Abstract
Background
Prucalopride is a selective serotonin receptor agonist with prokinetic activity, indicated for women with chronic constipation in whom laxatives have failed to provide adequate relief. Data suggests an improvement in about 50 % of such patients but whether the therapeutic effect is on patients with slow transit constipation (STC) and/or obstructed defaecation syndrome (ODS), or even those with constipation-predominant irritable bowel syndrome (IBS-C) is unknown. We therefore assessed whether there is any association between prucalopride efficacy and constipation type.
Methods
All patients receiving prucalopride between June 2010 and April 2012 at our institution were identified, and data analysed following a 4-week “test” period. Patients were sub-grouped as those suffering with ODS, STC, mixed (ODS and STC) or IBS-C based on symptomatology and investigations. Subjective assessment of patient satisfaction and continuation of medication were taken as positive outcomes and analysed for each sub-type along with any side effects.
Results
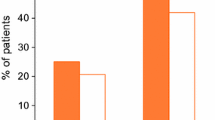
Sixty-nine patients met our criteria. Data were available for 59 women (median age 46 years, range 17–79 years). Sixty-five per cent of prescriptions came from colorectal surgeons. Overall, 25 out of 59 (42 %) patients improved, according to our criteria, after the 4-week trial period. Seventeen patients (29 %) had ODS, 26 (44 %) had STC, 7 (12 %) had mixed symptoms and 9 (15 %) had IBS-C. At 4 weeks, 10 out of 17 patients (59 %) with ODS had improved compared with 4 out of 9 patients (44 %) with IBS-C, 3 out of 7 patients (43 %) with mixed symptoms and 8 out of 26 (31 %) patients with STC. The underlying disorder did not predict whether or not a patient responded to the 4-week trial period (p = 0.32). Nine patients (15 %) experienced side effects that precluded further use.
Conclusions
Patients with all categories of constipation may respond to prucalopride. A trial regime may be indicated regardless of the aetiology of the constipation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic constipation is a common condition with around 15–17 % of adults reporting symptoms consistent with the Rome Diagnostic Criteria [1, 2]. It disproportionately affects women compared to men [2.2:1], with an increase in prevalence with age [1]. Not only can it inflict a heavy burden to the patient, but several analyses suggest that its effects, such as impaired quality of life, diminished vitality and decreased productivity, may represent a substantial socioeconomic burden [3, 4]. Moreover, only a minority of patients (27 %) are satisfied with current treatment options [5]. Using symptom-based criteria, constipation can be sub-classified and defined according to the Rome III guidelines [6, 7]. The sub-types include slow transit constipation (STC), obstructed defaecation syndrome (ODS), a mixture of the two and constipation-predominant irritable bowel syndrome (IBS-C), where transit may be normal.
There are no biological markers or specific tests for the diagnosis of IBS. Epidemiological studies show that 8–23 % of adults in the Western world have IBS of varying severity [6, 8]. The diagnosis is therefore based on identifying a cluster of clinical symptoms which must include abdominal pain or discomfort, as well as bloating [8].
Prucalopride (Resolor) is a selective, high-affinity serotonin (5-HT4) receptor agonist, licensed for use in the UK since 2010. It acts as a prokinetic and is indicated for the symptomatic treatment of chronic constipation in women in whom laxatives have failed to provide adequate relief. The drug is orally active and acts via a systemic mechanism initiating high-amplitude propagated contractions (HAPCs) in the colon [9]. According to National Institute for Health and Clinical Excellence (NICE) guidance, it should only be considered in women who have had no relief from constipation despite trying at least two different types of laxatives and lifestyle modification for at least 6 months [10].
Existing data suggests an improvement in about 50 % of patients but whether the effect is on patients with slow transit and/or obstructed defaecation or IBS-C is unknown. We therefore compared the association between efficacy and constipation sub-type.
Materials and methods
The study was approved as a service evaluation by the clinical effectiveness unit at Sheffield Teaching Hospitals. Patients attending our tertiary referral unit with constipation undergo a rigorous assessment process to exclude organic pathology before embarking on a specific management protocol. Those patients who fulfilled the NICE criteria for use of prucalopride were defined as having either ODS, STC, mixed (STC and ODS) or IBS-C based on symptomatology, investigations and the Rome III classification.
Diagnostic criteria for constipation were the following:
-
1.
Regular occurrence over at least 12 consecutive weeks with onset of symptoms at least 6 months prior to diagnosis of at least two symptoms taken from the Rome III criteria for functional constipation [6, 7]
-
2.
At least two of the following seen during repeated attempts at defaecation:
-
a.
Evidence of impaired evacuation based on balloon expulsion or imaging
-
b.
