differences with a 95% confidence interval?
-
9.
Did the control group use gold-standard treatment?
-
10.
Were the groups studied over the same time period?
-
11.
Did the two groups have the same baseline characteristics?
-
12.
Was the statistical analysis appropriate for the study design?
Data synthesis and analysis
The effect size was defined as the percentage of patients who achieved complete healing among all patients who received stem cell therapy. Heterogeneity was tested using Chi squared test (Q statistics), whereas the degree of heterogeneity was interpreted by quantifying inconsistency using the Higgins I2 value. Generally, an I2 value of 0–40% might not be important; 30–60% may represent moderate heterogeneity; 50–90% may represent substantial heterogeneity; and 75–100% may represent considerable heterogeneity. The overall effect size was examined via meta-analysis using a random effects model based on the information above. The cause of heterogeneity was investigated by subgroup analysis based on regrouping by homogeneous groups; one-study-removed meta-analysis was conducted to identify any possible heterogeneity due to excessive influence by a specific study. A cumulative meta-analysis was performed to determine possible heterogeneity caused by studies performed prior to a specific time period. Comprehensive meta-analysis (CMA) 2.2 (Biostat, Englewood, NJ, USA) was used for statistical analyses.
Publication bias
A funnel plot was used to assess publication bias, which can influence accumulation of evidence. A funnel plot assumes that small-scale studies will be distributed widely across the bottom of the graph, whereas large-scale studies will be narrowly distributed in the upper part. Thus, the distribution of effect sizes will appear symmetrically when there is no publication bias, and asymmetrically when it is present. Additionally, the extent of publication bias was quantified by Orwin’s fail-safe N value, which estimates the number of studies considered to have been unpublished, while assuming correction without publication bias. The fail-safe N method interprets the effect sizes based on statistical significance alone rather than practical significance, whereas the Orwin’s fail-safe N value allows researchers to pre-set the smallest effect size with clinical significance and the actual expected effect size, including studies that have disappeared.
Results
General characteristics of the studies included in the meta-analysis
Information on the selected articles is shown in Table 1. Of the 16 studies, 12 were single-group observational studies that examined only the patient group that received stem cell therapy with no control group. The remaining four were case–control studies, but only three of these were RCTs. Approximately, two-thirds of all published studies came from two countries: six from Spain and four from South Korea. Autologous and allogeneic stem cells were used in 12 and 4 studies, respectively. The number of stem cells and criteria for stem cell injection varied between studies; some studies adjusted the number of stem cells injected based on the fistula length, whereas others used a fixed number of cells regardless of the length. Therefore, there was nearly a ten-fold difference in the number of stem cells injected between some of the studies. Complete healing of perianal fistula was determined solely by clinical criteria in ten studies, whereas magnetic resonance imaging (MRI) was additionally used in six studies. The studies also applied different standards for the time point for determining complete healing of fistulas, ranging between 2 and 12 months after stem cell injection.
Total effect size of stem cell injection therapy for complex perianal fistulas
Complete healing rate, representing the total effect size of all 16 studies, was 62.8% (95% CI 53.5–71.2), indicating a relatively high success rate (Fig. 2). However, test results confirmed moderate heterogeneity with Q = 32.645, p = 0.005, and I2 = 54.05%. Additionally, no significant differences in total effect size were found in the forest plot of one-study-removed meta-analysis for checking bias due to a specific study (Fig. 3). In the forest plot of cumulative meta-analysis for checking bias on the overall results from differences in effect size at different research periods, studies conducted prior to 2012 showed relatively high complete healing rates. Accordingly, the total effect size was derived by excluding all studies published prior to 2012. However, the total effect size was 60.8% (95% CI 50.4–70.3), comparable to the effect size when pre-2012 studies were included (Fig. 4).
Subgroup analysis
Subgroup analysis was performed to determine whether heterogeneity was introduced by systematic errors among the studies.
