Abstract
Background
We investigated the association between positive surgical margin (PSM) status and biochemical recurrence (BCR) after robot-assisted radical prostatectomy (RARP) to develop a prognostic factor-based risk stratification model for BCR.
Methods
We analyzed the data of 483 patients who underwent RARP at our hospital between October 2010 and April 2019; 435 patients without neoadjuvant therapy were finally included. The BCR-free survival rate was determined using Kaplan–Meier analysis. Effects of the PSM status, including the number of PSMs, Gleason score (GS) at a PSM, and the maximum PSM length for BCR, were investigated using Cox regression analysis.
Results
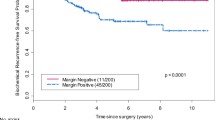
BCR was confirmed after RARP in 61 patients (14.0%), and PSM was confirmed in 74 patients (17.0%); PSM was a significant predictor of BCR (p < 0.001). The median number of PSMs was 2 (1–6), and the median maximum length of PSM was 6.0 (2.0–17.0) mm. Multivariable analysis showed lymph node invasion (p < 0.001), GS of ≥ 7 at a PSM (p = 0.022) and a maximum PSM length of > 6.0 mm (p = 0.003) were significant predictors of BCR. We classified the patients without lymph node invasion into good-, intermediate-, and poor-risk groups according to the other two risk factors (presence of 0, 1, and 2 factors, respectively) and rates of 1-year BCR-free survival (100.0, 72.7, and 48.1%, respectively).
Conclusion
Higher GS at PSM and greater length of PSM were significant predictors of BCR after RARP, and console surgeons should be careful to prevent PSM during RARP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radical prostatectomy (RP) is considered the standard surgical treatment of clinically localized prostate cancer (PCa), and robot-assisted radical prostatectomy (RARP) has been widely used globally in recent years. However, the rate of 5-year biochemical recurrence (BCR)-free survival is approximately 74–87% [1, 2]. Furthermore, after 10 and 20 years of follow-up, the rate of BCR-free survival rate decreased from 65.7 to 47.3%, and the majority of BCRs develop during the initial few years after RP [3, 4].
Numerous factors, including prostate-specific antigen (PSA) level, age, pathologic Gleason score (GS), pathologic T stage, pathologic N stage, positive surgical margin (PSM), seminal vesicle invasion, and lymphovascular invasion, have been reported to be independent predictors of BCR [1, 3,4,5,6,7]. With regard to the incidence of PSM according to type of surgery, Novara et al. reported PSM in 6.5–32% of patients in a contemporary series of RP, and the incidences of PSM after RARP, retropubic RP, and laparoscopic RP were similar [1]. Unfortunately, PSM may lead to clinical progression and poor cancer-specific survival [8]. Boorjian et al. reported that PSM increased the risk of BCR and local recurrence but was not independently associated with cancer-specific death or overall mortality by adding appropriate salvage treatment [9]. In other words, it is important to identify the patients who are at a high risk of early BCR after RP. Several recent reports have focused on the PSM status and BCR after RP, including RARP series [10,11,12,13,14]. Although BCR has been associated with length and location of PSM, GS at a PSM, and focality of PSM [10,11,12,13,14], it is still a matter of debate and the predictive value of the PSM status in the era of robotic surgery remains to be well investigated. Conversely, some recent studies have shown that RARP reduces the risk of PSM and rates of BCR compared with open RP [15,16,17]. In the current study, we investigated the association between the PSM status and BCR after RARP and develop a prognostic factor-based risk stratification model for early BCR according to the PSM status.
