Abstract
Background
Laparoscopic ventral mesh rectopexy (LVR) is gaining wider acceptance as the preferred procedure to correct internal as well as external rectal prolapse associated with obstructed defaecation syndrome and/or faecal incontinence. Very few reports exist on the use of biologic mesh for LVR. The aim of our study was to report the complication and recurrence rate of our first 100 cases of LVR for symptomatic internal rectal prolapse and/or rectocele using a porcine dermal collagen mesh.
Methods
Prospectively collected data on LVR for internal rectal prolapse were analysed. Surgical complications and functional results in terms of faecal incontinence (measured with the Faecal Incontinence Severity Index = FISI) and constipation (measured with the Wexner Constipation Score = WCS) at 3, 6 and 12 months were analysed. It was considered an improvement if FISI or WCS scores were reduced by at least 25 % and a cure if the FISI score decreased to <10 and the WCS decreased to <5.
Results
Between April 2009 and April 2013, 100 consecutive female patients (mean age 63 years, range 24–88 years) underwent LVR. All patients had internal rectal prolapse (grade III [n = 25] and grade IV [n = 75] according to the Oxford classification) and rectocele. Mean operative time was 85 ± 40 min. Conversion rate to open technique was 1 %. There was no post-operative mortality. Overall 16 patients (16 %) experienced 18 complications, including rectal perforation (n = 1), small bowel obstruction (n = 2), urinary tract infection (n = 8), subcutaneous emphysema (n = 3), wound haematoma (n = 2), long lasting sacral pain (n = 1) and incisional hernia (1). Median post-operative length of stay was 2 days. Ninety-eight out of 100 patients completed follow-up. At the end of follow-up, the mean FISI score improved from 8.4 (±4.0 standard deviation (SD) p = 0.003) to 3.3 ± 2.3 SD (p = 0.04). Incontinence improved in 37 out of 43 patients (86 %), and 31 patients (72 %) were cured. Similarly, the mean WCS score improved from 18.4 ± 11.6 SD to 5.4 ± 4.1 SD (p = 0.04). Constipation improved in 82 out of 89 patients (92 %), and 70 patients (79 %) were cured. No worsening of continence status, constipation or sexual function was observed. Fourteen patients (14 %) experienced persistence or recurrence of prolapse.
Conclusions
LVR using biologic mesh is a safe and effective procedure for improving symptoms of obstructed defaecation and faecal incontinence in patients with internal rectal prolapse associated with rectocele.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since its initial description by D’Hoore in 2004, laparoscopic ventral mesh rectopexy (LVR) has gained acceptance as a promising surgical treatment for rectal prolapse and internal rectal intussusception associated with obstructed defaecation syndrome (ODS) and faecal incontinence (FI) [1–4]. An increasing amount of published data show functional improvement in terms of FI (4–91 %), constipation (37–86 %) [2, 3, 5, 6] and dyspareunia and sexual dysfunction (39 %) [6] for patients with internal and external RP treated with LVR. These functional outcomes are usually obtained using synthetic mesh for rectal suspension which has been shown to increase the efficacy of the reconstructive procedure while reducing the recurrence rate by 30 % [7], compared with pexies without mesh.
However, this improvement of outcome goes hand in hand with the inherent risk of mesh-related complications, such as erosions and infections. This led to the ongoing debate about the use of biologic meshes in pelvic floor surgery. There is, however, currently no proof that one mesh is superior to the others [8]. Biologic meshes, serving as a collagen scaffold for soft tissue remodelling and regeneration of native tissue, may allow a safer reconstructive procedure, avoiding the use of a permanent foreign material, which may provide a nest for acute and chronic infection [8]. The in vivo durability of biologic mesh depends on the degree of cross-linking of its collagen component. In order to slow down degradation, additional cross-links are generated during the manufacturing process. For example, cross-linked dermal porcine collagen (Permacol™, Covidien, Mansfield, MA, USA) is one of the most widely used biologic meshes in pelvic floor surgery.
Very few reports on LVR using biologic mesh exist. However, these did show 82–95 % improvement of obstructed defaecation syndrome (ODS) symptoms and 73–95 % improvement of FI [9, 10]. The lack of comparative data does not allow us to establish the superiority of any biologic mesh in terms of surgical complications, as well as short- and longer-term functional outcomes [11].
In this critical appraisal, we report our experience with this abdominal, minimally invasive and nerve sparing technique, using porcine dermal collagen mesh.