Analysis based on the presence or absence of Crohn’s perianal fistula
In studies that examined only Crohn’s perianal fistulas, the overall complete healing rate was 64.1% (95% CI 52.3–74.5), while the homogeneity test results showed moderate heterogeneity with Q = 16.649, p = 0.055, and I2 = 45.9%. In studies that examined CPF, excluding Crohn’s perianal fistulas, the complete healing rate was 61.5% (95% CI 36.8–81.4), while the homogeneity test results suggested substantial heterogeneity with Q = 7.937, p = 0.047, and I2= 62.2% (Fig. 5).
Analysis based on the type of stem cells
In studies that used autologous stem cells, the complete healing rate was 69.4% (95% CI 55.9–80.2), while the homogeneity test results showed Q = 31.243, p = 0.001, and I2 = 64.8%. In studies that used allogeneic stem cells, the complete healing rate was 50.7% (95% CI 42.6–58.8), while the homogeneity test results were Q = 0.297, p = 0.961, and I2 = 0% (Fig. 6).
Analysis based on the method for calculating the number of stem cells injected
Between studies in which the number of stem cells injected was adjusted per length and size of the fistula and those that used a fixed number of stem cells, regardless of the length or size, the former group showed a relatively high complete healing rate 70.6% (95% CI 57.5–80.9), while the heterogeneity test results showed Q = 4.249, p = 0.373, and I2 = 5.8%. However, studies that used a fixed number of cells showed a relatively low complete healing rate of 59.4% (95% CI 49.1–69.0), while the heterogeneity test results showed Q = 20.477, p = 0.025, and I2 = 51.2% (Fig. 7).
Analysis based on the criteria for determining complete healing of perianal fistulas
Many studies determined complete healing of perianal fistulas based solely on physical examination. However, studies that used MRI, in addition to physical examination, showed a relatively low complete healing rate of 52.3% (95% CI 42.1–62.2), while the heterogeneity test results showed moderate heterogeneity with Q = 9.596, p = 0.088, and I2 = 47.9%. Studies that relied solely on clinical findings, such as physical examination results, showed complete healing rate of 71.4% (95% CI 62.2–79.2) and heterogeneity test results of Q = 6.670, p = 0.000, and I2 = 0% (Fig. 8).
Analysis based on the time point of determining complete healing of perianal fistulas
Studies that used postoperative 2 months as the time point for determining complete healing showed a complete healing rate of 71.4% (95% CI 60.9–80.0) and heterogeneity test results of Q = 4.300, p = 0.507, and I2 = 0%. Studies that used postoperative 6 months as the time point showed a complete healing rate of 53.5% (95% CI 42.8–64.0) and heterogeneity test results of Q = 11.537, p = 0.073, and I2 = 48.0% (Fig. 9).
Analysis based on randomization
Three RCTs showed a complete healing rate of 50.8% (95% CI 38.0–63.5) and heterogeneity test results of Q = 7.011, p = 0.030, I2 = 71.472 (Fig. 10). Combining these three RCTs and a non-RCT study that compared results with a control group [24] resulted in a total of four comparative studies, which were analyzed separately. Stem cell injection therapy increased the healing rate compared to control groups (OR 0.379, 95% CI 0.152–0.947, p = 0.038) with heterogeneity test results of Q = 10.976, p = 0.012, I2 = 72.666 (Fig. 11).
Risk of bias assessment of individual studies
The MINORS method was used to assess the risk of bias in individual studies. Studies with no control group (N = 12) showed a median score of 11 points (maximum = 16, range 6–14), whereas those with a control group (N = 4) showed a median score of 21 points (maximum = 24, range 20–24) (Table 2). The study by Garcia et al. [34] received two points (adequate) for each item, for a total score of 24 points, which was the maximum total possible, whereas the study by Piejko et al. [18] received the lowest score, six points.
