Abstract
Background
The aim of the present study was to assess the relationship between symptoms of obstructed defecation and findings on magnetic resonance (MR) defecography in males with obstructed defecation syndrome (ODS).
Methods
Thirty-six males with ODS who underwent MR defecography at our institution between March 2013 and February 2016 were asked in a telephone interview about their symptoms and subsequent treatment, either medical or surgical. Patients were divided into 2 groups, one with anismus (Group 1) and one with prolapse without anismus (Group 2). The interaction between ODS type and symptoms with MR findings was assessed by multivariate analysis for categorical data using a hierarchical log-linear model. MR imaging findings included lateral and/or posterior rectocele, rectal prolapse, intussusception, ballooning of levator hiatus with impingement of pelvic organs and dyskinetic puborectalis muscle.
Results
There were 21 males with ODS due to anismus (Group 1) and 15 with ODS due to rectal prolapse/intussusception (Group 2). Mean age of the entire group was 53.6 ± 4.1 years (range 18–77 years). Patients in Group 1 were slightly older than those in Group 2 (age peak, sixth decade in 47.6 vs 20.0%, p < 0.05). Symptoms most frequently associated with Group 1 patients included small volume and hard feces (85.0%, p < 0.01), excessive strain at stool (81.0%, p < 0.05), tenesmus and fecaloma formation (57.1 and 42.9%, p < 0.05); symptoms most frequently associated with Group 2 patients included mucous discharge, rectal bleeding and pain (86.7%, p < 0.05), prolonged toilet time (73.3%, p < 0.05), fragmented evacuation with or without digitation (66.7%, p < 0.005). Voiding outflow obstruction was more frequent in Group 1 (19.0 vs 13.3%; p < 0.05), while non-bacterial prostatitis and sexual dysfunction prevailed in Group 2 (26.7 and 46.7%, p < 0.05). At MR defecography, two major categories of findings were detected: a dyskinetic pattern (Type 1), seen in all Group 1 patients, which was characterized by non-relaxing puborectalis muscle, sand-glass configuration of the anorectum, poor emptying rate, limited pelvic floor descent and final residue ≥ 2/3; and a prolapsing pattern (Type 2), seen in all Group 2 patients, which was characterized by rectal prolapse/intussusception, ballooning of the levator hiatus with impingement of the rectal floor and prostatic base, excessive pelvic floor descent and residue ≤ 1/2. Posterolateral outpouching defined as perineal hernia was present in 28.6% of patients in Group 1 and were absent in Group 2. The average levator plate angle on straining differed significantly in the two patterns (21.3° ± 4.1 in Group 1 vs 65.6° ± 8.1 in Group 2; p < 0.05). Responses to the phone interview were obtained from 31 patients (18 of Group 1 and 13 of Group 2, response rate, 86.1%). Patients of Group 1 were always treated without surgery (i.e., biofeedback, dietary regimen, laxatives and/or enemas) which resulted in symptomatic improvement in 12/18 cases (66.6%). Of the patients in Group 2, 2/13 (15.3) underwent surgical repair, consisting of stapled transanal rectal resection (STARR) which resulted in symptom recurrence after 6 months and laparoscopic ventral rectopexy which resulted in symptom improvement. The other 11 patients of Group 2 were treated without surgery with symptoms improvement in 3 (27.3%).
Conclusions
The appearance of various abnormalities at MR defecography in men with ODS shows 2 distinct patterns which may have potential relevance for treatment planning, whether conservative or surgical.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the past two decades, magnetic resonance imaging (MRI) of the pelvis in males has been almost exclusively limited to the diagnosis and staging of pelvic malignancies, namely rectal and prostate cancers. Accordingly, much emphasis has been placed on detection of extramural and perivisceral extension of the disease, as well as fascial involvement and lymph node metastases, due to their relevance in total mesorectal excision and radical prostatectomy, respectively [1,2,3,4]. As a consequence, despite the plethora of references in the literature to the MRI features of evacuatory dysfunction [5,6,7,8,9], the utility of this diagnostic tool is poorly documented in males [10, 11] and the potential relevance of the examination for assessing the development of pelvic floor dysfunction has remained completely overlooked. The aim of the present study was to determine the relationship between evacuatory dysfunction and pelvic floor abnormalities in men with obstructed defecation syndrome (ODS) and to describe the technical details and diagnostic criteria for magnetic resonance (MR) defecography technique when applied to males with ODS.