Materials and methods
From April 2009 to April 2013, 100 consecutive patients with internal rectal prolapse were treated with LVR and entered into a prospective pelvic floor database. Patients with anterior compartment prolapse requiring concomitant bladder suspension were excluded from this report. Patients with severe utero-adnexal pathology in addition to prolapse (such as fibromatosis and endometriosis) and patients with external uterine prolapse first underwent hysterectomy performed by a gynaecologist, and rectal prolapse was reassessed months after recovery from surgery. All operations were performed at Tor Vergata University of Rome (Italy) by two colorectal surgeons (LF, PS) who trained in these procedures at John Radcliff Hospital in Oxford (UK) or by residents/fellows under their supervision.
The diagnosis of rectal prolapse and rectocele was made clinically (symptoms and proctoscopy) and confirmed by defecography and/or dynamic pelvic magnetic resonance imaging (MRI). All patients diagnosed with an external rectal prolapse were excluded from this series.
Proctograms were evaluated using the Oxford Prolapse Grading system (Table 1) [2, 12]. Rectoceles were classified as large if ≥4 cm and perineal descend severe if ≥7 cm on imaging. Anorectal function was evaluated using two different scores: the Wexner Constipation Score (WSC) and the Faecal Incontinence Severity Index (FISI). All patients underwent anal manometry. A full colonoscopy or computed tomography (CT) colonography to exclude colonic disease was performed, while a colonic transit study was used in young patients with severe constipation. Indications for surgery were grade III or IV rectal prolapse (internal rectal prolapse in the Oxford classification) at defecography with a FISI score ≥10 and/or a WCS ≥ 5. All patients had failed conservative management including bowel regimen, laxatives and a 12-week course of biofeedback therapy performed by a specialized pelvic floor therapist. All patients were evaluated by a urogynaecologist in order to study middle and anterior compartment involvement.
Written informed consent was obtained. All patients received a single dose of antibiotic (amoxycillin/clavulanic acid or cephalosporin in case of penicillin allergy) at induction. A urinary catheter was inserted.
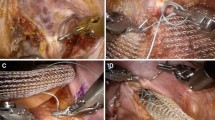
An anterolateral dissection was carried out between the rectum and the vagina starting from the sacral promontory, down to the levator ani muscle using a 4-trocar technique and a 30° scope (Fig. 1). A 3 × 18 cm tailored strip of biologic mesh (Permacol, TSL plc, UK) was positioned in this pocket at the level of the levator ani muscle and sutured to the anterior wall of the rectum using two parallel rows of non-absorbable 2-0 sutures (Tycron, Covidien, UK). Since 2012, in order to accelerate this step, a hole-belt puncher was used, creating holes within the mesh, as previously described [13]. During this manoeuvre, the rectum was gently and fully retracted cranially in order to visualize the levator ani muscle and the level of the first two distal sutures confirmed to be approximately at 2–3 cm above the dentate line by rectal examination or proctoscopy. The mesh was then secured on the sacral promontory using the ProTack™ device (Autosuture, Covidien, UK), and the vaginal vault (or cervix) was fixed to the mesh without traction by two additional absorbable sutures (vicryl 2-0) (Figs. 2, 3). Before suturing the posterior vaginal vault to the mesh, a posterior flat vaginal retractor was positioned and pulled in order to completely distend the vaginal apex. The peritoneum was closed using a running absorbable suture 2-0 (V-Lock, Covidien, UK). Drains were inserted only in special circumstances (i.e. extensive adhesiolysis, risk of bleeding due to therapeutic anticoagulation, rectal perforation). Post-operatively non-steroidal anti-inflammatory drugs and paracetamol were used. The urinary catheter was removed within 24 h from surgery, and fluid therapy discontinued to allow hospital discharge starting from post-operative day 2. Upon discharge patients were prescribed a high dose (3 times/day) of polyethylene glycol (Movicol, Norgine, Milan, Italy) and were weaned to 1/day by 6 weeks after surgery.
After surgery, patients were evaluated at 1 week, and 1, 3, 6 and 12 months by two of the authors (PS, LF).