Inappropriate contraction of the pelvic floor muscles (i.e., puborectalis or anal sphincter) or less than 20 % relaxation of basal resting sphincter pressure by manometry imaging or electromyography (EMG)
-
c.
Inadequate propulsive forces assessed by manometry or imaging.
-
a.
Investigations included a combination of colonic transit studies, defaecating proctogram and anorectal manometry. Patients with STC were characterised by a lack of an urge to defaecate with a transit marker study with slowing markers throughout the colon. Patients with outlet obstruction described a normal desire to defaecate but an inability to satisfactorily evacuate the rectum. Symptoms included difficult or prolonged attempts to defaecate with straining, incomplete and fragmented evacuation and the need for perineal support or digitation to initiate defaecation and anorectal discomfort. Some had both symptom patterns and were defined as mixed constipation. Others had predominantly abdominal discomfort and bloating with normal transit marker studies and were defined as IBS-C.
All patients receiving prucalopride in our institution between June 2010 and April 2012 were identified retrospectively via pharmacy records and data analysed following a 4-week “test” period as recommended by the manufacturers. A daily dose of 2 mg was prescribed for those under the age of 65 and 1 mg for those over 65 years of age. We compared efficacy with constipation sub-type. Patients were assessed according to 2 simple criteria: firstly, if they reported that they were satisfied with the effect of prucalopride and secondly, if they had asked for a repeat prescription after the initial test dose. A successful outcome was felt to have been achieved if the patient reported satisfaction with the treatment and had continued taking the drug. We also looked at the side effects as well as how many patients continued to use prucalopride after the 4-week test phase.
Statistics
The significance of differences in prucalopride efficacy between the constipation sub-types was analysed using Fisher’s exact test. A p value < 0.05 was considered statistically significant.
Results
Between June 2010 and April 2012, 69 patients were identified. Sixty-five per cent of prescriptions came from colorectal surgeons. Ten patients were excluded from the data analysis (no follow-up data n = 6, patients treated as inpatient for constipation n = 2, prescription for pseudo-obstruction n = 1 and failure to start medication despite receiving a prescription n = 1). Data was therefore available for 59 women (median age 46 years, range 17–79 years).
Seventeen women (29 %) had ODS, 26 (44 %) had STC, 7 (12 %) had mixed symptoms and 9 (15 %) had IBS-C. Overall improvement was noted in 25 women (42 %).
Nine out of 59 patients (15 %) experienced side effects that precluded further use within the 4-week test period. These included abdominal cramps (n = 4), nausea and vomiting (n = 2), headaches (n = 2), palpitations (n = 1) and diarrhoea (n = 1). No particular subgroup was more prone to a specific side effect.
At 4 weeks, 10 women with ODS (59 % of the ODS subgroup) were satisfied with the subjective improvement in their symptoms and asked to continue the medication. This compared to 8 with STC (31 % of STC subgroup), 3 with mixed symptoms (43 % of mixed subgroup) and 4 with IBS-C (44 % of IBS subgroup) (Table 1). There was no difference in efficacy between patients with different constipation sub-types (p = 0.32, Fisher’s exact test).
Median duration of follow-up was 5 months (range 2–9 months). During this period, 4 women stopped using prucalopride (16 % of prucalopride responders). In 2 women, the drug was no longer efficacious, 1 preferred to use an alternative laxative long-term and 1 woman stopped due to pregnancy. If the pregnant patient is excluded, the overall response rate to prucalopride in our heterogeneous group of patients with chronic constipation was 36 %.
Discussion
Prucalopride gained NICE recommendation for UK use in 2010 [10]. It is established as an agent beneficial for symptomatic treatment of chronic constipation in women in whom laxatives fail to provide adequate relief [11–13]. Not everyone tolerates the medication. We found 15 % of our patients had to give the medicine up due to side effects within the 4-week trial period. This was slightly more than in the trial by Camilleri et al. [11] where 8.2 % precluded any further use. For those who do tolerate the treatment, it does not always work. In our study, 42 % of patients felt that their symptoms had improved subjectively after the 4-week trial period. This is comparable to the trial by Camilleri et al. [11] where 34 % of patients improved after 4 weeks. Even when it is initially successful, patients do not always continue the therapy. Over our follow-up period, we noted a drop-out rate of 16 %, again similar to that documented by Camilleri.
The existing trials on prucalopride have all had broad eligibility criteria [11, 12]. The criteria used by NICE regarding eligibility for the therapy are also broad [10]. However, traditionally chronic constipation is divided into various categories depending on their symptoms [14]. Some patients have a slow transit of stool and characteristically describe the lack of desire to evacuate (STC), whilst others have the desire to evacuate but cannot push the stool out without excessive straining and/or using methods to support the pelvic floor or enhance evacuation (ODS). A third group has features consistent with IBS. There is some overlap of these categories, but differentiation of one sub-type from another is important particularly when deciding if some type of surgical intervention is warranted.