Patients and methods
Data from 483 consecutive patients with PCa who underwent RARP from October 2010 to April 2019 at our hospital were retrospectively analyzed. We excluded 47 patients who had received neoadjuvant hormonal treatment and 1 who had undergone follow-up at < 3 months; the remaining 435 patients were included in this study. The research protocol was approved by the institutional review board of Tottori University Hospital (20A085). We preoperatively evaluated these patients using pelvic magnetic resonance imaging, chest–pelvis computed tomography, and whole-body bone scanning according to their physicians’ discretion. The surgeries were performed by nine different surgeons, and anterograde surgery was performed via the transperitoneal approach using the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA, USA). Four nerve-sparing (NS) grade methods, described in previous studies [18,19,20] were used in all patients to preserve function and for tumor control in accordance with the National Comprehensive Cancer Network (NCCN) criteria [21]. The criteria for treatment with pelvic lymph node dissection (PLND) were based on the risk of lymph node (LN) metastases according to the European Association of Urology guideline or NCCN classification by the physicians’ discretion [21, 22]. Limited PLND and extended PLND were defined by Ploussard et al. [23]. We performed limited PLND in the early days of RARP until 2015; since 2016, after the learning curve period, we have performed extended PLND.
Patients in whom BCR were not confirmed after surgery did not receive immediate adjuvant hormonal treatment or pelvic radiation therapy. They underwent follow-up PSA measurements 1 month after surgery, then every 3 months during the first 2 years, every 6 months during the second to fourth years, and annually after the fifth year. BCR was defined by PSA levels of ≥ 0.2 ng/mL and secondary confirmation of increase after surgery. If the postoperative PSA levels were not < 0.2 ng/mL, the date of surgery represented the recurrence date. All surgical specimens were fixed in 10% neutral-buffered formalin, embedded in paraffin blocks, and stained with hematoxylin and eosin. The apex of the prostate was defined as the inferior-most 5–7-mm portion of the gland, and the base of the prostate as the superior-most 5–7-mm portion of the gland; the rest was defined as the mid-gland. The apex and the base of the prostate were divided into 3–5-mm sagittal sections and the mid-gland into 3–5-mm horizontal sections. Tumor characteristics were obtained from pathology reports by the pathologists at our institution. RP specimens were stained, and PSM was determined positive when cancer cells were in contact with the surface of the RP specimen on light microscopy, and one pathologist and two urologists individually evaluated the GS at PSM, the margin length, and the number of PSMs.
For the statistical analysis, we divided the patients by median age and the PSM status, including the number of PSMs, GSs at PSM, and the maximum length of PSM, and we divided PSA, pathological T stage, and pathological GS according to the risk categories of the NCCN classification [21]. We used the Kaplan–Meier method to evaluate the predictors of BCR, and Cox regression analysis to investigate the effects of age, PSA, pathologic T stage, pathologic GS, extraprostatic extension of the tumor, perineural invasion, lymphovascular invasion, seminal vesicle invasion, PSM, and lymph node invasion (LNI) on BCR. In addition, we used Cox regression analysis to examine the PSM status, including the number of PSMs, GS at PSM, and the maximum length of PSM along with the prognostic factors from the analyzed BCR data. To perform statistical analyses, we used IBM SPSS Statics for Windows (Version 25). All p values were two-sided, and values of < 0.05 were considered significant.
Results
The median follow-up period was 52.4 (5.4–111.6) months. Table 1 shows the clinical characteristics of the patients; the median age at surgery was 66 (48–78) years. Bilateral and unilateral NS techniques were performed in 22 (5.1%) and 147 patients (33.8%), respectively; bilateral and unilateral partial NS techniques were performed in 72 (16.6%) and 130 (29.9%), respectively; and non-NS techniques were performed in 64 (14.7%). Fifty-three patients (12.2%) did not undergo PLND; 163 (37.5%) underwent limited PLN; and 219 (50.3%) underwent extended PLND. The median number of LNs removed was 8 (0–23) during limited PLND and 17 (3–40) in extended PLND. PSM was confirmed in 74 patients (17.0%), and the status of the surgical margins was uncertain in 2 patients. Table 2 summarizes the pathological characteristics of the patients according to the PSM status after RARP. The overall incidence of PSM after RARP was 17.0%: 6.3% for stage pT2a, 10.0% for stage pT2b, 13.5% for stage pT2c, 39.7% for stage pT3a, 50% for stage pT3b, and 100% for stage pT4.