Thereafter, patients were seen once a year or if needed, depending on their clinical condition. Patients with a persistently abnormal or worsening WCS/FISI score underwent defecography or MRI defecography. Recurrence was defined as persistently abnormal or worsening WCS/FISI and abnormal defecography or MRI defecography. Mesh erosion was evaluated yearly using rigid proctoscopy and a standard gynaecological speculum. Sexual dysfunction was assessed using a validated questionnaire. Data on gender, age, mortality, morbidity (including erosion and sexual dysfunction), length of stay, recurrence, symptoms of ODS, WCS and FISI scores were prospectively collected. It was considered an improvement if FISI or WCS scores were reduced by at least 25 % and a cure if the FISI score decreased to <10 and the WCS decreased to <5 as in a previous publication [4].
Statistical analysis
The Chi-square test with odds ratio was used for categorical variables.
The paired t test was used to assess the differences between variables at baseline, and at 3 and 6 months or the end of follow-up. Kaplan–Meier analysis was used to assess recurrence-free probability. For the univariate analysis, a cut-off point of ≥5 for WCS score, ≥10 for FISI and ≥3 unsuccessful attempts per day for evacuation were used. A p value <0.05 was considered to be statistically significant.
Results
Median duration of follow-up was 20 months (range 6–54 months). Two patients were lost to follow-up. Patient characteristics are shown in Table 2. All patients were women with a mean age of 63 years (range 24–88 years). Mean body mass index (BMI) at surgery was 26 ± 5 kg/m2 (range 17–36 kg/m2). Overall 58 out of 98 patients (59 %) had undergone previous surgery including hysterectomy (n = 49), caesarian section (n = 24), surgery for anal fissure or haemorrhoids (n = 14), surgery for anal fistula (n = 2) and other gastrointestinal surgery (n = 17). Five patients had undergone previous stapled transanal rectal resection for ODS.
Mean duration of symptoms before surgery was 11 ± 9 years (range 2–31 years).
After the preoperative work-up and classification of prolapse using the Oxford Prolapse Grading system, 73 patients (74 %) were scheduled for surgery to correct a grade IV prolapse, and 25 were listed for correction of a grade III prolapse. A large rectocele ≥4 cm was present in 57 patients (58 %).
Results are summarized in Table 3. Mean operative time was 85 ± 40 min (range 50–160 min). There was one conversion to open technique due to severe pelvic adhesions secondary to a previous hysterectomy in a patient with a BMI of 36 kg/m2. There was no post-operative mortality. There was one intraoperative complication, a rectal perforation during the anterior dissection at the level of the levator ani muscle. This perforation was immediately repaired using 3-0 interrupted absorbable stitches (Caprosyn™, Covidien™), and the surgery was completed as planned. There were no surgical re-interventions during the patients’ hospital stay after LVR. Median post-operative length of stay was 2 days (range 2–5 days). Overall, 16 patients experienced 18 complications (18 %), including the rectal perforation. One patient (1 %) experienced incomplete small bowel obstruction (SBO) 25 days after surgery because of an adhesion between a ProTack™ clip and the terminal ileum and required laparoscopic adhesiolysis which was uneventful. Another patient was admitted to another hospital with the diagnosis of SBO and discharged after 2 days of medical treatment. These two patients were the sole two emergency room or hospital readmissions within 30 days observed in this series. Minor post-operative complications were observed in the remaining 15 patients as follows: eight urinary tract infections successfully treated with oral antibiotics, three subcutaneous emphysemas which resolved spontaneously, two wound haematomas treated conservatively, one case of persistent sacral pain, successfully treated with a 15-day course of low dose corticosteroids and painkillers, and one incisional hernia at the site of the 10-mm port in the right iliac fossa. There were no mesh-related complications. Out of the 98 patients who completed the 1-year follow-up, 73 (74 %) were sexually active and none of them reported sexual dysfunction.
Incontinence
The mean FISI score of the whole group before surgery was 8.4 (±4 standard deviation (SD); range 0–41) with a median of 6. Forty-three out of 98 (44 %) patients reported significant incontinence preoperatively with a FISI ≥ 10. Overall, considering the type of incontinence, six patients were incontinent to liquid stool, 18 were incontinent to gas, and 19 to mucous. At 3 months, the mean FISI improved to 4.4 (±2.9 SD; p = 0.01) with a median of 6 (range 0–34). At 6 months, the mean FISI improved to 3.2 (±2.2 SD; p = 0.03) with a median of 0 (range 0–34). At the end of follow-up, the mean FISI score was 3.3 (±2.3 SD; p = 0.04) (Table 4). In the 43 patients with a preoperative FISI score ≥10, incontinence improved in 33 (77 %) at 3 months and in 37 of 43 (86 %) at 6 and 12 months. Thirty-one (72 %) of these patients were cured. At the end of the follow-up, 12 patients (28 %) had abnormal FISI, 10 had persistent incontinence (23 %), and 2 had recurrence (5 %). No patients experienced worsening of incontinence at the end of the follow-up.