Publication bias analysis
Publication bias analysis using funnel plots showed asymmetrical distribution, indicating possible publication bias due to unpublished studies (Fig. 12). Accordingly, Orwin’s fail-safe N value was calculated under the assumption that the average complete healing rate in unpublished studies was 30% and combined complete healing rate was ≥ 50%. The results estimated the number of unpublished studies as four; thus, a small number of unpublished studies might not have significant impact on the overall results.
Discussion
Our meta-analysis revealed that using stem cell therapy to treat patients with CPF has shown notable success with respect to complete healing rate. Considering that all CPF cases included in the present study were recurrent cases that could not be treated with conventional surgical methods, the total complete healing rate of 62.8% is comparable to that of conventional treatment methods. Our findings suggest that stem cell therapy has significant potential for treating CPF.
There is a growing trend in stem cell research: only three studies were conducted prior to 2012, while 13 have been conducted since 2013. Given the proven safety and easy-to-use nature of the treatment, study results on the application of stem cells for CPF are expected to increase. Accordingly, our findings may help set the direction of such studies. However, the evidentiary power for the efficacy of stem cell therapy for treating CPF is somewhat weak. Among the studies, 14 were Phase I/II clinical trials that investigated the dose or safety of stem cells, while only two were Phase III trials that investigated the efficacy. Therefore, additional well-designed Phase III clinical trials are needed to broaden the clinical application.
In six studies with the largest study populations, the criteria for determining complete healing included MRI findings. When stricter criteria were applied, results showed a relatively low complete healing rate of 52.3%. This has a major implication for how the criteria for complete healing should be determined when similar studies related to complex perianal fistulas are planned.
Techniques of stem cell therapy have been reported to consist of injections in the fistula tract and internal opening in most studies, but protocols slightly differ from one study to another. In a few studies, detailed technique descriptions have not been provided. Borowski et al. [27] used a crisscross lattice technique to inject stem cells into the fistulae. The Spanish research group [17, 25, 29, 31, 34, 35] consistently described the injection technique, emphasizing that they injected no deeper than two mm using a long fine needle; half of the dose was injected around the internal opening and the other half through the external opening. The Korean group [20, 22, 28, 30] also consistently described the injection technique, which is characterized by sufficient curettage of the fistula tract and closure of the internal opening, followed by injection of stem cells in the opening and tracts, then the tract space is filled with a mixture of fibrin glue and stem cells. Lastly, Deitz et al. [19] described the filling of the fistula tract using a plug made of a combination of bioabsorbable matrix and stem cells.
The studies also showed differences in the number of stem cells injected, up to a 10-fold difference in some cases. Some studies, including our previous study [20], adjusted the number of stem cells injected based on the length and size of the fistula, whereas other studies injected a fixed number of cells regardless of the length. Moreover, some studies were designed to administer a second dose, with the cell number increased twofold if the first dose did not show any effect. Such differences in protocols were confirmed through the meta-analysis (70.6% vs. 59.4%); results suggest that adjusting the dose based on the fistula length and increasing the dose by twofold for a second administration if the first treatment fails would lead to a more reasonable study design.
Autologous stem cells showed a slightly higher complete healing rate in the subgroup meta-analysis. Thus, there should be no major difference in complete healing rates between the two types of stem cells, and the difference between the two types may be explained by differences in injection techniques. For autologous stem cells, after adipose tissues were harvested from the patient, the stem cells were cultivated and proliferated for a certain period before being injected back into the patient. Thus, the patient underwent two procedures. During the process of excising the abdominal adipose tissue, CPF was assessed; simultaneously, a preliminary treatment method such as seton drain was performed. After a given period, the patient received the stem cell injection [19, 21, 27]. This two-step surgical procedure may have affected the surgical outcome.