We confirmed that 61 patients (14.0%) had BCR after RARP during the follow-up period, and the rate of 5-year BCR-free survival was 84.6%. Table 3 shows the results of univariable and multivariable analyses of the associations between perioperative factors, including the pathological findings, and BCR. Univariable analysis revealed that a preoperative PSA level of ≥ 20, pathologic stage of ≥ T3a, pathologic GS of ≥ 8, extraprostatic extension, perineural invasion, lymphovascular invasion, seminal vesicle invasion, PSM, and LNI were significantly associated with BCR (p < 0.05 for all parameters). Multivariable regression analysis revealed that a pathologic GS of ≥ 8 (hazard ratio [HR], 2.036; 95% confidence interval [CI], 1.190–3.482; p = 0.009), lymphovascular invasion (HR 2.785; 95% CI 1.253–6.188; p = 0.012), PSM (HR 3.466; 95% CI 2.146–5.595; p < 0.001), and LNI (HR 4.617; 95% CI 2.299–9.273; p < 0.001) were significant negative predictors of BCR-free survival.
Table 4 lists the details of the characteristics of PSM stratified by GS, margin length, and number. Of the 74 patients with PSM, 39 (52.7%) had ≥ 2 PSMs and 35 (47.3%) had only one. Of the 35 patients with only one PSM, 19 (54.3%) had a PSM at the apex of the prostate, 7 (20.0%) in the mid-gland, and 9 (25.7%) at the base. The median maximum length of PSM was 6.0 (2.0–17.0) mm. According to multivariable regression analysis, including the PSM status and prognostic factors from the previous multivariable regression analysis for BCR, LNI (HR 11.948; 95% CI 3.803–37.535; p < 0.001), a GS of ≥ 7 at a PSM (HR 3.281; 95% CI, 1.190–9.045; p = 0.022), and maximum PSM length of > 6 mm (HR, 4.194; 95% CI, 1.620–10.858; p = 0.003) were significant negative predictors of BCR-free survival (Table 5). In addition, the same analysis excluding the patients with LNI also showed that higher GS at PSM (p = 0.010) and greater length of PSM (p = 0.004) were significant predictors of BCR. We classified the patients without LNI into good-, intermediate-, and poor-risk groups according to the other two PSM factors (presence of 0, 1, and 2 factors, respectively) and rates of 1-year BCR-free survival (100.0, 72.7, and 48.1%, respectively; Fig. 1a). Furthermore, we classified all patients into four risk groups (good, intermediate, poor, and very poor) according to all three risk factors, including LNI (presence of 0, 1, 2, and 3 factors, respectively) and rates of 1-year BCR-free survival (100.0, 72.8, 53.6, and 0%, respectively; Fig. 1b).
a Kaplan–Meier curves for biochemical recurrence-free survival among the patients without LNI by the risk stratification model according to Gleason score of ≥ 7 at a PSM and maximum PSM length of > 6. Good-, intermediate-, poor-risk group had 0, 1, and 2 factors, respectively. PSM: positive surgical margin. LNI: lymph node invasion. b Kaplan–Meier curves for biochemical recurrence-free survival by the risk stratification model according to Gleason score of ≥ 7 at a PSM, maximum PSM length of > 6, and the presence of LNI. Good-, intermediate-, poor-, and very poor-risk group had 0, 1, 2, and 3 factors, respectively. PSM: positive surgical margin. LNI: lymph node invasion
Discussion
We investigated the association between the presence of PSM and development of BCR after RARP and developed a prognostic factor-based risk stratification model for predicting early BCR according to the PSM status. In this study, the incidences of PSM did not significantly differ between the patients who underwent bilateral and unilateral NS procedures and those who underwent bilateral partial NS/unilateral partial NS/non-NS procedures (p = 0.932; data not shown). However, because aggressive NS techniques would increase the incidence of PSM, an increase in PSM incidence can be prevented by proper patient selection. Thompson et al. reported that RARP had a long learning curve with inferior outcomes initially, and then outcomes became progressively superior, including PSM incidence and urinary outcomes [24]. Novara et al. mentioned that several surgeon-related characteristics or procedure-related issues may play a major role in PSM incidence [1]. The incidence of PSM is reported to be between 5 and 46%, and this rate has been decreasing in large academic centers [13]. We believe that at high-volume centers, experienced surgeons should use aggressive NS techniques in RARP, whereas surgeons at low-volume centers should not use aggressive NS techniques in RARP during their learning curve period.