Constipation
The mean WCS before surgery was 18.4 (±11.6 SD; range 5–30) with a median of 16 (range 5–30). Eighty-nine out of 98 patients (91 %) reported an abnormal constipation score preoperatively with a WCS ≥ 5. Of those, 23 patients (26 %) presented with abnormal scores for both constipation and faecal incontinence. All 98 patients reported use of laxatives at least once a week, 85 (87 %) reported the need of enemas, and 31 (32 %) the need of digitation before surgery.
At 3 months, the mean WCS improved to 12 (±5 SD; p = 0.02) with a median of 10 (range 0–20). At 6 months, the mean WCS improved to 9.2 (±3.8 SD; p = 0.05) with a median of 9 (range 0–20). A further improvement was observed at the end of follow-up, when the mean WCS improved to 5.4 (±4.1 SD; p = 0.04), with a median of 7 (range 0–20) (Table 5). At the end of follow-up, constipation improved in 82 out of 89 patients (92 %) who had a preoperative WCS score >5. Seventy (79 %) were cured. At the end of the follow-up 24 patients reported the use of laxative once a week (24 %), 14 reported the need of enemas (14 %) and 6 the need for digitation (6 %). No patients experienced worsening of constipation.
Recurrent and persistent disease
Fourteen patients (14 %) experienced persistence (n = 3) or recurrence (n = 11) of the prolapse. A Kaplan–Meier curve showed the risk of recurrence was 16 % at 3 years (0.783–0.925, 0.95 CI) (Fig. 4). The peak of recurrences occurred between 24 and 36 months after surgery.
All 14 patients had preoperative constipation and five of them had an abnormal preoperative FISI score. Seven patients had no improvement of preoperative WCS scores, and all incontinent patients remained incontinent. In three patients prolapse, was considered to be persistent, while in all others it was considered to be recurrent. Six of these 14 patients were using digitation to evacuate before LVR, and all six continued to use digitation. Univariate analysis (Table 6) showed a correlation between preoperative unsuccessful attempts for evacuation (>3 in 24 h) and recurrence (p = 0.01). Similarly, severity of constipation seems to be associated with a higher risk of recurrence (p = 0.01). Moreover, pelvic floor descent >7 cm on proctogram seems to be associated with an increased risk of recurrence (p = 0.01).
A stapled transanal rectal resection (STARR) was offered to all 14 patients at least 1 year after LVR. Seven of them have undergone STARR. In three patients, a full anterior and posterior resection was performed using a 33-mm circular stapler (EEA, Covidien, Dublin). Two patients with residual posterior prolapse underwent only posterior resection. In two patients, STARR was performed using a single 36 mm stapler (TST 36-Megawindows, Touchstone Inc, China) for a full-thickness 360° rectal resection. All these procedures were uneventful, and five out seven patients (71 %) had resolution of symptoms and no recurrence at the end of the follow-up.
Discussion
In this series, we present our experience with LVR using biologics for the treatment of internal rectal prolapse associated with ODS and FI after failure of conservative medical therapy including oral laxatives, enemas and pelvic floor biofeedback. Although some reports showed success rates up to 90 % for both constipation and faecal incontinence [14], none of our patients had undergone previous colonic irrigation. Traditionally in our institution, if conservative medical management failed, surgery was offered.
Advantages of ventral rectopexy (VR) consist in the mobilization of the rectovaginal space down to the levator ani muscle and the anterior placement of a mesh, which is sutured distally to the anterior wall of the rectum as well as to the posterior wall of the vagina, and secured proximally to the sacral promontory [1]. In contrast to other methods of repair, posteriorly only a small patch of the promontorium needs to be freed with little risk of the more laterally running hypogastric nerves. The initial Orr-Loygue procedure consisted of a full anterior as well as posterior rectal mobilization, only sparing the lateral ligaments [15]. In 2004, D’Hoore and Penninckx [1] proposed a purely anterior rectal mobilization to overcome the risk of new-onset constipation, found in up to 50 % of patients who had undergone a posterior approach [16]. With the new technique of LVR, successful long-term results for the treatment of rectal prolapse have been reported [2, 5]. During the last 13 years, a few other authors have described this abdominal procedure as a safe solution for reducing prolapse- and intussusception-related symptoms, including obstructed defecation and faecal incontinence, and reported its association with low morbidity rates [2, 5, 16–18]. The LVR technique not only spares the pelvic nerves and therefore avoids rectal denervation inertia [19] but also offers the advantage of a concomitant repair of middle pelvic floor compartment pathologies like rectocele and enterocele [1]. So far, most studies have pointed out the role of LVR for external rectal prolapse, while studies on the results of LVR used for internal rectal prolapse are scant. However, Wijffels and colleagues reported the efficacy of this procedure for rectocele [4]. Since the dissection is conducted completely between the rectum and vagina, the concomitant rectocele is dissected and repaired by the mesh positioning. This may not differ from a transperineal rectocele repair with a mesh, except for the dissection direction [20].