This study has some limitations. The main limitation is that the meta-analysis was performed by considering the complete healing rate of perianal fistula in each study as a simple value. First, each study used different time points for determining the complete healing rate of perianal fistula. Considering the high recurrence rate of CPF, complete healing rate may be influenced significantly by the time point used to determine the complete healing rate. However, the meta-analysis was performed by simply considering the percentage of completely healed patients among all patients as a single value, without statistically supplementing this issue. When a meta-analysis technique is applied to observational studies, limitations in the study design are not reflected; in fact, the limitations of the observational studies may be obscured. Nevertheless, it is important to compile and organize cumulative and meaningful observational studies. Thus, meta-analyses using rigorous research selection criteria, together with existing methods based on conventional review, are being accepted and used widely [36, 37]. Although it would be difficult to represent the healing rates from 2, 6, and 12 months postoperatively as a single value, using such values may be acceptable for determining the overall trend.
Because different criteria were used for various key points, issues may be raised on whether it is appropriate to assess the total effect by a meta-analysis. Accordingly, we performed a subgroup analysis. However, the study methods were so diverse that few studies could be grouped. For example, the overview of individual studies (Table 1) depicts studies based on whether autologous or allogenic stem cells were used. However, Ciccocioppo et al. [38] used bone marrow-derived autologous stem cells, whereas Molendijk et al. [24] used bone marrow-derived allogenic stem cells. It is difficult to confirm if any bias was introduced in the results from differences between ADSCs and bone marrow-derived stem cells.
The present study used MINORS for qualitative assessment of each article, because this method is appropriate for qualitative assessment of non-controlled, single-group studies [39]. Only four studies had a control group and MINORS scores were relatively high, but the median score of 12 non-controlled, single-group studies showed a median value of 11 points (maximum = 16). Regarding the prospective calculation of sample size, only one study calculated the sample size. Regarding blinding during outcome assessment, only one study attempted to strengthen its blinding method by having a gastroenterologist/surgeon, who did not directly participate in the study, re-visit for outcome assessment.
Future studies should attempt to minimize bias pertaining to MINORS items. Study methods should use a dose based on the fistula length and size and administer the corresponding number of stem cells. Furthermore, a failed first round of stem cell therapy should be followed by a second round with a twofold higher number of stem cells.
One year after treatment might be the appropriate time point for determining complete healing and including MRI in the criteria for determining complete healing would ensure greater accuracy. Moreover, healing rate may be improved by administering stem cell therapy after a certain period following preliminary treatment, such as seton drain. RCT that includes a control group should be chosen, but since there is currently no standard treatment for CPF, a study with three groups, using treatment by rectal flap and fibrin glue, as the control groups may represent a clinical trial that provides better comparisons.
Conclusions
Stem cell injection may be a sound alternative for treating complex perianal fistulas that cannot be treated by conventional surgical methods. Stem cell therapy can be applied regardless of whether the fistulas are associated with CD. More favorable outcomes can be achieved by injecting autologous stem cells, with the number of stem cells being proportional to the fistula size. However, additional supportive evidence is needed through large-scale RCTs.
