Abstract
Purpose
The HER2-low breast cancer is a newly recognized entity with the clinical characteristics is yet to be defined. We hypothesized that HER2-low breast cancer could lead to an increased rate of brain metastases in patients with localized breast cancer. We tested this hypothesis in a large cohort of breast cancer patients with long follow-up.
Methods
We included 2686 adult breast cancer patients followed up in Hacettepe University Cancer Center. Patients with 1 + positive HER2 expression and 2 + HER2 expression with a negative FISH were categorized as HER2-low disease. We evaluated the brain metastasis risk with binary logistic regression analyses and reported odds ratios (OR) with 95% confidence intervals (CI).
Results
During a median 95.4 (IQR 72.6–123.1) month follow-up, 184 patients developed brain metastasis (6.9%). The brain metastases were developed in 5.1% of the patients with HER2-negative disease, 8.5% of the patients with HER2-low disease, and 10.1% of the patients with HER2-positive disease. A multivariable binary logistic regression model demonstrated an increased risk of brain metastasis in patients with HER2-low disease (OR: 1.611, 95% CI 1.055–2.460, p = 0.027) and in HER2-positive patients (OR: 1.837, 95% CI 1.308–2.580, p < 0.001). Additionally, HR + -HER2-low disease was associated with a decreased DFS compared to HR + -HER2-negative disease (p = 0.008).
Conclusion
In this study, we observed an increased risk of brain metastasis in localized breast cancer patients with HER2-low disease. We think that a high level of vigilance and a low threshold for brain imaging could benefit HER2-low breast cancer patients similar to the patients with HER-positive disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common malignancy and a leading cause of mortality in women with more than two million cases and 600,000 deaths in 2018 [1]. Breast cancer has four well-defined clinical subtypes, Luminal A, Luminal B, human epidermal growth factor receptor 2 (HER2) positive, and triple negative (TN), affecting the prognosis and guiding treatment decisions [2, 3]. The HER2-positive disease is a distinct subgroup characterized by the endocrine resistance and uncontrolled proliferation due to over-expression of epithelial growth factor receptors and clinically characterized by the high rate of brain metastases and early relapses [4,5,6,7]. While the HER2-positive disease was previously associated with poor survival outcomes, the survival outcomes are significantly improved with the advent of effective anti-HER2 treatments in the last 2 decades [8, 9].
The HER2-positive breast cancer was defined by a 3 + HER2 expression in immunohistochemistry (IHC) or in situ hybridization (ISH) positivity, and these patients are candidates for anti-HER2 treatments. However, the IHC 1 + and 2 + HER-positive breast cancers have a significant degree of HER-2 expression in cellular surfaces [10, 11], which could contribute to the disease progression. This notion led the researchers to test the anti-HER2 agents like trastuzumab and trastuzumab emtansine in these patients to improve outcomes, both in the metastatic and adjuvant settings [12, 13]. However, the results were largely disappointing and led to the limitation of clinical trials with anti-HER2 agents to HER2 3 + or HER2 ISH positive patients only. However, new-generation antibody–drug conjugates (ADC) like trastuzumab deruxtecan and trastuzumab duocarmazine demonstrated a significant degree of activity in patients with low HER2 expression in the metastatic setting [14, 15]. These findings reignited the interest in anti-HER2 treatments in hormone-positive (HR) and TN patients with 1 + and 2 + HER2 expression, and several clinical trials are being conducted in the HER2-low breast cancer with ADCs [16,17,18].
The HER2-low breast cancer is a newly recognized entity with the potential to be amenable to novel anti-HER2 agents. However, the clinical characteristics and the prognosis of this subtype are yet to be defined [16, 17]. Several studies evaluated the association between prognosis and low-level HER2 positivity in early breast cancer with variable HER2 expression cut-offs (> 0 + or 2 +) and methodologies (transcriptomic vs immunohistochemistry), and reported different affected subgroups (node-positive or negative/HR-positive or negative), leading to inconclusive results [19,20,21]. Besides, whether there are clinical similarities in metastasis patterns between HER2-positive and HER2-low disease was not evaluated. From these points, we hypothesized that HER2-low breast cancer could lead to decreased survival times and an increased rate of brain metastases in patients with localized breast cancer. In this study, we tested this hypothesis in a large cohort of breast cancer patients with long follow-up.