Although many symptom scoring systems and quality of life assessments exist for the objective assessment of outcome after interventions for constipation, we have avoided reporting this type of outcome. This is not only for the sake of simplicity, but also because we feel that reported satisfaction and request for continuation of the medication is a robust outcome by itself. This outcome measure combined with a subjective assessment of patient satisfaction we feel are strong indicators that the patient feels the medication is effective and is more meaningful than a complex analysis of constipation symptoms and/or quality of life domains. More importantly, the outcome measure described reflects clinical practice. We would discard prucalopride as an intervention and move on to alternative treatments if our outcome measure as described was not positive. We do not base this clinical decision on a symptom score or quality of life assessment tool and would presumably have poor compliance if we did.
Little data are available on prucalopride in the treatment of IBS-C. Tegaserod, an older generation, less selective, 5-HT4 agonist, was shown to be effective in the treatment of IBS-C in women [15–18] and licensed for use in 2002. However, it was later removed from the market owing to a risk of serious cardiovascular events [19]. Using a selective 5-HT4 agonist such as prucalopride should mitigate this side effect. However, to date, there have been no randomized trials that looked at its use specifically in IBS-C.
Given the prokinetic activity of prucalopride one would expect the greatest response to have been seen with STC. However, this was not the case. Our patients classified as having STC had the lowest response rate to prucalopride. Conversely, the ODS group who we expected to have a very low response had the largest percentage of responders. Why should this be the case? Perhaps the prokinetic activity resulting in a larger bulk of stool reaching the rectum more rapidly allows easier evacuation [20]. Alternatively is the aetiology more complex?. Bassotti has clearly shown that in some patients with obstructed defaecation, there is an abnormality of the enteric nervous system with a significant loss of the enteric glial cells. It is possible that prucalopride might act on the neurochemical function of the residual glial activity in these patients [21–23].
Although not statistically different to the response rate seen with other constipation sub-types, the fact that patients with ODS respond to prucalopride has important implications for this subgroup. Although causes of obstructed defaecation vary and include sensory disorders (e.g. megarectum and hyposensitivity) and functional problems (e.g. anismus and Hirschsprung’s), there is a large group of patients who may have mechanical abnormalities resulting in outlet obstruction and/or dissipation of force vector (‘lack of effective push’). This group of disorders has been the focus of significant attention in the surgical literature recently with several operations such as stapled transanal rectal resection (STARR) and ventral mesh rectopexy designed to correct the deformity [24–26]. Indeed, the reported success of these procedures has led some surgeons to believe that conservative management for this group is not warranted and if a potential mechanical cause of the obstructed defaecation is detected, this should be corrected first. This view is to a certain extent backed up by the literature hinting at a low response to conservative treatment [27]. Our response rate of 59 % in this group would tend to justify a trial of conservative therapy before considering surgery.
There is a group of patients with mixed slow transit and obstructed defaecation. Whether these patients truly have a combined aetiology or that one condition leads to the other is difficult to establish. They can also be a difficult group to treat, particularly if surgery is contemplated as, for instance, correction of any mechanical outlet obstruction may not resolve the slow transit component of the disorder. The potential for some patients to respond to prucalopride adds to the armamentarium of options for this group.
There are some potential drawbacks to our study. This is a retrospective observational trial and has the limitations of such a trial. There is no doubt that our numbers are small. There is also no doubt that there is significant overlap of symptoms among each subgroup, making accurate classification of constipation type difficult. We also did not attempt to report objectively the improvement in constipation symptoms.
Conclusions
There is, however, one clear message that cannot be disputed. Patients with all types of constipation and most pertinently ODS have the potential to respond to prucalopride to their satisfaction and therefore at least delay or even avoid surgical intervention.
