Abstract
Background
Hepatocellular carcinoma (HCC) is the most common primary liver cancer in the worldwide. Sorafenib is approved for first-line therapy against advanced HCC, but chemo-resistance is still a leading cause of tumor relapse and treatment failure in HCC. Thus, there is a significant clinical need to identify effective strategies to overcome drug resistance on the disease.
Methods
The protein and mRNA expression of TRIM37 in HCC cell lines and patient tissues were determined using Real-time PCR and Western blot, respectively. HCC tissue samples were analyzed by IHC to investigate the association between TRIM37expression and the clinicopathological characteristics of HCC patients. Functional assays, such as MTT, FACS, and Tunel assay, are used to determine the oncogenic role of TRIM37 in human HCC progression. Furthermore, western blotting and luciferase assay were used to determine the mechanism of TRIM37promotes chemoresistance in HCC.
Results
We found that both the mRNA and protein expression of TRIM37 was markedly upregulated in HCC cell lines and tissues, especially in Sorafenib-resistance HCC tissues. Moreover, high TRIM37 expression was associated with poor prognosis with HCC patients. TRIM37 overexpression confers Sorafenib resistance on HCC cells; however, inhibition of TRIM37 sensitized HCC cell lines to Sorafenib cytotoxicity. Additionally, TRIM37 upregulated the levels of AKT activity and phosphorylated AKT, thereby activating canonical AKT signaling.
Conclusion
Our findings suggest that targeting TRIM37 signaling may represent a promising strategy to enhance Sorafenib response in HCC patients with chemoresistant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and account for more than 800,000 deaths in the worldwide each year [1, 2]. Liver transplantation and hepatic resection with adjuvant chemo-therapy are the major treatments for HCC [3]. Sorafenib and Lenvatinib are clinically approved for first-line chemo-therapy against advanced, unresected HCC, but yield modest survival benefits [4, 5]. Currently, the survival rate remains gloomy, with survival rates of only 10–20% at 5 years, because of the late diagnosis and poor prognosis due to intrahepatic metastasis and high rate of recurrence [6,7,8]. Thus, there is an urgent need to develop effective diagnostic methods and therapeutic strategies to improve current HCC treatment modality.
Constitutive activation of Akt signaling is common in a wide range of human solid tumors and plays a central role in tumorigenesis by tipping the balance towards cell survival, proliferation and growth [9, 10]. At this point, comprehensive understanding of the regulatory mechanisms of Akt signaling might provide new clues for the development of targeted cancer therapies. Aside from amplification or gain-of-function mutations in EGFR, RAS, PI3K and Akt can lead to constitutive activation of Akt signaling [11,12,13,14,15], which confers poor prognosis in various types of human cancer, defect in terminating Akt signaling has also been demonstrated to be involved in initiation and progression of human cancers. However, how the inhibitory effects at diverse levels are concomitantly disrupted in tumor cells to exhibit constitutively activated Akt signaling remains largely unclear.
Tripartite motif containing 37 (TRIM37) is a novel E3 ubiquitin ligase which comprises a RING-B-box-coiled-coil domain (RBCC), TRAF domain (TD), and polyacidic domain [16, 17]. It has been recently reported that TRIM37 plays vital roles in various biological processes, and significantly contributes to tumor metastasis and primary tumor growth [18,19,20,21,22]. Moreover, several studies reported that TRIM37 acts as a positive regulator of cell proliferation in lung cancer cells by activation of Akt pathway and knockdown of TRIM37 suppresses the proliferation, migration [23] and invasion of glioma cells through the inactivation of PI3K/Akt signaling pathway [24]. However, the function and molecular mechanism of TRIM37 in HCC were now rarely reported. In the current study, we unveiled a novel function of TRIM37 in HCC chemo-resistance by regulating AKT signaling. We found that TRIM37 confers Sorafenib resistance on HCC cells and inhibition of TRIM37-sensitized HCC cell lines to Sorafenib cytotoxicity. Mechanically, overexpression of TRIM37 upregulated AKT activity and the levels of phosphorylated AKT subsequently activating canonical AKT signaling. Our findings suggest that TRIM37 signaling may represent a promising strategy for Sorafenib-resistant HCC patients and enhance therapeutic effect for this malignant tumor.