Methods
Study cohort
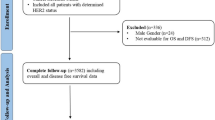
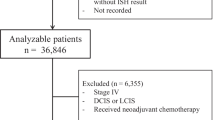
For this retrospective cohort study, we evaluated the data of 3151 adult breast cancer patients followed up between 01.01.2000 and 30.12.2016 in Hacettepe University Cancer Center. We included all patients treated within the prespecified dates other than patients treated in clinical trials, patients with incomplete clinical and survival data, and patients with metastatic disease at baseline. HER2 status was determined via IHC and expression levels were graded as negative, 1 + , 2 + and 3 + according to relevant ASCO CAP guidelines [22] at the time of tumor evaluation. Fluorescent in situ hybridization (FISH) was performed for the IHC 2 + cases. Patients with 1 + positive HER2 expression and 2 + HER2 expression with a negative FISH were categorized as HER2-low disease as previously suggested [16, 22]. Due to prohibitions in our country, the HER2 statuses were not re-evaluated for the study.
We recorded baseline demographics (age, sex, marital status), menopause status, patient weight and height, tumor T-N-M stage, tumor hormone, and HER2 expression status, lymphovascular invasion (LVI), perineuronal invasion (PNI), surgery and radiotherapy history, comorbidities and regular medications together with survival data. Additionally, the presence or absence of brain metastasis development during follow-up was recorded. The patients were diagnosed with brain metastasis with the imaging studies that were conducted due to symptoms and no routine cranial imaging was performed during the follow-up, as suggested in the guidelines [23, 24]. Comorbidities were categorized according to Charlson Comorbidity Index (CCI) [25]. The disease-free survival (DFS) events were defined as the development of metastases or new breast tumors under follow-up and/or death. The overall survival (OS) time was defined as the period from diagnosis to the last follow-up and/or death, and DFS time was defined as the period between diagnosis to disease progression and/or death.
Statistical analyses
We presented the descriptive characteristics by the medians and interquartile ranges (IQR) for continuous variables and percentages for categorical variables. We compared baseline characteristics with Kruskal–Wallis and Chi-square tests. We evaluated the predisposing factors for brain metastasis development with Chi-square and Fisher’s exact tests. We constructed a binary logistic regression model for brain metastasis development with the statistically significant parameters in the univariate analyses. We used Kaplan–Meier survival curves and Cox regression analyses for univariate and multivariable survival analyses, respectively. We reported odds ratios (OR) and hazard ratios (HR) with 95% confidence intervals (CI) for the variables included in the multivariable models. We used Statistical Package for Social Sciences version (SPSS) version 25.0 (IBM Inc., Armonk, NY, USA) in the analyses and considered p values below 0.05 statistically significant.
Results
Baseline characteristics
After excluding patients with incomplete data (n = 171) and patients with metastatic disease at baseline (n = 294), we included a total of 2686 patients who followed up between the prespecified dates. The cohort's median age was 48 (IQR 41–56), and 49.3% of the patients were premenopausal at diagnosis. Most patients had T1 or T2 disease (84%) and more than half of the patients had node positive disease (53.3%) (Table 1). Six hundred seventy-three patients (25.1%) had HER2-positive disease, while 1723 (64.1%) had HR + and 290 (10.8%) had TN breast cancer. Hypertension was the most frequent comorbidity (22.5%), and the median CCI was 1. The HER2 expression was 0 in 1625 (60.5%), + 1 in 172 (6.4%), 2 + in 265 (9.9%) and + 3 in 624 (23.2%) patients. Additionally, HER2 FISH was positive in the 49 patients with + 2 HER2 expression. The HER2 1 + and 2 + patients were included together as the HER2-low group in the analyses (n = 388). The large portion of patients with HER2-low disease had HR positivity (n = 347, 89.6%), while TN-HER2-low disease was present in the minority (n = 41, 10.6%). Compared with HER2-negative tumors, HER2-low and HER2-positive tumors had higher T and N stages (Table 2). The median age of HER2-negative cohort was slightly higher and the frequency of hypertension was higher in HER2-negative and HER2-low patients compared to HER2-positive patients (Table 2).
