Abstract
Background
It was the aim of this prospective study to analyze both the feasibility and preliminary results of video-assisted anal fistula treatment (VAAFT) combined with advancement flap repair for complex fistulas in Crohn's disease.
Methods
All patients with perianal Crohn's disease suffering from complex fistulas who underwent definitive surgery using VAAFT combined with advancement flap repair were prospectively enrolled in the study. Only complex fistulas with concurrent stable disease and without any evidence of severe inflammatory activity or perianal sepsis were treated using the VAAFT technique. Patients with Crohn's proctitis or prior proctectomy were not candidates for the procedure. VAAFT was performed by using the VAAFT equipment (Karl Storz, Tuttlingen, Germany). Key steps included visualization of the fistula tract and/or side tracts using the fistuloscope and correct localization of the internal fistula opening under direct vision with irrigation. Diagnostic fistuloscopy was followed by advancement flap repair. In addition to feasibility, primary end points included detection of side tracts, success and continence status (assessed by the Cleveland Clinic Incontinence Score). Success was defined as closure of both internal and external openings, absence of drainage without further intervention and absence of abscess formation. Follow-up information was derived from clinical examination 3, 6 and 9 months postoperatively.
Results
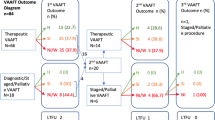
Within a 3-month observation period (September to November 2011), VAAFT was attempted in 13 patients with Crohn's associated complex fistulas. The completion rate was 85 % (11/13). In these 11 patients (median age 34 years, 64 % females), complex fistulas were transsphincteric (8), suprasphincteric (2) and recto-vaginal (1). Forty-six percent (5/11) had concomitant therapy with biologic drugs. In 36 % (4/11), VAAFT was performed with fecal diversion. Median duration of surgery was 22 (range 18–42) minutes. Using VAAFT, additional side tracts not detected preoperatively could be identified in 64 % (7/11). No morbidity occurred. After a mean follow-up of 9 months, the success rate was 82 % (9/11). No deterioration of continence was documented (Cleveland Clinic Incontinence Score 2.4 vs. 1.6, p > 0.05).
Conclusion
Preliminary results of the addition of the VAAFT technique to advancement flap repair in Crohn's fistulas demonstrate that this leads to a high identification rate of occult side tracts with encouraging short-term healing rates. Moreover, a completion rate of 85 % seems promising.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The crucial issue in the surgical treatment of complex anorectal fistulas associated with Crohn’s disease is to provide high rates of definitive closure without significant impairment of continence. As traditional techniques such as advancement flap repair have been shown to be effective in a high proportion of patients, but associated with notable failure rates particularly related to the dynamic course of Crohn’s disease [1–3], surgeons continue to search for innovative, minimally invasive procedures to improve healing rates. Initial experiences on the use of VAAFT reported by Meinero and Mori [4] have shown that the technique has promising results in patients with complex anal fistulas. As regards the challenging issue of rectovaginal fistulas, failure and reoperation rates as well as a high incidence of recurrence have a major impact on continence status and quality of life in patients with Crohn’s disease [5, 6]. As it is generally accepted that both recurrence and reoperation rates extremely high in cases of complex fistulas associated with Crohn’s disease [5–7], it was the aim of this prospective study to analyze both the feasibility and short-term efficacy of the VAAFT technique combined with transrectal advancement flap repair for the closure of complex fistulas in Crohn’s disease.
Materials and methods
Study design
All patients with fistulizing perianal Crohn’s disease suffering from complex fistulas who underwent surgery using the VAAFT technique combined with transrectal advancement flap repair were prospectively enrolled in this study. Inclusion criteria included trans- or suprasphincteric and rectovaginal fistulas. In patients with rectovaginal fistulas, only fistulas of the lower two-thirds of the rectovaginal space were candidates for the procedure, VAAFT was only used if Crohn’s disease was stable and there was no evidence of severe inflammation activity, rectal stricture or concomitant perianal sepsis. Additionally, patients who had Crohn’s proctitis or prior proctectomy were not scheduled for the VAAFT procedure (Table 1). Primary end points of this study included feasibility (completion of the procedure), identification of additional side tracts and success or failure. Success was defined as closure of both internal and external openings, absence of drainage without further intervention and absence of abscess formation. Informed consent for this innovative technique was obtained from all patients. The study was self-funded, so no financial support was received.