Materials and methods
Patients
The clinical series of all male patients with impaired evacuation referred to our diagnostic center between March 2013 and February 2016 to undergo MR defecography, was reviewed. These men were telephoned by the staff nurses (G.M, P.G.) and asked to provide information regarding their current disease status, and type and date of any conservative treatment or pelvic surgery. The original MR images were retrieved from the local diagnostic imaging archiving system and compared with symptoms listed by the patient in a form filled out in the waiting room at the time of the examination. Indications for the examination included impaired evacuation and ODS. Symptoms of ODS include difficulty in expulsion, straining at stool for more than 25% of the time, prolonged toilet time, hard feces, feeling of unsatisfactory emptying and need for self digitation [12]. When present, associated symptoms of lower urinary tract symptoms (LUTS) such as difficulty in initiating voiding (hesitancy), prolonged voiding time > 40 s, reduced urinary stream, and urgency/frequency were recorded. Prior to imaging, during the preliminary interview, all patients gave written informed consent to the examination and were coached on the details of the two subsequent portions of the examination, i.e., phase 1 (dynamic) and phase 2 (static). More precisely, they were instructed to start rectal emptying at will and just notify the examiner via intercom to allow for contemporary acquisition of images during two subsequent series of 120 and 60 s, respectively, with an interval of 60-s relaxing time. Thereafter, a different dynamic maneuver was explained which consisted of a Valsalva maneuver, to be maintained without interruption. The specific instructions for performing it properly were the following: “first take a deep breath so as to maintain enough air inside the chest then keep your mouth and nose closed and bear down to produce your maximal pelvic strain, starting now and holding that position without interrupting the maneuver for 10 s until told to breathe and relax.” Finally, after completion of the dynamic phase, patients were instructed to relax and just maintain the fixed position on the diagnostic table to allow for phase 2 (static) image acquisition of pelvic anatomy.
Imaging technique
MRI studies were performed (M.B.) on a 1.5 T, horizontally oriented scanner (Philips; Achieva Nova model, The Netherlands) using a SENSE XL TORSO, four elements, surface coil wrapped around the patient’s pelvis. According to a previously described technique [13], rectal cleansing 3 h before the examination was required in all cases unless patients reported spontaneous evacuation in the morning. Just before imaging, patients were asked to void so as to have their bladder empty; then, they were placed on the MRI table in the left lateral horizontal (Sims) position and up to 300 ml of acoustic transmission gel was injected as rectal contrast material via a 3-mm-wide rubber catheter. After the probe was withdrawn, patients were turned supine and a water-proof pad was placed beneath the exposed buttocks to collect any material, before patients were positioned within the gantry opening to start acquisition of images. In order to document rectal expulsion (examination phase 1) in both the sagittal and coronal plane, the balanced turbo field echo (BTFE) pulse sequence is employed (TR, 2.7 ms; TE, 1.3 ms; 45° flip angle; 30-mm-thick section; FOV, 300 mm; 256 × 256 matrix and two averages; 1 im/0.768 s over 43 s) using the so-called single slice, multiple maneuver technique. In simple terms, this technique consists of exciting the same midsagittal or midcoronal body section properly chosen from the region of interest continually during a rest–strain emptying cycle at a rate of 1 image/every 0.786 s. The adoption of this strategy at the very beginning of the examination proved effective for reducing the rate of evacuation failure caused by excessive delay and/or vanishing sensation. However, if this occurred, patients were asked to have a bowel movement in the toilet (so-called toilet test) and were re-examined in the diagnostic room immediately afterward. Then, moving through the steady-state Valsalva portion of the dynamic examination, the “multiple slice, single maneuver” technique was used (TR, 4.1 ms; TE, 1.4 ms; flip angle, 45°; 10-mm-thick section; 256 × 256 matrix and two averages; FOV, 300 mm; 2.7 s/slice over 13 s). In practice, after selecting a couple of different, contiguous 10-mm-thick body sections relative to the symphysis pubis, two straining series were obtained which allowed comparison with those at rest using the same anatomic landmarks for evidence of (a) any geometrical deformity or enlargement of the levator hiatus and (b) impingement of various organs within it. Finally, the static pelvic anatomy was depicted (examination phase 2) in all three planes (sagittal, axial and coronal) using the turbo spin echo (TSE) T2-weighted pulse sequence (TR, range 3000–6000 ms; TE, 100 ms; flip angle, 90°; thickness, 3–4-mm, reconstruction matrix, 576 and 1 average; FOV, 350 mm, acq. time, 3:44 min; total images, 35). The field of view extends from the anal verge (bottom level) to the upper margin of the iliac crest (upper level) and from the sacrococcygeal bone (backward) to the anterior margin of the pubic bone (forward) so as to include the anal sphincter complex, perianal region, levator plate and ischiorectal fossae, the prostate gland and seminal vesicles, the distal gut and the urinary bladder. A complete summary of the imaging protocol adopted is presented in Table 1.