Materials and methods
Cell lines and cell culture
Normal hepatocyte cell line, LO2, was from the American Type Culture Collection (ATCC, Manassas, VA, USA) and were cultured under the conditions stated by the manufacturer. The HCC cell lines were grown in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS, Invitrogen), at 37 °C in a 5% CO2 atmosphere in a humidified incubator.
Tissue specimens
Our study included 53 HCC patients who received Sorafenib chemotherapy, patients diagnosed as HCC through pathological examinations and treated from January 2005 to January 2010 in the First Affiliated Hospital of Sun Yat-sen University (Supplementary Table 1). For the use of the clinical materials for research purposes, prior patient consent and approval were obtained from the Institutional Research Ethics Committee. All patients received standard Sorafenib-based chemotherapy. Chemoresistance or chemosensitivity was defined as relapse or progression within one year or after 1 year from the last chemotherapy, respectively. The ten HCC tissues and the matched adjacent noncancerous tissues were frozen and stored in liquid nitrogen until further use.
Plasmids, virus constructs, and retroviral infection of target cells
Full-length human TRIM37-coding sequences were amplified by PCR and cloned into the pSin-EF2 vector. Human TRIM37-targeting short hairpin RNA (shRNA) oligonucleotides sequences were cloned into pSuper-retro-puro to generate pSuper-retro-TRIM37-RNAi(s). The TRIM37 shRNA sequences were: RNAi#1, TTCGAGAATATGATGCTGTGG; and RNAi#2, TTTGCGAGTAAGTCCAAACGG (synthesized by Invitrogen). Transfection of siRNAs or plasmids was performed using the Lipofectamine 3000 reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instruction. Retroviral production and infection were performed as described previously. Stable cell lines expressing indicated genes were selected for 10 days with 0.5 μg/ml puromycin 48 h after infection. The primers used were listed as follows.
Western blot analysis
Western blotting was performed according to the manufacturer’s instruction, using the primary antibodies, anti-TRIM37 (Abcam), anti-p-Akt (Ser473), anti-p-Akt (Thr308), anti-Akt, anti-cleaved caspase3, anti-cleaved PARP antibodies (Cell Signaling, Danvers, MA, USA). Following the initial western blot assay, the membranes were stripped and re-probed with anti-α-tubulin (Sigma, Saint Louis, MO, USA) as a protein loading control.
Immunohistochemistry (IHC)
Immunohistochemical analysis was performed to study altered protein expression in 53 human HCC tissues according previous report. Paraffin-embedded tissues were analyzed using IHC with anti-TRIM37 antibody (Abcam, 1:200). The degree of immunostaining of formalin-fixed, paraffin-embedded sections was reviewed and scored separately by two independent pathologists uninformed of the histopathological features and patient data of the samples. The scores were determined by combining the proportion of positively-stained tumor cells and the intensity of staining. The scores given by the two independent pathologists were combined into a mean score for further comparative evaluation. Tumor cell proportions were scored as follows: 0, no positive tumor cells; 1, < 10% positive tumor cells; 2, 10–35% positive tumor cells; 3, 35–75% positive tumor cells; 4, > 75% positive tumor cells. Staining intensity was graded according to the following standards: 1, no staining; 2, weak staining (light yellow); 3, moderate staining (yellow brown); 4, strong staining (brown). The staining index (SI) was calculated as the product of the staining intensity score and the proportion of positive tumor cells. Using this method of assessment, we evaluated protein expression in malignant lesions by determining the SI, with possible scores of 0, 2, 3, 4, 6, 8, 9, 12, and 16. Samples with a SI ≥ 8 were determined as high expression and samples with a SI < 8 were determined as low expression. Cut-off values were determined on the basis of a measure of heterogeneity using the log-rank test with respect to overall survival.
Cytotoxicity assay
The sensitivity to sorafenib of HCC cells was determined using the MTT assay as previously described. Briefly, 2 × 103 cells were seeded onto 96-well plates and incubated at 37 °C overnight. Cells were then transfected with different concentrations of sorafenib (0–100 μM). After incubation for 72 h, 50 μl of the MTT solution (0.15%) was added to each well, and the plates were further incubated for 2 h. 100 ml of DMSO was added to solubilize the MTT formazan product. Absorbance at 540 nm was measured with a Falcon microplate reader (BD-Labware). Dose–response curves were plotted on a semi-log scale as the percentage of the control cell number, which was obtained from the sample with no drug exposure. IC50 was determined by the intersection of the sorafenib concentration and the midpoint of the 570-nm reading.