Survival analyses
During a median 95.4 (IQR 72.6–123.1) months follow-up, 539 (20.1%) patients died, and 710 (26.4%) patients had any DFS events. The median OS and DFS were not reached. The T3–T4 primary, lymph node positivity, diabetes, and higher CCI (2 vs. 0–1) were associated with decreased OS (p < 0.001 for each) in univariate analyses. Similarly, these factors were associated with decreased PFS (p < 0.001 for T3–T4 primary, lymph node positivity and higher CCI and p = 0.004 for diabetes) in univariate analyses. The patients with LVI or PNI had decreased OS and DFS, while the survival difference between normal and overweight patients did not reach statistical significance (p = 0.092 for OS and p = 0.439 for DFS). Low levels of HER2 expression (vs. remaining cohort including HER2-negative and HER2-positive patients) did not have a statistically significant association with OS (p = 0.981) and DFS (p = 0.294). Similarly, the patients with HER2-low disease had similar OS compared to HER2-negative patients (p = 0.393). In contrast, low-level HER2 expression was associated with decreased DFS compared to negative HER2 expression (Fig. 1). The adverse effect of low-level HER2 expression was statistically significant in HR + -HER2-low disease (p = 0.008), while the difference did not reach statistical significance in TN-HER2-low disease (p = 0.242). A multivariable model was constructed with statistically significant variables in univariate OS analyses. In multivariable analyses, all included parameters other than the history of diabetes retained a significant negative association with OS. The DFS analyses were consistent with OS analyses (Table 3).
Evaluation of brain metastasis risk
During the follow-up, 184 patients developed brain metastasis (6.9%). The risk of brain metastases development was increased in premenopausal patients and node-positive patients, and patients with T3–T4 tumors. Additionally, brain metastasis development risk was increased in HER2-low and HER2-positive patients (p = 0.001). The brain metastases were developed in 5.1% of the patients with HER2-disease, 8.5% of the patients with HER2-low disease, and 10.1% of the patients with HER2-positive disease (Fig. 2). A multivariable binary logistic regression model demonstrated an increased risk of brain metastasis in patients with HER2-low disease (OR: 1.611, 95% CI 1.055–2.460, p = 0.027) and in HER2-positive patients (OR: 1.837, 95% CI 1.308–2.580, p < 0.001) (Table 3, Fig. 3). The increased risk of brain metastasis remained significant in patients with HR + -HER2-low disease (OR: 1.655, 95% CI 1.041–2.664, p = 0.033). The association between brain metastasis development risk and HER2-low disease did not reach statistical significance in patients with TN-HER2-low disease (OR: 1.590, 95% CI 0.568–4.450, p = 0.377).
Discussion
Breast cancer is the second leading cause of brain metastasis and development of brain metastases is related to significant mortality [26,27,28]. The risk of brain metastasis is subtype-dependent in breast cancer with a predilection for increased risk in HER2-positive tumors [29, 30]. The brain tropism in HER2-positive tumors is possibly related to an interplay of several factors in the tumor microenvironment and blood–brain barrier [31], and HER2 oncogene postulated to be at the center of these interplay [31, 32]. The HER2-low breast cancers also contain a significant level of HER2 receptors on the cell surface [11]. However, whether a similar tropism for brain metastases is present in HER2-low breast cancers is unknown.