Image analysis
MR images were analyzed by one radiologist experienced in pelvic floor imaging (V.P.) according to well-established diagnostic criteria existing in the radiological literature [14,15,16,17,18,19]. More specifically, images were analyzed for evidence of any bulge extending more than 20 mm beyond the expected line of the anterior rectal wall, which was defined as rectocele. Lateral and posterior bulging was defined as perineal hernia to indicate that the likely defect occurred through the levator ani muscle rather than the anterior midline. Mucosal prolapse was defined as an internal rectal wall folding, up to 3 mm in thickness, occurring in the anterior or posterior margin which did not show a tendency to migrate distally on straining or during evacuation. Conversely, intussusception was defined as any descending full-thickness invagination of the rectal wall greater than 3 mm, not reaching beyond the anal verge as an external rectal prolapse. Although sometimes visible in the anterior or posterior margin, it most frequently affects the rectum circumferentially and is called intrarectal when it remains within the rectum, and intra-anal if its apex penetrates the anal canal. Also, a prominent puborectalis impression during attempted defecation, whether transient or persistent, coupled with failure of the anorectal angle and/or the anal canal to open was considered indicative of dyskinetic contraction affecting the levator ani muscle, the anal sphincter complex or both. In addition, a typical geometrical deformity of the gut profile during expulsion of contrast was noted, consisting of a more or less symmetric and persistent narrowing at the anorectal junction which produced a “sand-glass-like” appearance. With regard to the emptying of rectal contrast, special care was taken to analyze the evacuation performance in order to differentiate normal subjects, who empty their rectum rapidly and completely, from those who have prolonged, incomplete or even failed evacuation. More particularly, quantification of evacuation performance was attempted by registering the time taken to initiate anal canal opening from the start signal given by the examiner, the rate of evacuation during image acquisition, and the final amount of contrast retained after no less than 3 attempts. Overall, the evacuation performance was classified as effective (residue, less than 1/3 within the standard average time of 3 min), unsatisfactory (residue, ≥ 1/2 the total volume injected), or overtly ineffective (residue, ≥ 2/3 even after the toilet test). On midsagittal dynamic images, quantification of visceral descent during emptying was performed measuring (in mm) the deepest vertical distance of the bladder neck, base of prostate gland and rectal floor relative to the reference line (zero line), defined as the horizontal line drawn on the screen tangent to the inferior border of the symphysis pubis (Figs. 1, 2a, b). This was preferred to the more common pubococcygeal line (PCL), i.e., the line joining the inferior border of the symphysis to the last sacrococcygeal joint because it is less dependent on the inclination/configuration of spine, and virtually identical to the hymen plane which is the universally accepted anatomical reference for genital prolapse in use all over the world. In addition the levator plate angle was measured as the angle between the slope of the iliococcygeus muscles, which fuse at the midline anterior to the coccyx to form the levator plate, and a horizontal reference line tangent to the levator plate drawn on the screen. The width of the levator hiatus was also measured as the H-line, from the pubis to the posterior anorectal junction. Finally, on axial images diffuse bulging of the levator ani muscle at the level of the pubic symphysis, resulting in an abnormal increase in the area of the pelvic hiatus, was termed “ballooning.” On static MR images, the identity of various anatomic layers, including the peritoneal reflections, linear condensations of the endopelvic fascia, periprostatic, mesorectal and Denonvilliers’ fascia [20], were noted.