Akt activity assay
Cells were serum-starved and treated with EGF (10 ng/ml) or insulin (100 nM). To measure kinase activities in cells or tumor tissues, Akt was precipitated by a specific anti-Akt antibody. The immune complexes were then incubated with a biotinylated peptide substrate, which became phosphorylated in the presence of activated Akt. The phosphorylated substrates, which reflected the activity of Akt kinase in the extract, were then quantified with the K-LISA AKT Activity Kit (Calbiochem, Darmstadt, Germany) that comprises a primary antibody recognizing the phosphorylated substrate peptides.
Apoptosis assay
For evaluation of apoptosis, PE Annexin V Apoptosis Detection Kit I (BD Pharmingen) was used. Briefly, 1 × 106 HCC cells were plated in 10-cm plates and incubated for 24 h. Treatment was started with sorafenib (10 μM) for 24 h. Cell morphology was assessed by phase-contrast microscopy. Then, cells were removed from plate by trypsin–EDTA, washed twice with PBS, and re-suspended with binding buffer at 106 cells/ml. FITC Annexin V and propidium iodide were added (each at 5 μl/105 cells). Cells were incubated for 15 min at room temperature in the dark. Percentage of apoptosis was analyzed with an EPICS XL flow cytometer (Beckman-Coulter). Each sample was analyzed in triplicate.
Statistical analysis
Statistical tests for data analysis included Fisher’s exact test, log-rank test, Chi square test, and Student’s 2-tailed t test. Multivariate statistical analysis was performed using a Cox regression model. Statistical analyses were performed using the SPSS 11.0 statistical software package. Data represent mean ± SD. P < 0.05 was considered statistically significant.
Microarray data process and visualization
Microarray data were downloaded from the TCGA database: (http://www.tcga.org/).
GSEA was performed using GSEA 2.0.9: (http://www.broadinstitute.org/gsea/).
Results
TRIM37 overexpression correlates with progression and poor prognosis in human hepatocellular carcinoma
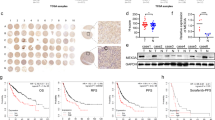
By analysing the published mRNA expression profiles (the cancer genome atlas TCGA dataset, https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga), we found that TRIM37 mRNA was significantly upregulated in HCC tissues compared with normal tissues (P < 0.01, Fig. 1a-b). Moreover, hepatocellular carcinoma patients with higher TRIM37 expression had a shorter survival time and demonstrated an earlier relapse time (P < 0.05; Fig. 1c). Consistently, real-time PCR and western blotting analyses revealed that TRIM37 was markedly overexpressed in hepatocellular carcinoma cell lines at both the protein and mRNA levels, compared with normal human liver cell line (THLE3) (Fig. 1d-e and supplemental Fig. 1a-b). HCC chemotherapy resistance is one of the major reasons for poor prognosis in cancer patients. Interestingly, we found that TRIM37 expression was elevated in sorafenib resistance—but lower in sorafenib-sensitive HCC specimens (Fig. 1f), and higher TRIM37 expression demonstrated an earlier relapse disease-free survival time in HCC patient treatment with sorafenib (Fig. 1g), suggesting that overexpression of TRIM37 might be involved in sorafenib chemotherapy failure in HCC.