Surgical technique
All patients were examined preoperatively in the proctologist’s office. Patients who had seton drainage due to previous drainage of concomitant abscess were examined with endoanal ultrasound to exclude residual anorectal abscess. Magnetic resonance imaging (MRI) was not used routinely. Focussing on surgical strategy, patients who had seton drainage were scheduled for definitive surgery after a minimum time interval of 6–8 weeks and after interdisciplinary discussion with gastroenterologists. Fistulas in which the fistula tract was located in the upper two-thirds of the external sphincter were treated by rectal advancement flap repair in addition to fistulectomy. On the day of surgery, bowel preparation was performed by enema. No patient underwent mechanical bowel preparation. Single-dose antibiotic prophylaxis (cefotaxime and metronidazole) was mandatory. Procedures were either performed under general or spinal anesthesia (according to patients’ preference) and in the lithotomy position. Surgery included a diagnostic (VAAFT) and therapeutic phase (transrectal advancement flap repair).
The VAAFT procedure
Video-assisted anal fistula treatment was performed by using the VAAFT equipment (Karl Storz, Tuttlingen, Germany) (Fig. 1). For the diagnostic phase, the technique introduced by Meinero was used [4]. Key steps included excision of the external (perianal) opening of the fistula tract, insertion of the fistuloscope, visualization of the fistula tract and/or side tracts using the fistuloscope and correct localization of the internal fistula opening under direct vision with irrigation (Fig. 2a–c). If potential side tracts were identified, fistula tissue was destroyed by using electrocautery or brushing. In contrast to the Meinero technique, the diagnostic phase was followed by fistula excision and transrectal advancement (full-thickness) flap repair.
The flap procedure
Following diagnostic fistuloscopy by using VAAFT, it was crucial to excise the fistula tract running from the external opening to the external anal sphincter and to have sufficient external drainage of the wound. Using a Parks or Simms retractor, a full-thickness flap consisting of mucosa, submucosa and the internal sphincter was mobilized from the level of the dentate line to 2–6 cm cephalad. The base of the flap was approximately twice the width of its apex. The crypt-bearing tissue around the internal opening was excised, and the fistula tract was cored out of the sphincters. The defect of the internal anal sphincter was closed with absorbable sutures (Vicryl 2/0®, Ethicon Endo-Surgery, Norderstedt, Germany). After excision of its apex, the full-thickness flap was advanced and sutured without any tension to the “neodentate line” or to the subcutaneous parts of the external sphincter where appropriate using absorbable sutures (Vicryl 3/0®, Ethicon Endo-Surgery, Norderstedt, Germany). The technique has been described previously [8].
Postoperatively, immediate oral feeding (regular food) was possible and no further antibiotics were given. Patients were discharged on the 2nd postoperative day and were instructed to avoid strenuous activity for 14 days.
Follow-up study
All patients were followed up at 2 weeks after surgery to monitor regular wound healing. Specific follow-up information was derived from clinical examination 3, 6 and 9 months postoperatively. This follow-up was performed in an outpatient setting with regular clinical examination (including digital rectal examination, proctoscopy, evaluation of the fistula status by using a fistula probe) and assessment of success or failure. Continence status was assessed using the Cleveland Clinic Incontinence Score [9].
Results
Within a 3-month observation period (September to November 2011), VAAFT combined with advancement flap was attempted in 13 patients with complex fistulas associated with Crohn’s disease. The completion rate was 85 % (11/13). In 2 patients with a narrow anal canal following chronic inflammation, the internal opening could not be clearly identified or reached by using the fistuloscope. As regards the remaining 11 patients (median age 34 years, 64 % females), complex fistulas were transsphincteric (8), suprasphincteric (2) and recto-vaginal (1). Forty-six percent (5/11) had concomitant therapy with biologic drugs (infliximab, adalimumab). In 36 % (4/11), VAAFT was performed with fecal diversion. Median duration of surgery was 22 (range 18–42) minutes. Using VAAFT, additional side tracts not detected preoperatively could be identified in 64 % (7/11). Additional side tracts were subcutaneous (2), intersphincteric (1) and in the direction of the levator muscle (4). No procedural morbidity occurred. After a mean follow-up of 9 months, the success rate was 82 % (9/11). Stoma reversal was possible in 75 % (3/4). No deterioration of continence was documented (Cleveland Clinic Incontinence Score 2.4 vs. 1.6, p > 0.05). Results are summarized in Tables 2 and 3.