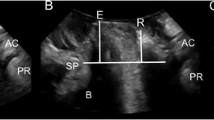
T2-weighted MR midsagittal image: method for drawing the horizontal reference line (continuous red line) to quantify the vertical distance of prostatic base (dotted yellow line) and rectal floor (dotted white line) at rest and on straining. The positions of prostate base and bladder neck are coincident. Sy = symphysis pubis; Bl = bladder; R = rectum
Statistical analysis
Simple statistics of mean, median, standard deviation (SD) and range were calculated for all data. The interaction among various symptoms in the group with anismus and prolapse/intussusception was assessed by multivariate analysis for categorical data using a hierarchical log-linear (HLL) model and compared with findings at MRI. A p value less than 0.05 was considered statistically significant. Calculations were performed with SPSS/PC + software.
Results
Patient characteristics
Thirty-six male patients with a mean age of 53.6 ± 4.1 years (range 18–77 years) were included in the study. On the basis of presenting symptoms and results of the physical examination, including digital rectal examination and ano-proctoscopy, the patient population was subdivided into two different categories, as follows: patients with anismus (Group 1): n = 21 (58.3%) and patients with prolapse/intussusception (Group 2): n = 15 (41.6%) patients) 4 of whom also had mild intermittent anismus. Overall, patients with anismus were older than those with prolapse/intussusception (mean age 57.1 years ± 3.2 vs 42.9 years ± 4.9; p < 0.05) and there was an age peak in the sixth decade 47.6 vs 20.0% (p < 0.05).
Presenting symptoms
With regard to the association among various presenting symptoms (Table 2), small volume and hard feces, excessive straining at stool and tenesmus occurred more frequently in Group 1, while mucous discharge and/or bleeding and pain, prolonged toilet time and fragmented defecation due to a feeling of incomplete evacuation with or without digitation were more frequent in Group 2. Voiding outflow obstruction was more common in Group 1 (19.0 vs 13.3%, p < 0.05), while non-bacterial prostatitis and sexual complaints were significantly more common in Group 2 (26.7 and 46.7 vs 4.8% and 0, respectively, p < 0.05).
MRI findings
The MR study was well tolerated by all patients who were all able to perform the various maneuvers during image acquisition (mean time, 21 ± 4 min), according to the instructions received. When the MR images were analyzed (Table 3), all Group 1 patients showed a persistent impression of the puborectalis muscle at the posterior margin of the anorectal junction on emptying, together with minimal pelvic organ descent and lack of levator plate angle widening; these findings were associated with ≥ 2/3 contrast retention after emptying in 85.7% and a typical deformity of the anorectal configuration, with an asymmetric “sand-glass” appearance (Fig. 3) in 52.4% of cases. On the other hand, all Group 2 patients had single or double folding of the rectal wall upon itself, i.e., intussusception occurring concurrently with detachment of the Denonvilliers’ fascia (Fig. 4), coupled with hiatus ballooning and impingement of the prostate base and seminal vesicles. More precisely (Table 4), a significant difference was registered between the two groups with regard to (a) the levator plate angle (21.3° ± 4.1 vs 65.6° ± 8.1, p < 0.05); (b) the anteroposterior diameter of the levator hiatus, i.e., H-line (34.1 mm ± 5.3 vs 80.4 mm ± 6.2, p < 0.05); (c) the degree of rectal floor and prostate base descent on straining (20.2 mm ± 8.1 vs 31.2 mm ± 6.3; and − 34.6 mm ± 5.2 vs − 10.4 mm ± 9.1, respectively, p > 0.05); (d) the time to start anal opening (≤ 180 s. in 38.8 vs 13.8%, p < 0.05) and the final residue (≥ 2/3 in 85.7 vs 20.0%, p < 0.05). Interestingly, an outpouching of the anterior rectal wall occurred in 3 cases [2 in Group 1 (9.5%) and 1 in Group 2 (6.7%), p ns]. However, the outpouching was never seen to exceed 15 mm and therefore it was not diagnosed as rectocele according to our predefined criteria. Rather, the lateral and/or posterior walls were affected in 6 patients (28.6%) all of them in Group 1 (dyskinetic pattern). Posterolateral outpouchings were best seen on coronal and axial dynamic BTFE images and were considered consistent with perineal hernia (Fig. 5a, b). On midsagittal MR images, the position of the bladder neck and prostatic base virtually coincided; the descent of the latter on straining was taken as representative of both measurements. Finally, a close correlation was found between the downward displacement of the base of the prostate and the downward displacement of the rectal floor on emptying (Fig. 6), suggesting a possible interaction between these two variables. On static MR images, with the exception of fascia around the inferior hypogastric plexus, which appeared on T2-weighted MR parasagittal images as high signal intensity rectangular, mesh-like, fenestrated structures, all other peritoneal reflections and fascial condensations were invariably seen as low signal intensity structures; among them, the median umbilico-vesical ligament (urachus), the peritoneal reflection over the surface of the bladder, the seminal vesicles and anterior surface of rectum, the Denonvilliers’ fascia, mesorectal fascia and rectosacral (Waldeyer’s) fascia were consistently identified on sagittal images up to 71.3% of cases.