Overexpression of TRIM37 correlates with HCC progression and poor prognosis. a, b Expression profiling of mRNAs showing that TRIM37 is upregulated in HCC tissues (T) compared to normal tissues. c Kaplan–Meier analysis of overall (left panel) or disease-free (right panel) survival curves from TCGA dataset for HCC patients with low TRIM37 expression or high TRIM37 expression. *P < 0.05. d Western blotting analysis of TRIM37 expression in THLE3 and 7 HCC cell lines. e Western blotting analysis of TRIM37 expression in eight HCC tissues (T) and two non-tumor tissues (ANT), α-Tubulin was used as a loading control. f IHC staining indicating the TRIM37 protein expression. g The Kaplan–Meier survival curves compare HCC patients with low and high TRIM37 expression levels (n = 53; P < 0.05)
Upregulation of TRIM37 confers sorafenib resistance in HCC in vitro
Interestingly, GSEA analysis revealed that TRIM37 overexpression was strongly correlated with gene signatures associated with anti-apoptosis-based signatures, suggesting that TRIM37 overexpression may contribute to sorafenib-induced apoptosis role in HCC (Fig. 2a). To determine the effect of TRIM37 on HCC chemoresistance, HCC-LM3 and HepG2 cancer cell lines that stably expressed TRIM37 were established (Fig. 2b). Overexpression of TRIM37 in HCC cells (IC50 values were LM3: 23.1 μM and HepG2:43.5 μM, respectively) enhanced sorafenib resistance compared with the vector control (IC50 values were LM3: 4.3 μM, and HepG2:6.9 μM, respectively; P < 0.01) (Fig. 2c). Furthermore, the Annexin V and Tunel staining assay show that the percentage of apoptotic cells in TRIM37-overexpression HCC cells treated with sorafenib was much lower compared than that in control cells (Fig. 2d–e). Furthermore, we also found that overexpressing TRIM37 or silencing TRIM37 only resulted in slightly change of apoptotic rate of HCC cells without any treatment (Supplementary Fig. 2). Interestingly, the protein level of Cleaved caspase3 and Cleaved PARP was significantly decreased in TRIM37 overexpression HCC cells (Fig. 2f). The above results indicated that upregulation of TRIM37 confers sorafenib resistance to HCC cells.
Upregulation of TRIM37 conferred HCC to sorafenib resistance in vitro. a GSEA plot, indicating a significant correlation between the mRNA levels of TRIM37 expression in HCC and the apoptosis resistance gene signatures in published datasets. b Western blotting analysis of TRIM37 in the indicated cells. α-tubulin was used as a loading control. c IC50 of sorafenib in the indicated cells. d Annexin V-FITC and PI staining of the indicated cells treated with sorafenib (10 μM) for 24 h. Each bar represents the mean ± SD of three independent experiments. e Representative micrographs (left) and quantification of Tunel positive signaling in the indicated assay. * P < 0.05. f Western blotting analysis of cleaved caspase3 and PARP in the indicated cells. α-tubulin was used as a loading control
Downregulation of TRIM37 enhances the ability of sorafenib sensitive in HCC in vitro.
We further examined the effect of TRIM37 inhibition on sorafenib chemoresistance. Endogenous TRIM37 expression was silenced using a short hairpin RNA (shRNA; Fig. 3a). Consistent with the overexpression results, IC50 Annexin V and Tunel staining assays show that the percentage of apoptotic cells in TRIM37-suppression HCC cells treated with sorafenib was much higher compared than that in control cells (Fig. 3b-d). In addition, the protein levels of cleaved Caspase3 and Cleaved PARP were significantly increased in TRIM37-suppression HCC compared with control cells (Fig. 3e). Taken together, these results suggest that TRIM37 downregulation induced sorafenib sensitivity to HCC cells in vitro.
Upregulation of TRIM37 conferred HCC to sorafenib resistance in vivo. a Western blotting analysis of TRIM37 in the indicated cells. α-tubulin was used as a loading control. b IC50 of sorafenib in the indicated cells. c Annexin V-FITC and PI staining of the indicated cells treated with sorafenib (10 μM) for 24 h. Each bar represents the mean ± SD of three independent experiments. d Representative micrographs (left) and quantification of Tunel positive signaling in the indicated assay. * P < 0.05. e Western blotting analysis of cleaved caspase3 and PARP in the indicated cells. α-tubulin was used as a loading control
Upregulation of TRIM37 activates the AKT signaling pathway in HCC
To better understand the mechanism underlying TRIM37-overexpressed-induced chemoresistance, Gene ontology (GO) enrichment analysis was performed and showed that AKT signaling pathway was enriched in TRIM37 up-regulated genes in TCGA dataset (Fig. 4a). These results suggest that TRIM37 may play crucial roles in AKT signaling pathway regulation. As expected, the Akt activity was significantly induced in TRIM37-transduced cells but decreased in TRIM37-silenced HCC cells (Fig. 4b). Meanwhile, the strength and duration of Akt activity induced by EGF or insulin were dramatically prolonged in TRIM37-transduced cells but rapidly reduced in TRIM37-silenced HCC cells (Fig. 4c). The expressions of p-Akt expression were dramatically increased in TRIM37-transduced cells and rapidly reduced in TRIM37-silenced cells (Fig. 4d). Furthermore, the expression levels of numerous well-characterized AKT downstream genes were shown to be increased in TRIM37-overexpressing cells, but were lower in TRIM37-silenced cells (Fig. 4d), indicating that TRIM37 overexpression sustains PI3K/Akt signaling in HCC cells.