References
Corman ML, Nicholls RJ, Fazio VW (2013) Corman’s colon and rectal surgery, 6th edn. Lippincott Williams & Wilkins, Philadelphia
Parks AG, Stitz RW (1976) The treatment of high fistula-in-ano. Dis Colon Rectum 19:487–499
Steele SR, Kumar R, Feingold DL, Rafferty JL, Buie WD (2011) Practice parameters for the management of perianal abscess and fistula-in-ano. Dis Colon Rectum 54:1465–1474. https://doi.org/10.1097/DCR.0b013e31823122b3
Cadeddu F, Salis F, Lisi G, Ciangola I, Milito G (2015) Complex anal fistula remains a challenge for colorectal surgeon. Int J Colorectal Dis 30:595–603. https://doi.org/10.1007/s00384-014-2104-7
Disease sub-category statistics. Healthcare Big Data Opening System 2017. http://opendata.hira.or.kr/op/opc/olap3thDsInfo.do. Accessed 18 Apr 2018
Loungnarath R, Dietz DW, Mutch MG, Birnbaum EH, Kodner IJ, Fleshman JW (2004) Fibrin glue treatment of complex anal fistulas has low success rate. Dis Colon Rectum 47:432–436
Balciscueta Z, Uribe N, Balciscueta I, Andreu-Ballester JC, Garcia-Granero E (2017) Rectal advancement flap for the treatment of complex cryptoglandular anal fistulas: a systematic review and meta-analysis. Int J Colorectal Dis 32:599–609. https://doi.org/10.1007/s00384-017-2779-7
Chuang-Wei C, Chang-Chieh W, Cheng-Wen H, Tsai-Yu L, Chun-Che F, Shu-Wen J (2008) Cutting seton for complex anal fistulas. Surgeon 6:185–188
van Koperen PJ, D’Hoore A, Wolthuis AM, Bemelman WA, Slors JF (2007) Anal fistula plug for closure of difficult anorectal fistula: a prospective study. Dis Colon Rectum 50:2168–2172
Grimaud JC, Munoz-Bongrand N, Siproudhis L et al (2010) Fibrin glue is effective healing perianal fistulas in patients with Crohn’s disease. Gastroenterology 138:2275–2281. https://doi.org/10.1053/j.gastro.2010.02.013
Zuk PA, Zhu M, Mizuno H et al (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7:211–228. https://doi.org/10.1089/107632701300062859
Zuk PA, Zhu M, Ashjian P et al (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295. https://doi.org/10.1091/mbc.e02-02-0105
Rodriguez AM, Pisani D, Dechesne CA et al (2005) Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J Exp Med 201:1397–1405. https://doi.org/10.1084/jem.20042224
Aurich H, Sgodda M, Kaltwasser P et al (2009) Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut 58:570–581. https://doi.org/10.1136/gut.2008.154880
Gonzalez MA, Gonzalez-Rey E, Rico L, Buscher D, Delgado M (2009) Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology 136:978–989. https://doi.org/10.1053/j.gastro.2008.11.041
Kolle SF, Fischer-Nielsen A, Mathiasen AB et al (2013) Enrichment of autologous fat grafts with ex vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. Lancet 382:1113–1120. https://doi.org/10.1016/S0140-6736(13)61410-5
Panes J, Garcia-Olmo D, Van Assche G et al (2016) Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase three randomised, double-blind controlled trial. Lancet 388:1281–1290. https://doi.org/10.1016/S0140-6736(16)31203-X
Piejko M, Romaniszyn M, Borowczyk-Michalowska J, Drukala J, Walega P (2017) Cell therapy in surgical treatment of fistulas. Preliminary results. Polski przeglad Chirurgiczny 89:48–51
Dietz AB, Dozois EJ, Fletcher JG et al (2017) Autologous mesenchymal stem cells, applied in a bioabsorbable matrix, for treatment of perianal fistulas in patients with Crohn’s disease. Gastroenterology 153:59–62.e52. https://doi.org/10.1053/j.gastro.2017.04.001
Choi S, Ryoo SB, Park KJ et al (2017) Autologous adipose tissue-derived stem cells for the treatment of complex perianal fistulas not associated with Crohn’s disease: a phase II clinical trial for safety and efficacy. Tech Coloproctol 21:345–353. https://doi.org/10.1007/s10151-017-1630-z
Wainstein C, Quera R, Kronberg U et al (2016) Mesenchymal stem cells and platelet-rich plasma in the treatment of patients with perineal Crohn’s disease. Int J Colorectal Dis 31:725–726. https://doi.org/10.1007/s00384-015-2221-y
Park KJ, Ryoo SB, Kim JS et al (2016) Allogeneic adipose-derived stem cells for the treatment of perianal fistula in Crohn’s disease: a pilot clinical trial. Colorectal Dis 18:468–476. https://doi.org/10.1111/codi.13223