MR defecography in a 57-year-old man with a 6-month history of tenesmus, excessive strain at stool and weight sensation at the perineal region: balanced turbo filed echo midsagittal image a taken at the beginning of emptying after a 30-s delay from the command to start the movement; note the persistent impression of the puborectalis muscle on the posterior anorectal junction coupled with the typical “sand-glass” deformity of the distal gut, consistent with Type 1 dyskinetic obstructive defecation. b Image taken just 10 s later a showing the progressive closure of the anal outflow by the puborectalis muscle (arrow) until interruption of the stream without any further emptying despite repeated attempts. Sy = symphysis pubis; Bl = bladder; Pr = prostate; R = rectum
MR defecography in a 35-year-old man with a 5-year history of fragmented evacuation, mucous discharge and painful evacuation: a quick emptying of contrast material showing double infolding of the rectal wall consistent with rectoanal intussusception starting at the point of detachment of Denonvilliers’ fascia (arrow); b corresponding axial dynamic image taken at the upper level of the levator hiatus (see dotted line, a) showing ballooning of the levator hiatus with impingement of bladder, seminal vesicles (arrow) and rectum, closely resembling the condition commonly observed in women with descending perineum syndrome. Sy = symphysis pubis; Bl = bladder; Sv = seminal vesicles; R = rectum
Follow-up interviews
A total of 31 (86.1%) responses were obtained 18/21 of Group 1 and 13/15 of Group 2. Interviews were carried out at a median time interval of 18 months (range, 9–35 months) from imaging. Patients with anismus after confirmation by MRI were always treated without surgery (i.e., biofeedback, dietary regimen, laxatives and/or enema) which resulted in symptoms being partially relieved in 12/18 cases (66.6%) and unchanged in the remaining 6/18 cases (33.3%). Of the patients in Group 2, 2/13 (15.3%) underwent surgical repair, consisting of stapled transanal rectal resection (STARR) which resulted in symptom recurrence after 6 months and laparoscopic ventral rectopexy which resulted in symptom improvement, respectively. The other 11 were treated without surgery with symptoms improvement in 3 (27.3%).
Discussion
In the last 2 decades, technical advances in MRI, have made this diagnostic tool an accurate method for the study of pelvic floor anatomy and evacuation disorders. Reasons for its wide acceptance and rapid spread all over the world include lack of ionizing radiation, multiplanarity, and the ability to provide high-quality images of soft tissues clearly demonstrating the behavior of pelvic viscera during evacuation of rectal contrast, an examination called MR defecography. Much has been written about the less physiological nature of the examination when compared to X-ray defecography. The patient is usually studied supine, but when patients are asked to evacuate contrast on the table, the ability to do so can be taken as evidence of adequate straining. Most authors today agree about the superior reproducibility of different quantitative measurements made by MRI of all relevant parameters and the increase in diagnostic confidence as regards various abnormalities affecting pelvic floor structures. Until recently, however, this examination has been almost exclusively employed in women with pelvic floor dysfunction [5,6,7,8,9] for staging of pelvic organ prolapse, investigation of trauma to the pelvic floor during vaginal delivery and pre-/postoperative evaluation of ODS to name a few.