Minor complications after LVR may range from 4 to 8 %, according to the literature [4, 21, 22].
Our overall complication rate of 16 % was comparable to these data, being mainly due to minor complications. However, we observed two SBO one of which required surgery and adhesiolysis, due to entrapment of the small bowel mesentery in the ProTack™ clips. In this case, the sacral promontory was not completely covered by peritoneum at re-operation [22].
This emphasizes the importance of peritoneum completely covering all foreign material, mesh or otherwise.
Wijffels et al. [3, 12, 23–26] demonstrated the safety of this technique for the elderly compared with previously used more extensive procedures for rectal prolapse.
Our population was relatively young (mean age: 63 years), due to a large referral of patients with anorexia nervosa, which is possibly an under-recognized cause of rectal prolapse [27, 28]. The complication rate in this younger age group was not lower than that in older age groups from other studies. On the other hand, it should be noted that anorexic patients may have immune system problems due to undernourishment.
Nevertheless, the post-operative mean hospital stay in our study was only 2 days, comparable with that reported by experienced centres [2, 4, 12].
With regard to recurrence of rectal prolapse, LVR provides good results. D’Hoore et al. [2] reported a recurrence rate of only 5 % in a subgroup of 42 patients with a 5-year follow-up. The recent literature has indicated that the average recurrence rate is 4–5 % (range 0–27 %).
In our study, an overall incidence of recurrent or persistent internal prolapse of 14 % was observed. This seems higher than in other centres. It may be partly due to study bias since our group included many anorexic patients, among whom constipation exceeds 80 % and some degree of constipation remains after the BMI is re-established [29]. As a matter of fact, three patients who experienced persistence and recurrence were anorexic. Despite this, anorexia seems not be a risk factor for failure. Moreover, six out of 14 recurrences were observed in the first 20 patients treated, and the learning curve might be responsible for these. This may explain the longer-term curve drop on the Kaplan–Meier curve. It is also possible that early recurrences or persistence of anatomical abnormalities is secondary to technical failures, though a mesh-related problem cannot be excluded. However, according to our univariate analysis of preoperative factors influencing recurrence, the most important risk factors seem to be pelvic floor descent on defecography as well as severe constipation preoperatively. This could be explained by global pelvic floor weakness and probably pudendal neuropathy. Further investigation before surgery in this subgroup of patients may be important, as well as consideration for alternative treatments.
LVR provides good functional outcomes. Post-operative constipation usually improves in 25 % of patients, while new-onset constipation after surgery occurs in about 3 % of patients. FI decreases in up to 50 %, while new-onset post-operative FI is very rare. However, if ‘pure’ ventral rectopexy series are considered, the reduction in post-operative constipation is even greater and new-onset constipation is very rare: FI may improve in up to 91 % of the cases and constipation in up to 86 % of the cases when synthetic mesh is used, at least in short-term follow-up. These percentages are reduced to about 80 % when longer-term follow-up is considered [2, 4, 5, 29–31].
Looking at studies using the same technique, but with biologic instead of synthetic mesh, improvement rates can reach 95 % for constipation and for incontinence, although it must be noted that these results are observed with short-term follow-up [9, 10].
In the present study, 91 % of the patients with a grade III and IV internal rectal prolapse complained about preoperative constipation. Post-operative improvement of constipation was about 80 % at the end of follow-up, and we did not observe any new-onset constipation. These results are comparable to those reported in the literature cited above, considering the longer follow-up of our series.
Significant incontinence was evident in 44 % of our study population, and it was significantly improved in 86 % (cured in 72 %) of the patients after surgery. Similar to findings reported in the literature, these improvements were immediately evident starting 3 months after surgery and improved during the first 6 months. No further improvements were observed thereafter, as previously reported [10].