In the present study, we observed a 61% increased risk of brain metastasis in HER2-low breast cancer compared to HER2-negative patients. If this association is supported by prospective evidence, the patients with HER2-low breast cancers could be candidates for new therapeutic strategies in the adjuvant setting to prevent brain metastasis. The increased brain metastasis risk did not reach statistical significance in the TN group. We think that the small sample size (n = 289) and a limited number of events (n = 26) could be the reasons for the lack of association, and the association between HER2 expression and brain metastasis risk should be evaluated in larger TN breast cancer cohorts. In contrast, the brain metastasis risk was significantly increased in the HR + -HER2-low group independent of tumor T and N stages. While the HR + tumors conventionally have a better prognosis and a lower brain metastasis risk, low levels of HER2 expression could define a biologically more aggressive subtype with an increased brain metastasis risk, possibly due to similar biologic mechanisms with HER2-positive tumors.
We observed similar OS in patients with HER2-low tumors and HER2-negative tumors, while the DFS was significantly lower in patients with HER2-low tumors, especially in the HR + -HER2-low subgroup (Fig. 1). The effect of low-level HER2 expression on survival was investigated in several studies in localized breast cancer and demonstrated conflictive results [21, 33, 34]. In one of the pioneer reports by Camp et al., both the high and normal levels of HER2 expression were associated with poor outcomes, while the intermediate HER2 expression was associated with better DFS in a cohort of 300 breast cancer patients. The authors used a histogram to define the HER2 expression and reported that 71.3% of the breast cancers had intermediate HER2 expression [35]. A latter report with a moderate sample size (n = 91) suggested that any level of HER2 expression was associated with decreased DFS (p = 0.001) and OS (p = 0.001) in node lymph-positive breast cancer patients [21], albeit the HER2-low disease was comprised a significantly lower portion of their cohort (14% HER2 1 + and 5% HER2 2 +) similar to our study. The negative prognostic effect was more pronounced in the HR + -HER2-positive cohort [21], similar to our study. Rossi et al. included a larger cohort of patients (n = 1150) with HER2-negative and HER2-low disease and observed lower DFS in HER2 2 + patients compared to HER2-negative and HER2 1 + patients. In the study, the 1 + HER2 expression was higher than our cohort (39% vs. 6.4%), while the 2 + HER2 expression rates were similar (10% vs. 9.9%). The adverse effect of 2 + HER2 expression was time dependent and observed after an early survival advantage [34]. Interestingly, in the most comprehensive study (> 5000 patients), researchers from Germany reported lower DFS (HR: 1.217, 95% CI 1.052–1.408, p = 0.008) but similar breast cancer-specific survival (HR = 1.045, 95% CI 0.926–1.178, p = 0.474) in HR + patients with moderate HER2 expression [36]. However, the DFS difference did not reach statistical significance in HR− patients. These data [36] and our observations in the HR + subgroup only made us think that HER2-low status could be an additional prognostic factor in HR + patients, which traditionally have a better prognosis than the TN disease [37, 38].
Our study has several limitations. First, the study's retrospective nature and the small number of patients in some subgroups limited the generalizability. Our study was conducted in a tertiary reference center that may have created shifts in our case distribution as the increased percentage of younger patients. The 1 + HER2 expression was possibly underreported in our study as recent studies reported more than 30% 1 + HER expression levels [39], while 2 + HER expression rates in our study were similar to the literature [36, 39]. This issue could prevent our results’ generability, although we think that our results are still hypothesis-generating. We could not exclude the possibility of an undetected brain metastasis due to a lack of regular interval cranial imaging to detect brain metastasis in accordance with the international oncology guidelines. Additionally, the maturation rates for OS and DFS levels were low limiting the power of survival analyses. However, despite these limitations, we observed a significant increase in the risk of brain metastasis in patients with HER-2-low breast cancer. To best our knowledge, this is the first report on the brain metastasis risk in this newly defined disease phenotype.