Studies investigating the appearance of pelvic floor dysfunction in males are scarce and poorly detailed. The first, published in 1987, was the classic paper of Skomorowska et al. [21] who reported the existence of anatomical differences between genders in the measurement of anorectal angle and pelvic floor descent. Subsequently, in 1991, Cavallo et al. [22] described the imaging features of rectocele in 8 men. Using a combination of X-ray defecography and computed tomography, they showed that the pocket was located between the prostatic apex and the urogenital diaphragm. Ten years later, Chen et al. [23] in their investigation of 234 males with evacuation dysfunction and an average duration of symptoms of 10.3 years, looked at the association between defecography and clinical-physiological findings. They demonstrated the presence of rectocele in 48% of cases, almost equally located anteriorly or posteriorly and coupled with prior prostatectomy in up to 40% of cases. In addition, a dyskinetic puborectalis was the most common associated feature (65%), followed by rectoanal intussusception (23%). More recently, Savoye-Collet et al. [24] in their reappraisal of the influence of gender on the development of abnormalities on defecography in patients with constipation and pelvic floor disorders, compared the results of 66 men with those of a 198 women. Interestingly, no significant difference between genders was found for the diagnosis of intussusception (57.6% in men vs 44.9% in women), while a perineal descent of 30 mm rather than 20 mm at rest and a rectocele were more frequently seen in women (50 vs 20%, p < 0.005; and 44.4 vs 4.5%, p < 0.001, respectively). Finally, in 2014 Andrade et al. [25] after reviewing the most common abnormalities seen at conventional defecography in 24 men and 266 women, reported that dyskinetic puborectalis syndrome, was most common in men (37 vs 9.4%, p < 0.01; OR 5.78) whereas excessive perineal descent at rest or on evacuation was most frequently observed in women in their 50 s (60.3 and 81.8%, respectively, p < 0.001; OR 6.8). Most surprisingly, the authors reported the presence of rectocele in up to 100% of males, even though they admitted that the depth of rectal outpouching was less than 2 cm in all cases. Recently Mikuma et al. conducted an MR defecography study on a group of healthy men [26]. However, to the best of our knowledge no study has explored the spectrum of abnormalities in men with ODS, as seen on MR defecography.
Based on robust anatomical and functional discriminants identifiable on sagittal, coronal and axial MR images, we identified two distinctive abnormal patterns: Type 1, was a dyskinetic pattern, accounting for 58.3% of cases, characterized by a combination of features which included (a) a persistent impression of the puborectalis muscle at the posterior aspect of the anorectal junction, with or without the so-called sand-glass deformity of the gut contour (see Fig. 3); (b) delay in starting anal opening and weak stream of contrast during the entire emptying phase or no emptying at all despite repeated attempts; (c) lesser downward displacement of pelvic organs on straining; and (d) a greater residue of contrast (≥ 2/3 the volume injected). Additional features included posterior and lateral outpouching consistent with perineal hernia, limited downward displacement of pelvic organs on straining relative to the reference line, lack of anal canal widening, and an average H-line on emptying of 40 ± 5 mm. Most commonly, Type 1 pattern was associated with symptoms such as tenesmus, small and hard feces, excessive straining at stool and prolonged toilet time. At the other end of the spectrum, Type 2 prolapsing pattern accounted for 41.6% of the patient population examined and included the following: (a) rectoanal intussusception of the rectal wall in which the anterior inversion point always started at the exact point and time of its detachment from the Denonvilliers fascia (Fig. 4); (b) excessive descent of the rectal floor and prostatic base on straining; and (c) a smaller residue of contrast after emptying (exceeding half of the volume injected in no more than 20% of cases). Additional findings included, ballooning of the levator ani hiatus with impingement of pelvic organs closely resembling the picture commonly seen in the female population with descending perineum syndrome; and an average H-line of 70 mm ± 5. Accordingly, the most frequent symptoms associated with Type 2 pattern, were mucous discharge, pain, fragmented evacuation and digitation to help defecation.
Through a more in-depth analysis and interpretation of Type 1 dyskinetic pattern, it is interesting to note that the most relevant abnormalities seen in the present study were largely consistent with those described by Spazzafumo et al. [19] in a study which included 581 patients with evacuation dysfunctions, 155 of whom were men, evaluated with multiple correspondence analysis (MCA) of defecography signs. In their paper, the authors interpreted the close association among delayed emptying, high residue, and dyskinetic outlet obstruction as an indication for a rehabilitation program. The major contribution made by the current study is the exclusive association of the dyskinetic pattern (Group 1) with the presence of posterolateral outpouching of the rectal wall through a focal defect of the levator ani muscle, which is a unique feature of dynamic axial MR images (see Fig. 5) to be interpreted more properly as perineal hernia.