Our results confirm the unique role of LVR in ameliorating or preserving continence and in treating constipation, with a negligible risk of a new-onset constipation, making traditional sigmoid resection in constipated patients unnecessary. Indeed, post-operative constipation may remain or develop even after sigmoid resection along with a mortality rate as high as 10 % [32, 33].
Regarding the use of biologic or synthetic mesh, one can say, looking at the functional outcome data above, that they both do the initial mechanical job. Only data from very long follow-ups can establish whether all biologic meshes can sustain this mechanical support following their degradation. Certainly, some biologic meshes seem to be inferior to others [34].
Some studies showed inferior functional outcome results and graft-related complications in colposuspension with biologic mesh compared with synthetic mesh [35].
Therefore, whether biologic meshes cause less mesh-related problems needs to be proven in larger-scale well-defined studies.
According to the literature, polypropylene, polyester or polytetrafluoroethylene meshes have been used routinely. Use of a foreign material to fix or suspend the rectum is intended to give more initial mechanical support until tissue remodelling has occurred and to stimulate more fibrous tissue formation than an ordinary suture rectopexy. The main disadvantage of using synthetic mesh is the risk of post-implantation mesh-related complications. Among these, pelvic sepsis has been reported in 2–16 % of patients who underwent prosthetic rectopexy [36].
The risk of pelvic sepsis is higher if a resection is performed and if a pelvic haematoma is present [37]. Other complications that have been described are rectal stricture, recto-vaginal fistula, mesh erosion into the vagina, rectum and bladder, pelvic pain and dyspareunia, due to nerve irritation and chronic inflammation [38]. The 2008 National Institute for Health and Clinical Excellence review of surgery for pelvic organ prolapse showed that mesh-related complications depend on the type of mesh used and on the duration of follow-up. As demonstrated by this review, erosion rates for biologic meshes (xenografts) were nil, while they increased to 7 % for synthetic and to 14 % for combined synthetic ones. On the other hand, the failure rate was higher for biologic meshes than for synthetic ones (23 vs. 9 %) [38].
A 2011 safety communication of the US Food and Drug Administration concerning pain, mesh infections and mesh erosion through the vagina after mesh implantation for pelvic organ prolapse and stress urinary incontinence surgery called the use of meshes for this indication into question. However, one should emphasize that these reports concern the transvaginal and partially blind placement of the meshes [39], which is distinctly different from our LVR technique with an excellent view of the placement of the mesh in relation to vagina and rectum. We believe that the biologic mesh between the rectum and vagina positioned after a full lower anterior dissection corrects rectocele and reduces the risk of mesh erosion and/or infection.
So far, no clear conclusion in favour of one mesh over the other can be drawn in terms of short- and long-term safety of synthetic mesh in pelvic reconstructive surgery since most of the reported studies are retrospective or uncontrolled series. This is even more difficult for rectal prolapse surgery due to the different approaches and study designs used, as well as different lengths of follow-up. Following LVR, the risk of mesh detachment, infection or erosion into the rectum or the vagina exists but it is extremely rare [40].
When the mesh is completely covered by the peritoneum, mesh -related complications are theoretically avoided [2].
However, the risk of mesh erosion into the vagina can be as high as 21 % and according to a large review on sacrocolpopexy is 3.4 % [36].
Mesh placement closer to the vaginal wall instead of the rectum might explain this difference [12]. One study also emphasized the partially blind insertion route as a very prominent risk factor for mesh complications like erosion [41].
Although the risk of mesh-related complications after LVR is very low, the possibly devastating clinical effects tempt us to search for an alternative to synthetic mesh. Biologic meshes were introduced with the hope that results would improve. Moreover, biologic meshes have been used in infected areas, and we believe that their use could additionally minimize the risk of pelvic infections and that this could justify their costs [37].
This study has some limitations, such as the need for a longer follow-up and the lack of a psychological evaluation of the patients, considering the strong impact that psychiatric comorbidities have on pelvic floor dysfunction [42].
Conclusions
Our results confirm the safety of LVR for repair of internal rectal prolapse in terms of functional outcome as well as recurrence. In addition, the use of biologic mesh in our series with intermediate follow-up provided functional results similar to those reported for series where traditional synthetic mesh was used for rectal prolapse repair.