Garcia-Arranz M, Herreros MD, Gonzalez-Gomez C et al (2016) Treatment of Crohn’s-related rectovaginal fistula with allogeneic expanded-adipose derived stem cells: a phase I–IIa clinical trial. Stem Cells Transl Med 5:1441–1446. https://doi.org/10.5966/sctm.2015-0356
Molendijk I, Bonsing BA, Roelofs H et al (2015) Allogeneic bone marrow-derived mesenchymal stromal cells promote healing of refractory perianal fistulas in patients with Crohn’s disease. Gastroenterology 149:918–927. https://doi.org/10.1053/j.gastro.2015.06.014
Garcia-Olmo D, Guadalajara H, Rubio-Perez I, Herreros MD, De-La-Quintana P, Garcia-Arranz M (2015) Recurrent anal fistulae: limited surgery supported by stem cells. World J Gastroenterol 21:3330–3336. https://doi.org/10.3748/wjg.v21.i11.3330
Cho YB, Park KJ, Yoon SN et al (2015) Long-term results of adipose-derived stem cell therapy for the treatment of Crohn’s fistula. Stem Cells Transl Med 4:532–537. https://doi.org/10.5966/sctm.2014-0199
Borowski DW, Gill TS, Agarwal AK, Tabaqchali MA, Garg DK, Bhaskar PUD (2015) Adipose tissue-derived regenerative cell-enhanced lipofilling for treatment of cryptoglandular fistulae-in-ano: the ALFA technique. Surg Innov 22:593–600. https://doi.org/10.1177/1553350615572656
Lee WY, Park KJ, Cho YB et al (2013) Autologous adipose tissue-derived stem cells treatment demonstrated favourable and sustainable therapeutic effect for Crohn’s fistula. Stem Cell 31:2575–2581. https://doi.org/10.1002/stem.1357
de la Portilla F, Alba F, Garcia-Olmo D, Herrerias JM, Gonzalez FX, Galindo A (2013) Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis 28:313–323. https://doi.org/10.1007/s00384-012-1581-9
Cho YB, Lee WY, Park KJ, Kim M, Yoo HW, Yu CS (2013) Autologous adipose tissue-derived stem cells for the treatment of Crohn’s fistula: a phase I clinical study. Cell Transl 22:279–285. https://doi.org/10.3727/096368912X656045
Herreros MD, Garcia-Arranz M, Guadalajara H, De-La-Quintana P, Garcia-Olmo D (2012) Autologous expanded adipose-derived stem cells for the treatment of complex cryptoglandular perianal fistulas: a phase III randomized clinical trial (FATT 1: fistula advanced therapy trial 1) and long-term evaluation. Dis Colon Rectum 55:762–772. https://doi.org/10.1097/DCR.0b013e318255364a
Guadalajara H, Herreros D, De-La-Quintana P, Trebol J, Garcia-Arranz M, Garcia-Olmo D (2012) Long-term follow-up of patients undergoing adipose-derived adult stem cell administration to treat complex perianal fistulas. Int J Colorectal Dis 27:595–600. https://doi.org/10.1007/s00384-011-1350-1
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62:1006–1012
Garcia-Olmo D, Herreros D, Pascual I et al (2009) Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum 52:79–86. https://doi.org/10.1007/DCR.0b013e3181973487
Garcia-Olmo D, Garcia-Arranz M, Herreros D, Pascual I, Peiro C, Rodriguez-Montes JA (2005) A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum 48:1416–1423. https://doi.org/10.1007/s10350-005-0052-6
Greenland S (1994) Can meta-analysis be salvaged? Am J Epidemiol 140:783–787
Stockdale AJ, Chaponda M, Beloukas A et al (2017) Prevalence of hepatitis D virus infection in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health 5:e992–e1003. https://doi.org/10.1016/S2214-109X(17)30298-X
Ciccocioppo R, Bernardo ME, Sgarella A et al (2011) Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut 60:788–798. https://doi.org/10.1136/gut.2010.214841
Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J (2003) Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 73:712–716
Funding
This study received no external grants or funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Data availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Choi, S., Jeon, B., Chae, G. et al. The clinical efficacy of stem cell therapy for complex perianal fistulas: a meta-analysis. Tech Coloproctol 23, 411–427 (2019). https://doi.org/10.1007/s10151-019-01994-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-019-01994-z