Conclusion
In this study, we observed an increased risk of brain metastasis in localized breast cancer patients with HER2-low disease and a lower DFS in HR + -HER2-low disease. We think that a high level of vigilance and a low threshold for brain imaging could benefit HER2-low breast cancer patients similar to the patients with HER-positive disease until our observation is further tested in the larger prospective datasets.
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Szymiczek A, Lone A, Akbari MR (2021) Molecular intrinsic versus clinical subtyping in breast cancer: a comprehensive review. Clin Genet 99:613–637
van Maaren MC, de Munck L, Strobbe LJA et al (2019) Ten-year recurrence rates for breast cancer subtypes in the Netherlands: a large population-based study. Int J Cancer 144:263–272
Kennecke H, Yerushalmi R, Woods R et al (2010) Metastatic behavior of breast cancer subtypes. J Clin Oncol 28:3271–3277
Rouanet P, Roger P, Rousseau E et al (2014) HER2 overexpression a major risk factor for recurrence in pT1a-bN0M0 breast cancer: results from a French regional cohort. Cancer Med 3:134–142
Lin NU, Amiri-Kordestani L, Palmieri D et al (2013) CNS metastases in breast cancer: old challenge, new frontiers. Clin Cancer Res 19:6404–6418
Brufsky AM, Mayer M, Rugo HS et al (2011) Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res 17:4834
Patel A, Unni N, Peng Y (2020) The changing paradigm for the treatment of HER2-positive breast cancer. Cancers 12:2081
Slamon DJ, Clark GM, Wong SG et al (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177–182
Hendriks BS, Klinz SG, Reynolds JG et al (2013) Impact of tumor HER2/ERBB2 expression level on HER2-targeted liposomal doxorubicin-mediated drug delivery: multiple low-affinity interactions lead to a threshold effect. Mol Cancer Ther 12:1816–1828
Onsum MD, Geretti E, Paragas V et al (2013) Single-cell quantitative HER2 measurement identifies heterogeneity and distinct subgroups within traditionally defined HER2-positive patients. Am J Pathol 183:1446–1460
Fehrenbacher L, Cecchini RS, Geyer CE Jr et al (2019) NSABP B-47/NRG oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1 + or 2 +. J Clin Oncol 38:444–453
Burris HA, Rugo HS, Vukelja SJ et al (2010) Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)—positive breast cancer after prior HER2-directed therapy. J Clin Oncol 29:398–405
Modi S, Park H, Murthy RK et al (2020) Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low–expressing advanced breast cancer: results from a phase Ib study. J Clin Oncol 38:1887–1896
Banerji U, van Herpen CML, Saura C et al (2019) Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol 20:1124–1135
Tarantino P, Hamilton E, Tolaney SM et al (2020) HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol 38:1951–1962
Eiger D, Agostinetto E, Saúde-Conde R et al (2021) The exciting new field of HER2-low breast cancer treatment. Cancers 13 (5):1015
Marchiò C, Annaratone L, Marques A et al (2021) Evolving concepts in HER2 evaluation in breast cancer: heterogeneity, HER2-low carcinomas and beyond. Semin Cancer Biol 72:123–135
Koscielny S, Terrier P, Daver A et al (1998) Quantitative determination of c-erbB-2 in human breast tumours: potential prognostic significance of low values. Eur J Cancer 34:476–481
Dittadi R, Brazzale A, Pappagallo G et al (1997) ErbB2 assay in breast cancer: possibly improved clinical information using a quantitative method. Anticancer Res 17:1245–1247
Gilcrease MZ, Woodward WA, Nicolas MM et al (2009) Even low-level HER2 expression may be associated with worse outcome in node-positive breast cancer. Am J Surg Pathol 33:759–767
Wolff AC, Hammond MEH, Allison KH et al (2018) HER2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update Summary. J Oncol Pract 14:437–441