Discussion
Although many efforts have been made to improve outcome of definitive surgery for complex anal fistulas associated with Crohn’s disease including the use of medical therapy, precise diagnostic tools, the application of “biologicals” (systemic and local) and innovations in surgical technique (e.g., plug procedure), surgery for Crohn’s related fistulas remains challenging [10–19]. Therefore, the key question regarding surgery for complex anal fistulas associated with Crohn’s disease is whether factors predictive of success or failure can be identified. Active inflammation, undrained perianal sepsis, undetected side tracts and rectovaginal fistula in particular have been shown to negatively affect outcome. Failure rates after flap repair for fistulizing Crohn’s disease are very much higher than after repair for complex fistulas of cryptoglandular origin: Mizrahi et al. [1] reported a success rate of 59.6 % (after a mean follow-up of 40 months) after flap repair for both cryptoglandular and Crohn’s fistulas. As expected, a significantly higher recurrence rate (57.1 %) was observed after flap repair of complex fistulas associated with Crohn’s disease than after repair of fistulas without Crohn’s disease (33.3 %). Similar results (50 % recurrence rate) were reported by Sonoda et al. [2] with a higher risk of recurrence in Crohn’s disease and rectovaginal fistulas. Additionally, long-term follow-up shows that recurrence rates after surgery for complex fistulas in Crohn’s disease are nearly 50 % and continuously increase over time [3].
Because of the high recurrence rates of complex fistulas associated with Crohn’s disease and because the author was personally impressed by the introduction of the VAAFT technique initially described by Meinero [4], it was the aim of this prospective study to analyze both feasibility and preliminary results of VAAFT combined with advancement flap repair for complex fistulas in Crohn's disease. To provide a relatively homogenous patient cohort, patients with severe proctitis, rectal stricture and horse-shoe fistulas were excluded from this pilot study. Since one aim of the study was to assess the feasibility of this novel approach, clear inclusion and exclusion criteria were formulated. Within the observation period, 7 patients met the exclusion criteria.
As regards equipment and technique, the procedural steps were identical to those used by Meinero for the diagnostic phase [4]. Key steps included excision of the external (perianal) opening of the fistula tract, insertion of the fistuloscope, visualization of the fistula tract and/or side tracts using the fistuloscope and correct localization of the internal fistula opening under direct vision with irrigation. In contrast to the Meinero technique (destruction of the main fistula tract by electrocautery), after diagnostic phase and after potential destruction of fistula tissue in side tracts with electrocautery or brushing, a “conventional” advancement flap repair was performed. In the beginning, there was some doubt as to whether the fistuloscope could be inserted and moved through the fistula tract. In two patients with a narrow anal canal and postinflammatory scarring, the diagnostic phase using the fistuloscope was not possible. However, fistuloscopy with identification of the internal opening was feasible in 11 of 13 procedures (completion rate 85 %). It is important that fistuloscopy is performed under direct vision and without excessive force; otherwise, “iatrogenic” or false tracts can be created and continence disorders will follow. Therefore, strict adherence to the technical criteria formulated by Meinero (e.g., surgery advisable if the fistula is active) is important [4].
Respecting these technical rules, additional side tracts not detected preoperatively (clinically or by endosonography) could be identified in 64 % (7/11) of patients. In these patients, additional fistula tracts were either destroyed by brushing (n = 4) or by electrocautery (n = 3). It can only be speculated whether the identification and therapy of these side tracts or residual fistula tissue had a significant impact on the current healing rates, because the study was limited by its small sample size, the absence of a control group, the positive selection bias (e.g., proctitis, horse-shoe fistulas excluded) and finally, the unknown effects of additional therapies in general (e.g., use of biologic drugs). Moreover, the follow-up was too short to provide definite healing rates. However, after a mean follow-up of 9 months, the success rate was 82 % (9/11), and stoma reversal was performed in 3 out of 4 patients. It has to be admitted that mean follow-up after stoma reversal was only 3 months in these patients. No procedural morbidity occurred, and no deterioration of continence was documented (Cleveland Clinic Incontinence Score 2.4 vs. 1.6, p > 0.05).
Further evaluation of the VAAFT technique is necessary to clearly define its role. Therefore, increased sample sizes, long-term follow-up and the “ideal” indications for the technique have to be discussed. In particular, in complex anal fistulas in which the internal opening could not be identified, there is another potential role for VAAFT. Moreover, comparative studies (e.g., comparison of VAAFT with preoperative MRI) are mandatory. At present, the current results are not generally conclusive, but it seems that the addition of VAAFT to advancement flap repair can enrich the surgical armamentarium in treating complex anal fistulas associated with Crohn’s disease.