References
Higgins PD, Johanson JF (2004) Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol 99:750–759
Peppas G, Alexiou VG, Mourtzoukou E, Falagas ME (2008) Epidemiology of constipation in Europe and Oceania: a systematic review. BMC Gastroenterol 8:5
Glia A, Lindberg G (1997) Quality of life in patients with different types of functional constipation. Scand J Gastroenterol 32:1083–1089
Irvine EJ, Ferazzi S, Pare P, Thompson WG, Rance L (2002) Health-related quality of life in functional GI disorders: focus on constipation and resource utilization. Am J Gastroenterol 97:1986–1993
Tack J, Dubois D, Schenk F (2009) Only 27% of patients with chronic constipation are satisfied with current treatment options. Gut 58(Suppl. II):181
Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC (2006) Functional bowel disorders. Gastroenterology 130:1480–1491
Bharucha AE, Wald A, Enck P, Rao S (2006) Functional anorectal disorders. Gastroenterology 130:1510–1518
Drossman DA, Camilleri M, Mayer EA, Whitehead WE (2002) AGA technical review on irritable bowel syndrome. Gastroenterology 123:2108–2131
De Schryver AM, Andriesse GI, Samsom M, Smout AJ, Gooszen HG, Akkermans LM (2002) The effects of the specific 5HT(4) receptor agonist, prucalopride, on colonic motility in healthy volunteers. Aliment Pharmacol Ther 16:603–612
National Institute for Health and Clinical Excellence. TA211 Constipation (women)—prucalopride: guidance. 15 Dec 2010
Camilleri M, Kerstens R, Rykx A, Vandeplassche L (2008) A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med 358:2344–2354
Quigley EM, Vandeplassche L, Kerstens R, Ausma J (2009) Clinical trial: the efficacy, impact on quality of life, and safety and tolerability of prucalopride in severe chronic constipation – a 12-week, randomized, double-blind, placebo- controlled study. Aliment Pharmacol Ther 29:315–328
Tack J, van Outryve M, Beyens G, Kerstens R, Vandeplassche L (2009) Prucalopride (Resolor) in the treatment of severe chronic constipation in patients dissatisfied with laxatives. Gut 58:357–365
Attaluri A, Rao S (2006) Diagnostic and therapeutic approaches to patients with constipation. Practical Gastroenterol 30:30–47
Chey WD, Pare P, Viegas A, Ligozio G, Shetzline MA (2008) Tegaserod for female patients suffering from IBS with mixed bowel habits or constipation: a randomized controlled trial. Am J Gastroenterol 103:1217–1225
Layer P, Keller J, Loeffler H, Kreiss A (2007) Tegaserod in the treatment of irritable bowel syndrome (IBS) with constipation as the prime symptom. Ther Clin Risk Manag 3:107–118
Evans BW, Clark WK, Moore DJ, Whorwell PJ (2007) Tegaserod for the treatment of irritable bowel syndrome and chronic constipation. Cochrane Database Syst Rev CD003960
Novick J, Miner P, Krause R et al (2002) A randomized, double-blind, placebo-controlled trial of tegaserod in female patients suffering from irritable bowel syndrome with constipation. Aliment Pharmacol Ther 16:1877–1888
US FDA public health advisory tegaserod maleate (marketed Zelnorm™). http://www.fda.gov/CDER/DRUG/advisory/tegaserod.htm. (Accessed July 2008)
Bannister JJ, Davidson P, Timms JM, Gibbons C, Read NW (1987) Effect of stool size and consistency on defaecation. Gut 28:1242–1245
Bassotti G, Villanacci V, Nascimbeni R et al (2012) Increase of colonic mast cells in obstructed defaecation and their relationship with enteric glia. Dig Dis Sci 57:65–71
Bassotti G, Villanacci V, Bellomi A et al (2012) An assessment of enteric nervous system and estroprogestinic receptors in obstructed defecation associated with rectal intussusception. Neurogastroenterol Mot 24:155–161
Bassotti G, Villanacci V, Nascimbeni R et al (2007) Colonic neuropathological aspects in patients with intractable constipation due to obstructed defecation. Mod Pathol 20:367–374
D’Hoore A, Penninckx F (2006) Laparoscopic ventral recto(colpo)pexy for rectal prolapse: surgical technique and outcome for 109 patients. Surg Endosc 20:1919–1923
Slawik S, Soulsby R, Carter H, Payne H, Dixon AR (2008) Laparoscopic ventral rectopexy, posterior colporrhaphy and vaginal sacrocolpopexy for the treatment of recto-genital prolapse and mechanical outlet obstruction. Colorectal Dis 10:138–143
Collinson R, Wijffels N, Cunningham C, Lindsey I (2010) Laparoscopic ventral rectopexy for internal rectal prolapse: short-term functional results. Colorectal Dis 12:97–104
Lehur PA, Stuto A, Fantoli M et al (2008) ODS II Study Group. Outcomes of stapled transanal rectal resection versus biofeedback for the treatment of outlet obstruction associated with rectal intussusception and rectocele: a multicenter, randomized, controlled trial. Dis Colon Rectum 51:1739
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jadav, A.M., Mcmullin, C.M., Smith, J. et al. The association between prucalopride efficacy and constipation type. Tech Coloproctol 17, 555–559 (2013). https://doi.org/10.1007/s10151-013-1017-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-013-1017-8