In this study, a pathologic GS of ≥ 8, lymphovascular invasion, PSM, and LNI were significant negative predictors of BCR-free survival, according to multivariable regression analysis. These results are corroborated by several reports [1, 4, 6, 7, 16, 25]. In addition, we examined the PSM status, including the number of PSMs, GS at a PSM, and the maximum length of PSM, along with the prognostic factors from the previous multivariable regression analysis for BCR. We found that LNI, a GS of ≥ 7 at a PSM, and maximum PSM length of > 6 mm were significant negative predictors of BCR-free survival. These results showed that the PSM status influenced BCR strongly compared with an overall GS of ≥ 8. Although biopsy GS before RARP is not necessarily the same as pathologic GS after RARP, we suggest that greater caution should be taken to prevent PSM in cases in which GS at biopsy is high.
Because the correlation between LNI and BCR was very strong in this study and in a previous report [4], we developed a prognostic factor-based risk stratification model for early BCR according to the PSM status in patients without LNI. Consequently, we found that the good-risk group (GS of 6 at a PSM and maximum PSM length of ≤ 6 mm) had significantly higher rates of BCR-free survival than the intermediate-risk group (GS of ≥ 7 at a PSM or maximum PSM length of > 6 mm), especially within 1 year after RARP (p < 0.05). Our results confirmed that the rate of BCR-free survival among patients without PSM was similar to that of the good-risk group after RARP. This good-risk group may comprise patients with PCa and pathologic PSM who had no BCR without additional adjuvant therapy after RP during the open surgery era. Furthermore, by including the presence of LNI to the prognostic factor-based risk stratification model, we could find patients with early BCR within a year after RARP. Although the association between PSM and cancer-specific survival remains controversial, we believe that early prediction of BCR according to the PSM status is important in decisions regarding salvage or adjuvant treatment for preventing the progression of PCa.
The effect of PSM location on BCR is even more controversial [12, 26]. Yossepowitch et al. reported that a posterolateral PSM conferred the highest risk of BCR, according to a review of 73 publications on open surgery [26]. Among the 35 patients with a single PSM in our study, those with a PSM at the apex of the prostate tended to have a lower rate of BCR than those with PSM in the mid-gland and at the base of the prostate; however, the difference was not significant (p = 0.055; data not shown). We have previously reported that when cancer is suspected at the apex on magnetic resonance imaging, a PSM at the apex of prostate is more likely to be present [27]. In accordance with that report, we usually attempt to cut the urethra at the apex of the prostate more distally in these cases. For this reason and because of anatomic rationale, we speculated that in the case of PSM at the apex of the prostate, the residual tumor volume is very small.
Although a high number of PSMs have been reported to be significantly associated with BCR [11, 12], our study confirmed a tendency for patients with a maximum PSM length of > 6 mm to be significantly more prone to BCR than those with a high number of PSMs. However Kates et al. reported that a lower GS at a PSM is independently associated with a shorter margin [14], there was no significant correlation between GS ≥ 7 at a PSM and maximum length of PSM > 6 mm in this study (p = 0.939, data not shown). Because of the difficulty in identifying the site of cancer in the prostate during RARP, careful surgical maneuvering is necessary to avoid PSM of > 6 mm, particularly on the posterolateral side of the prostate.