Not by chance, the total absence of anterior rectocele in our study is in stark contrast with both the 100% rate reported by Andrade et al. [25] and the 48% rate reported by Chen et al. [23]. When attempting to explain such a dramatic difference, it is important to keep in mind that these authors considered any outpouching < 2 cm in depth, measured perpendicular to the expected position of the anterior rectal wall, to be a rectocele which may lead to overdiagnosis of the abnormality, whatever the imaging modality used.
With regard to Type 2 prolapsing pattern, the advantage of MR defecography over evacuation proctography in the depiction of symptomatic intussusception was clearly described by Dvorkin et al. in their elegant paper on 10 patients, 4 of whom were male, using open MRI [10]: MRI allowed better characterization of various morphological features, including the thickness of rectal wall infolding and the depth of pelvic floor descent although it missed 3 of 10 intussusceptions seen on evacuation proctography. The present study, however, has added some important new features to the list such as evidence that the inversion point of the intussusception began exactly at the site of detachment of the rectal wall from the Denonvilliers’ fascia in all cases and was coupled with the development of abnormal hiatus ballooning and impingement of pelvic organs. On the basis of such observations, we hypothesize that the onset of rectoanal intussusception might be the effect of an excessive sliding motion occurring on the surfaces of structures in contact with one another due to repetitive overload until the forces which normally ensure adhesion are overcome. This hypothesis is supported by the evidence of a close correlation between the rectal floor descent and prostatic base descent on straining (see Fig. 6) and suggests the possibility that some of the LUTS seen more frequently in patients with the prolapse pattern share a common etiology.
We also described the association of LUTS with these 2 distinct MRI patters: symptoms consistent with urinary obstruction such as hesitancy, prolonged voiding time > 60 s and residue in the bladder > 150 ml were more common in Group 1 patients while non-bacterial prostatitis and complaints interfering with sexual activity (erectile performance or even painful ejaculation), which were most commonly associated with Group 2 patients and the MR Type 2 prolapsing pattern (see Table 2).
Another contribution of the present study was the evidence that the boundaries of the levator hiatus in men are potentially subjected to the same overload deformities already known in women. This despite the absence of pelvic floor trauma related to vaginal delivery. Although the clinical relevance of such a finding regarding the origin of pelvic floor dysfunction in men remains to be established, this observation highlights the importance of including a detailed analysis of the endopelvic fascia and fat recesses as a routine and crucial portion of the MR examination of the male pelvis. In men, similarly to what occurred in women, the main value of MR defecography seems to be related to the ability to detect the presence of unsuspected abnormalities amenable of conservative or surgical treatment and to help the clinician plan treatment.
Limitations of the study are in the retrospective nature and in the inherent limits of the arbitrary classification of the patients in the 2 groups of anismus and rectal/prolapse/intussusception. In fact, it is known that these 2 groups may overlap. Moreover 14% of patients were lost to follow-up and phone call as follow-up method may not accurately reflect the treatment and its outcome.
Conclusions
The absence of childbirth-related trauma to the pelvic floor musculature makes it easier to understand the origin of rectal prolapse and ODS in men. Evidence is given by the present research that the two most relevant variants of ODS can be linked to an unbalance of forces acting at level of the rectal outlet, as follows: Type 1 (dyskinetic) results from increase in binding forces (i.e., muscle contraction and angulation) creating an obstacle to the free movement of the rectum and to the outflow of rectal content. This variant affects most frequently patients older than 50 years and is amenable to biofeedback and conservative medical treatment. At the other end of the spectrum Type 2 (prolapse) is found, which is almost equally distributed in adults and young people and is characterized by decreased strength of the binding forces that normally ensure stability and maintains proper attachment of the anorectal junction during emptying. Although the cause is still unknown, a sort of excessive “sliding layers” mechanism can be hypothesized leading to detachment of moving surfaces (i.e., the rectal wall, Denonvilliers’ and mesorectal fascia) subjected to repetitive overload from above. To repair such a defect, surgery may be included among the therapeutic options.