Conclusion
Preliminary results of the VAAFT technique combined with advancement flap repair in fistulas associated with Crohn’s disease demonstrate that the addition of VAAFT to advancement flap repair leads to a high identification rate of occult side tracts with encouraging short-term healing rates. Moreover, a completion rate of 85 % seems promising.
References
Mizrahi N, Wexner SD, Zmora O et al (2002) Endorectal advancement flap: are there predictors of failure? Dis Colon Rectum 45:1616–1621
Sonoda T, Hull T, Piedmonte MR, Fazio VW (2002) Outcomes of primary repair of anorectal and rectovaginal fistulas using the endorectal advancement flap. Dis Colon Rectum 45:1622–1628
Löffler T, Welsch T, Mühl S, Hinz U, Schmidt J, Kinele P (2009) Long-term success rate after surgical treatment of anorectal and rectovaginal fistulas in Crohn's disease. Int J Colorectal Dis 24:521–526
Meinero P, Mori L (2011) Video-assisted anal fistula treatment (VAAFT): a novel sphincter-saving procedure for treating complex anal fistulas. Tech Coloproctol 15:417–422
Hannaway CD, Hull TL (2008) Current considerations in the management of rectovaginal fistula from Crohn's disease. Colorectal Dis 10:747–755
Andreani SM, Dang HH, Grondona P, Khan AZ, Edwards DP (2007) Rectovaginal fistula in Crohn’s disease. Dis Colon Rectum 50:2215–2222
Joo JS, Weiss EG, Nogueras JJ, Wexner SD (1998) Endorectal advancement flap in perianal Crohn’s disease. Am Surg 64:147–150
Schwandner O (2011) Obesity is a negative predictor of success after surgery for complex anal fistula. BMC Gastroenterol 11:61
Jorge JM, Wexner SD (1993) Etiology and management of fecal incontinence. Dis Colon Rectum 36:77–97
Tozer PJ, Burling D, Gupta A, Phillips RK, Hart AL (2011) Review article: medical, surgical and radiological management of perianal Crohn's fistulas. Aliment Pharmacol Ther 33:5–22
Bourikas LA, Koutroubakis IE (2010) Anti-TNF and fistulizing perianal Crohn's disease: use in clinical practice. Curr Drug Targets 11:187–197
Karmiris K, Bielen D, Vanbeckevoort D et al (2011) Long-term monitoring of infliximab therapy for perianal fistulizing Crohn's disease by using magnetic resonance imaging. Clin Gastroenterol Hepatol 9:130–136
Bode M, Eder S, Schürmann G (2008) Perianal fistulas in Crohn's disease—biologicals and surgery: is it worthwhile? Z Gastroenterol 46:1376–1383
van der Hagen SJ, Baeten CG, Soeters PB, Russel MG, Beets-Tan RG, van Gemert WG (2005) Anti-TNF-alpha (infliximab) used as induction treatment in case of active proctitis in a multistep strategy followed by definitive surgery of complex anal fistulas in Crohn's disease: a preliminary report. Dis Colon Rectum 48:758–767
Hyder SA, Travis SP, Jewell DP, McC Mortensen NJ, George BD (2006) Fistulating anal Crohn's disease: results of combined surgical and infliximab treatment. Dis Colon Rectum 49:1837–1841
El-Gazzaz G, Hull T, Church JM (2012) Biological immunomodulators improve the healing rate in surgically treated perianal crohn's fistulas. Colorectal Dis 14:1217–1223
Schwandner O, Fuerst A, Kunstreich K, Scherer R (2009) Innovative technique for the closure of rectovaginal fistula using Surgisis mesh. Tech Coloproctol 13:135–140
Sciaudone G, Di Stazio C, Limongelli P et al (2010) Treatment of complex perianal fistulas in Crohn disease: infliximab, surgery or combined approach. Can J Surg 53:299–304
Duff S, Sagar PM, Rao M, Dolling S, Sprakes M, Hamlin PJ (2011) Infliximab and surgical treatment of complex anal Crohn's disease. Colorectal Dis 14:972–976
Acknowledgments
The author thanks PD Dr. Christoph Isbert, Department of Surgery, University of Wuerzburg, for providing the drawings of anal fistula.
Conflict of interest
No conflict of interest exists.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schwandner, O. Video-assisted anal fistula treatment (VAAFT) combined with advancement flap repair in Crohn’s disease. Tech Coloproctol 17, 221–225 (2013). https://doi.org/10.1007/s10151-012-0921-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-012-0921-7