The clinical course of PCa and PSM is not lethal and is heterogeneous; it may exhibit no clinical progression, even in the absence of adjuvant treatment. In some patients with a low GS and a small PSM, RARP is curative, and we recommend that close observation without immediate adjuvant radiation therapy to be considered in such patients within 12 months after RARP. Because the redundant immediate adjuvant radiation therapy may cause adverse effects, such as radiation cystitis, radiation proctitis, and a second primary cancer, and because it entails unnecessary medical costs, we do not strongly recommend immediate adjuvant radiation therapy in such cases.
This study has several limitations. First, it was a retrospective study at a single institution, and some selection biases toward patients who underwent RARP with NS technique and PLND were present. Second, although all RP specimens were evaluated by pathologists at our hospital, no central pathologic review was performed. Third, we included 99 patients (22.8%) with short postoperative follow-up periods less than 2 years, and the median follow-up period of 52.4 months was too short to assess long-term BCR in patients with PCa after RARP. Fourth, because of the small number of patients with solitary PSM in this study, the relationship between the location of PSM and BCR was unclear, and further investigation including survival information and long-term outcomes is needed. In addition, the PSM in this study tended to be longer than in other studies, and more care must be taken to prevent PSM during RARP. There are two factors which may affect PSM status, one is the patient's factors, such as T stage and the tumor location, the other one is the surgeon's factors, such as skills, experience and procedure employed. The impact of PSM status when predicting worse outcomes in clinical scenario is not simple and may be the surgeon or institution specific. However, we confirmed that the 1-year BCR-free survival rates in good-, intermediate-, and poor-risk groups were 100.0, 72.7, and 48.1%, respectively, and our stratification model could identify which patients should receive immediate adjuvant therapy after RARP.
Conclusion
Higher GS at PSM and longer PSM, and LNI were significant predictors of BCR after RARP, and we developed a simple risk stratification model according to the PSM status to predict early BCR. Immediate adjuvant therapy could be considered for poor-risk patients, and console surgeons should be extremely careful to prevent PSM during RARP.
References
Novara G, Ficarra V, Mocellin S et al (2012) Systematic review and meta-analysis of studies reporting oncologic outcome after robot-assisted radical prostatectomy. Eur Urol 62:382–404
Murphy DG, Kerger M, Crowe H et al (2009) Operative details and oncological and functional outcome of robotic-assisted laparoscopic radical prostatectomy: 400 cases with a minimum of 12 months follow-up. Eur Urol 55:1358–1366
Liesenfeld L, Kron M, Gschwend JE et al (2017) Prognostic factors for biochemical recurrence more than 10 years after radical prostatectomy. J Urol 197:143–148
Morizane S, Honda M, Shimizu R et al (2020) Small-volume lymph node involvement and biochemical recurrence after robot-assisted radical prostatectomy with extended lymph node dissection in prostate cancer. Int J Clin Oncol 25:1398–1404
Badani KK, Reddy BN, Moskowitz EJ et al (2018) Lymph node yield during radical prostatectomy does not impact rate of biochemical recurrence in patients with seminal vesicle invasion and node-negative disease. Urol Oncol 36:e311–e316
Wilczak W, Wittmer C, Clauditz T et al (2018) Marked prognostic impact of minimal lymphatic tumor spread in prostate cancer. Eur Urol 74:376–386
Boorjian SA, Thompson RH, Siddiqui S et al (2007) Long-term outcome after radical prostatectomy for patients with lymph node positive prostate cancer in the prostate specific antigen era. J Urol 178:864–870 (discussion 861-870)
Chalfin HJ, Dinizo M, Trock BJ et al (2012) Impact of surgical margin status on prostate-cancer-specific mortality. BJU Int 110:1684–1689