Gradishar WJ, Moran MS, Abraham J et al (2021) NCCN Guidelines® Insights: breast cancer, version 4.2021: featured updates to the NCCN guidelines. J Natl Compr Cancer Netw 19:484–493
Cardoso F, Paluch-Shimon S, Senkus E et al (2020) 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 31:1623–1649
Charlson ME, Pompei P, Ales KL et al (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Achrol AS, Rennert RC, Anders C et al (2019) Brain metastases. Nat Rev Dis Prim 5:5
Steindl A, Kreminger J, Moor E et al (2020) 363O Clinical characterization of a real-life cohort of 6001 patients with brain metastases from solid cancers treated between 1986–2020. Ann Oncol 31:S397
Cheng X, Hung MC (2007) Breast cancer brain metastases. Cancer Metastasis Rev 26:635–643
Berghoff A, Bago-Horvath Z, De Vries C et al (2012) Brain metastases free survival differs between breast cancer subtypes. Br J Cancer 106:440–446
Sun MS, Liu YH, Ye JM et al (2021) A nomogram for predicting brain metastasis in patients with de novo stage IV breast cancer. Ann Transl Med 9:853
Zimmer AS, Van Swearingen AED, Anders CK. HER2-positive breast cancer brain metastasis: a new and exciting landscape. Cancer Rep (Hoboken) 2020; e1274.
Gupta P, Srivastava SK (2014) HER2 mediated de novo production of TGFβ leads to SNAIL driven epithelial-to-mesenchymal transition and metastasis of breast cancer. Mol Oncol 8:1532–1547
Agostinetto E, Rediti M, Fimereli D et al (2021) HER2-low breast cancer: molecular characteristics and prognosis. Cancers 13:2824
Rossi V, Sarotto I, Maggiorotto F et al (2012) Moderate immunohistochemical expression of HER-2 (2+) without HER-2 gene amplification is a negative prognostic factor in early breast cancer. Oncologist 17:1418–1425
Camp RL, Dolled-Filhart M, King BL et al (2003) Quantitative analysis of breast cancer tissue microarrays shows that both high and normal levels of HER2 expression are associated with poor outcome. Can Res 63:1445
Eggemann H, Ignatov T, Burger E et al (2015) Moderate HER2 expression as a prognostic factor in hormone receptor positive breast cancer. Endocr Relat Cancer 22:725–733
Howlader N, Cronin KA, Kurian AW et al (2018) Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiol Biomark Prev 27:619
Fallahpour S, Navaneelan T, De P et al (2017) Breast cancer survival by molecular subtype: a population-based analysis of cancer registry data. CMAJ Open 5:E734–E739
Schettini F, Chic N, Brasó-Maristany F et al (2021) Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. Npj Breast Cancer 7:1
Funding
The authors received no financial support for this article.
Author information
Authors and Affiliations
Contributions
DCG and SA have planned the work. DCG, MBK, BF, MO, HCY, KK, NK, OD, AU, and SA participated in patient care and data collection. All authors, namely DCG, MBK, BF, MO, HCY, KK, NK, OD, AU, and SA participated have made significant and substantive contributions to the reporting of the work. All authors have participated in the review of relevant literature, drafting of the manuscript, review and revisions of the final draft. DCG, MBK and SA have analysed the data and determined the main conclusions. DCG has prepared the first draft of the manuscript. All authors reviewed and participated in the preparation of the revised and final version of the manuscript. DCG and SA are responsible for the overall content as guarantors. All co-authors qualify the criteria for authorship according to Vancouver protocol.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Guven, D.C., Kaya, M.B., Fedai, B. et al. HER2-low breast cancer could be associated with an increased risk of brain metastasis. Int J Clin Oncol 27, 332–339 (2022). https://doi.org/10.1007/s10147-021-02049-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-021-02049-w