References
D’Hoore A, Cadoni R, Penninckx F (2004) Long-term outcome of laparoscopic ventral rectopexy for total rectal prolapse. Br J Surg 91:1500–1505
D’Hoore A, Penninckx F (2006) Laparoscopic ventral recto(colpo)pexy for rectal prolapse: surgical technique and outcome for 109 patients. Surg Endosc 20:1919–1923
Wijffels N, Cunningham C, Dixon A, Greenslade G, Lindsey I (2001) Laparoscopic ventral rectopexy for external rectal prolapse is safe and effective in the elderly. Does this make perineal procedures obsolete? Colorectal Dis 13:561–566
Collinson R, Wijffels N, Cunningham C, Lindsey I (2010) Laparoscopic ventral rectopexy for internal rectal prolapse: short-term functional results. Colorectal Dis 12:97–104
Auguste T, Dubreuil A, Bost R, Bonaz B, Faucheron JL (2006) Technical and functional results after laparoscopic rectopexy to the promontory for complete rectal prolapse. Prospective study in 54 consecutive patients. Gastroenterol Clin Boil 30:659–663
Abet E, Lehur PA, Wong M (2012) Sexual function and laparoscopic ventral rectopexy for complex rectocoele. Colorectal Dis 14:721–726
Ahmad M, Sileri P, Franceschilli L (2012) The role of biologics in pelvic floor surgery. Colorectal Dis 14(Suppl 3):19–23
Shah BC, Tiwari MM, Goede MR et al (2011) Not all biologics are equal! Hernia 15:165–171
Wahed S, Ahmad M, Mohiuddin K, Katory M, Mercer-Jones M (2012) Short term results for laparoscopic ventral rectopexy using biologic mesh for pelvic organ prolapse. Colorectal Dis 14:1242–1247
Sileri P, Franceschilli L, De Luca E (2012) Laparoscopic ventral rectopexy for internal rectal prolapse using biologic mesh: postoperative and short-term functional results. J Gastrointest Surg 16:622–628
Smart NJ, Pathak S, Boorman P (2013) Synthetic or biologic mesh use in laparoscopic ventral mesh rectopexy-a systematic review. Colorectal Dis 15:650–654
Boons P, Collinson R, Cunningham C, Lindsey I (2010) Laparoscopic ventral rectopexy for external rectal prolapse improves constipation and avoids de novo constipation. Colorectal Dis 12:526–532
Sileri P, Franceschilli L, Gaspari AL (2012) Saving time stitching thick biologic mesh during laparoscopic ventral rectopexy. Tech Coloproctol 16:393–394
Koch SM, Melenhorst J, van Gemert WG, Baeten CG (2008) Prospective study of colonic irrigation for the treatment of defaecation disorders. Br J Surg 95:1273–1279
van den Esschert JW, van Geloven AA, Vermulst N, Groenedijk AG, de Wit LT, Gerhards MF (2008) Laparoscopic ventral rectopexy for obstructed defecation syndrome. Surg Endosc 22:2728–2732
Bachoo P, Brazzelli M, Grant A (2000) Surgery for complete rectal prolapse in adults. Cochrane Database Syst Rev (2):CD001758
Formijne Jonkers HA, Poierrié N, Draaisma WA (2013) Laparoscopic ventral rectopexy for rectal prolapse and symptomatic rectocele: an analysis of 245 consecutive patients. Colorectal Dis 15:695–699
Maggiori L, Bretagnol F, Ferron M (2013) Laparoscopic ventral rectopexy: a prospective long-term evaluation of functional results and quality of life. Tech Coloproctol 17:431–436
Prasad ML, Pearl RK, Abcarian H, Orsay CP, Nelson RL (1986) Perineal proctectomy, posterior rectopexy, and postanal levator repair for the treatment of rectal prolapse. Dis Colon Rectum 29:547–552
Smart NJ, Mercer-Jones MA (2007) Functional outcome after transperineal rectocele repair with porcine dermal collagen implant. Dis Colon Rectum 50:1422–1427
Samaranayake CB, Luo C, Plank AW, Merrie AE, Plank LD, Bissett IP (2010) Systematic review on ventral rectopexy for rectal prolapse and intussusception. Colorectal Dis 12:504–512
Faucheron JL, Voirin D, Riboud R et al (2012) Laparoscopic anterior rectopexy to the promontory for full-thickness rectal prolapse in 175 consecutive patients: short- and long-term follow-up. Dis Colon Rectum 55:660–665
Madiba TE, Baig MK, Wexner SD (2005) Surgical management of rectal prolapse. Arch Surg 140:63–73