References
Hricak H, Williams RD, Spring DB et al (1983) Anatomy and pathology of the male pelvis by magnetic resonance imaging. Am J Roentgenol 141:1101–1110
Myers RP, Cahill DR, Kay PA et al (2000) Puboperineales: muscular boundaries of the male urogenital hiatus in 3D magnetic resonance imaging. J Urol 164:1412–1415
Brown G, Kirkham A, Williams GT et al (2004) High resolution MRI of the anatomy important in total mesorectal excision of the rectum. Am J Roentgenol 182:431–439
Kiyoshima K, Yokomizo A, Yoshida T et al (2004) Anatomical features of periprostatic tissue and its surroundings: a histological analysis of 79 radical retropubic prostatectomy specimens. Jpn J Clin Oncol 34:463–468
Lienemann A, Anthuber C, Baron A et al (1997) Dynamic MR colpocystorectography assessing pelvic floor descent. Eur Radiol 7:1309–1317
Comiter CV, Vasavada SP, Barbaric ZL et al (1999) Grading pelvic prolapse and pelvic floor relaxation using dynamic magnetic resonance imaging. Urology 54:454–457
Stoker J, Halligan S, Bartram CI (2001) Pelvic floor imaging. Radiology 218:621–641
Fielding JR (2002) Practical MR imaging of female pelvic floor weakness. Radiographics 22:295–304
Cortes E, Reid WMN, Singh K et al (2004) Clinical examination and dynamic magnetic resonance imaging in vaginal vault prolapse. Obstet Gynecol 103:41–46
Dvorkin LS, Hetzer F, Scott SM et al (2004) Open-magnet MR defecography compared with evacuation proctography in the diagnosis and management of patients with rectal intussusception. Colorect Dis 6:45–53
Tomita R, Igarashi S, Fujisaki S et al (2010) Significance of defecography in the diagnosis and evaluation of male patients with defecation disorders. Hepatogastroenterology 57:220–223
Altomare DF, Spazzafumo L, Rinaldi M et al (2008) Set-up and statistical validation of a new scoring system for obstructed defecation syndrome. Colorectal Dis 10:84–88
Piloni V, Tosi P, Vernelli M (2013) MR-defecography in obstructed defecation syndrome (ODS): technique, diagnostic criteria and grading. Tech Coloproctol 17:501–510
Mahieu P, Pringot J, Bodart P (1984) Defecography: II. Contribution to the diagnosis of defecation disorders. Gastrointest Radiol 9:253–261
Kujipers HC, Bleijenberg G (1985) The spastic pelvic floor syndrome: a cause of constipation. Dis Colon Rectum 28:6669–6672
Ekberg O, Mahieu PHG, Bartram CI et al (1990) Defecography: dynamic radiological imaging in proctology. Gastroenterol Int 3:93–99
Piloni V, Amadio L, Marmorale C (1991) Defecography in obstructed defecation. A unifying concept for fecal blockade syndrome. Coloproctology 13:118–122
Wexner SD (1991) Rectal prolapse and intussusception. In: Beck DE, Welling D (eds) Manual of patient care in colorectal surgery. Little Brown, Boston, pp 191–212
Spazzafumo L, Piloni V (1999) Rectal constipation and clinical decision-making: multiple correspondence analysis of defecographic findings. Tech Coloproctol 4:117–121
Fritsch H, Hotzinger H (1995) Tomographical anatomy of the pelvis, visceral pelvic connective tissue, and its compartments. Clin Anat 8:17–24
Skomorowska E, Hegedus V (1987) Sex differences in anorectal angle and perineal descent. Gastrointest Radiol 12:353–355
Cavallo G, Salzano A, Grassi R et al (1991) Rectocele in males: clinical, defecographic, and CT study of singular cases. Dis Colon Rectum 34:964–966
Chen HH, Iroatulam A, Alabaz O et al (2001) Associations of defecography and physiologic findings in male patients with rectocele. Tech Coloproctol 5:157–161
Savoye-Collet C, Savoye G, Koning E et al (2010) Gender influence on defecographic abnormalities in patients with posterior pelvic floor disorders. World J Gastroenterol 16(4):462–466
Andrade LC, Correia H, Semedo LC et al (2015) Conventional videodefecography: pathologic findings according to gender and age. Eur J Radiol 1:1–5
Mikuma N, Namagawa M, Morita K et al (1998) Magnetic resonance imaging of the male pelvic floor. The anatomical configuration and dynamic movement in healthy men. Neurourol Urodyn 17:591–597
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Piloni, V., Bergamasco, M., Melara, G. et al. The clinical value of magnetic resonance defecography in males with obstructed defecation syndrome. Tech Coloproctol 22, 179–190 (2018). https://doi.org/10.1007/s10151-018-1759-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-018-1759-4