Boorjian SA, Karnes RJ, Crispen PL et al (2010) The impact of positive surgical margins on mortality following radical prostatectomy during the prostate specific antigen era. J Urol 183:1003–1009
Iremashvili V, Pelaez L, Jorda M et al (2019) A comprehensive analysis of the association between Gleason score at a positive surgical margin and the risk of biochemical recurrence after radical prostatectomy. Am J Surg Pathol 43:369–373
Keller EX, Bachofner J, Britschgi AJ et al (2019) Prognostic value of unifocal and multifocal positive surgical margins in a large series of robot-assisted radical prostatectomy for prostate cancer. World J Urol 37:1837–1844
Sooriakumaran P, Ploumidis A, Nyberg T et al (2015) The impact of length and location of positive margins in predicting biochemical recurrence after robot-assisted radical prostatectomy with a minimum follow-up of 5 years. BJU Int 115:106–113
Brimo F, Partin AW, Epstein JI (2010) Tumor grade at margins of resection in radical prostatectomy specimens is an independent predictor of prognosis. Urology 76:1206–1209
Kates M, Sopko NA, Han M et al (2016) Importance of reporting the Gleason score at the positive surgical margin site: analysis of 4,082 consecutive radical prostatectomy cases. J Urol 195:337–342
Srougi V, Bessa J Jr, Baghdadi M et al (2017) Surgical method influences specimen margins and biochemical recurrence during radical prostatectomy for high-risk prostate cancer: a systematic review and meta-analysis. World J Urol 35:1481–1488
Fujimura T, Fukuhara H, Taguchi S et al (2017) Robot-assisted radical prostatectomy significantly reduced biochemical recurrence compared to retro pubic radical prostatectomy. BMC Cancer 17:454
Hu JC, Gandaglia G, Karakiewicz PI et al (2014) Comparative effectiveness of robot-assisted versus open radical prostatectomy cancer control. Eur Urol 66:666–672
Takenaka A, Tewari AK (2012) Anatomical basis for carrying out a state-of-the-art radical prostatectomy. Int J Urol 19:7–19
Hinata N, Sejima T, Takenaka A (2013) Progress in pelvic anatomy from the viewpoint of radical prostatectomy. Int J Urol 20:260–270
Yumioka T, Honda M, Kimura Y et al (2017) Influence of multinerve-sparing, robot-assisted radical prostatectomy on the recovery of erection in Japanese patients. Reprod Med Biol. https://doi.org/10.1002/rmb2.12063
Mohler J, Bahnson RR, Boston B et al (2010) NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Cancer Netw 8:162–200
Heidenreich A, Bellmunt J, Bolla M et al (2011) EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol 59:61–71
Ploussard G, Briganti A, de la Taille A et al (2014) Pelvic lymph node dissection during robot-assisted radical prostatectomy: efficacy, limitations, and complications-a systematic review of the literature. Eur Urol 65:7–16
Thompson JE, Egger S, Bohm M et al (2014) Superior quality of life and improved surgical margins are achievable with robotic radical prostatectomy after a long learning curve: a prospective single-surgeon study of 1552 consecutive cases. Eur Urol 65:521–531
Gandaglia G, De Lorenzis E, Novara G et al (2017) Robot-assisted radical prostatectomy and extended pelvic lymph node dissection in patients with locally-advanced prostate cancer. Eur Urol 71:249–256
Yossepowitch O, Bjartell A, Eastham JA et al (2009) Positive surgical margins in radical prostatectomy: outlining the problem and its long-term consequences. Eur Urol 55:87–99
Yao A, Iwamoto H, Masago T et al (2014) The role of staging MRI in predicting apical margin positivity for robot-assisted laparoscopic radical prostatectomy. Urol Int 93:182–188
Acknowledgements
The authors thank the medical engineering, nursing, and anesthesia staff at Tottori University Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Morizane, S., Yumioka, T., Makishima, K. et al. Impact of positive surgical margin status in predicting early biochemical recurrence after robot-assisted radical prostatectomy. Int J Clin Oncol 26, 1961–1967 (2021). https://doi.org/10.1007/s10147-021-01977-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-021-01977-x