William C, Cirocco MD (2010) Altemeier procedure for rectal prolapse: an operation for all ages. Dis Colon Rectum 53:1618–1623
Duthie GS, Bartolo DC (1992) Abdominal rectopexy for rectal prolapse: a comparison of techniques. Br J Surg 79:107–113
Riansuwan W, Hull TL, Bast J, Hammel JP, Church JM (2010) Comparison of perineal operations with abdominal operations for full-thickness rectal prolapse. World J Surg 34:1116–1122
Rexnik Z, Vishne TH, Kristt D, Alper D, Ramadan E (2001) Rectal prolapse: a possible under-recognized complication of anorexia nervosa amenable to surgical correction. Int J Psychiatry Med 31:347–352
Sileri P, Iacoangeli F, Staar F et al (2012) Nervosa anorexia leads to defaecatory disorders compared to general population. Gastroenterology 5:S1072–S1073
Silvis R, Gooszen HG, van Essen A, de Kruif AT, Janssen LW (1999) Abdominal rectovaginopexy: modified technique to treat constipation. Dis Colon Rectum 42:82–88
Verdaasdonk EG, Bueno de Mesquita JM, Stassen LP (2006) Laparoscopic rectovaginopexy for rectal prolapse. Tech Coloproctol 10:318–322
Portier G, Iovino F, Lazorthes F (2006) Surgery for rectal prolapse: orr-Loygue ventral rectopexy with limited dissection prevents postoperative-induced constipation without increasing recurrence. Dis Colon Rectum 49:1136–1140
Yakut M, Kaymakcioglu N, Simsek A, Tan A, Sen D (1998) Surgical treatment of rectal prolapse. A retrospective analysis of 94 cases. Int Surg 83:53–55
Lechaux JP, Lechaux D, Perz M (1995) Results of Delorme’s procedure for rectal prolapse. Advantages of modified technique. Dis Colon Rectum 38:301–307
Gaertner WB, Bonsack ME, Delaney JP (2007) Experimental evaluation of four biologic prostheses for ventral hernia repair. J Gastrointest Surg 11:1275–1285
Clearhout F, De Ridder D, Van Beckevoort D et al (2010) Sacrocolpopexy using xenogenic acellular collagen in patients at increased risk for graft-related complications. Neurourol Urodynam 29:563–567
Peppas G, Gkegkes ID, Makris MC, Falagas ME (2010) Biologic mesh in hernia repair, abdominal wall defects, and reconstruction and treatment of pelvic organ prolapse: a review of the clinical evidence. Am Surg 76:1290–1299
Athanasiadis S, Weyand G, Heiligers J (1996) The risk of infection of three synthetic materials used in rectopexy with or without colonic resection for rectal prolapse. Int J Colorectal Dis 11:42–44
Hamoudi-Badrek A, Greenslade GL, Dixon AR (2013) How to deal with complications after laparoscopic ventral mesh rectopexy (LVMR): lessons learnt from a tertiary referral centre. Colorectal Dis 15:707–712
FDA Safety Communication (2011) UPDATE on serious complications associated with transvaginal placement of surgical mesh for pelvic organ prolapse. July 13, 2011
Franklin ME Jr, Treviño JM, Portillo G, Vela I, Glass JL, González JJ (2008) The use of porcine small intestinal submucosa as a prosthetic material for laparoscopic hernia repair in infected and potentially contaminated fields: long-term follow-up. Surg Endosc 22:1941–1946
Brubaker L, Norton PA, Albo ME et al, Urinary Incontinence Treatment Network (2011) Adverse events over two years after retropubic or transobturator midurethral sling surgery: findings from the trial of midurethral sling (TOMUS) study. Am J Obstet Gynecol 205:498.e1–498.e6
Pescatori M, Spyrou M, Pulvirenti d’Urso A (2007) A prospective evaluation of occult disorders in obstructed defecation using the ‘iceberg diagram’. Colorectal Dis 9:452–456
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Franceschilli, L., Varvaras, D., Capuano, I. et al. Laparoscopic ventral rectopexy using biologic mesh for the treatment of obstructed defaecation syndrome and/or faecal incontinence in patients with internal rectal prolapse: a critical appraisal of the first 100 cases. Tech Coloproctol 19, 209–219 (2015). https://doi.org/10.1007/s10151-014-1255-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-014-1255-4