TRIM37 up-regulation activates the AKT signaling pathway in HCC. a GSEA plot, indicating a significant correlation between the mRNA levels of TRIM37 expression in HCC and the AKT-activated gene signatures in published datasets. b Analysis of luciferase reporter activity in the indicated cells after transfection with 100 ng pAKT-luciferase plasmids or control-luciferase plasmid. c Relative Akt activity in the indicated cells, which were serum-starved and subsequently stimulated with 10ng ml-1 EGF (left panel) or 100 nM insulin (right panel) for indicated times. d Western blotting analysis of the expression levels of indicate proteins in the indicated cells. a-tubulin was used as a loading control. e Real-time PCR analysis demonstrating an apparent overlap between AKT–dependent gene expression and TRIM37–regulated gene expression. The pseudo color represents an intensity scale for TRIM37 versus vector or TRIM37 siRNA versus control siRNA, calculated by log2 transformation
Akt signaling pathway is required for TRIM37-induced chemoresistance
Next, we investigated whether TRIM37-mediated HCC sorafenib resistance occurs through AKT signaling activation. As shown in Fig. 5a, the IC50 of sorafenib inhibited by TRIM37 overexpression on HCC was significantly decreased by transfection of an AKT siRNA or treatment with an AKT inhibitor (LY294002). Meanwhile, we found that blockade of the AKT pathway significantly abrogates the chemoresistance of TRIM37 on HCC cell lines, as determined by Annexin V and Tunel staining assays compared with that in the control group (Fig. 5b-c). Taken together, these results indicate that activation of the AKT signaling pathway exerted functional effects of TRIM37 on HCC chemoresistance.
AKT signaling pathway is required for TRIM37-induced chemoresistance. a Quantification of colony numbers in HCC cells transfected with vector, AKT-siRNA or treated with the AKT inhibitor, as determined by IC50. b Quantification of sorafenib-induced TUNEL-positive cells in HCC cells transfected with vector, AKT-siRNA or treated with the AKT inhibitor. Each bar represents the mean ± SD of three independent experiments. c Annexin V-FITC and PI staining of the indicated cells transfected with the vector, AKT-siRNA or treated with the AKT inhibitor. d Expression analysis (left) and correlation (right) of TRIM37 expression and p-Akt (Ser473) and p-Akt (Thr308) in eight freshly collected HCC tissue samples (T); α-Tubulin was used as loading controls. Each bar represents the mean ± SD of three independent experiments
Clinical relevance of TRIM37-induced AKT activation in HCC
The clinical relevance of TRIM37 expression and AKT activation was further characterized in HCC. As showed in Fig. 5d, e, TRIM37 levels in eight freshly collected clinical HCC samples were positively correlated with p-AKT(Ser-473) (r = 0.62, P = 0.037) signals and p-AKT(Thr-308) signals (r = 0.66, P = 0.031). These data further support the notion that TRIM37 up-regulation confers HCC sorafenib resistance and activation of the AKT signaling pathway that may lead to a poor clinical outcome for patients with HCC.
Discussion
In the current study, we provide evidence that TRIM37 plays an important role in sorafenib resistance in HCC and the regulation of the AKT signalling pathway. TRIM37 expression was significantly increased in sorafenib-resistant HCC tissues and TRIM37 overexpression enhanced sorafenib resistance, but TRIM37silencing restored the sensitivity of HCC cells to sorafenib. Moreover, we found that TRIM37 enhanced sorafenib resistance by upregulating AKT activity and the levels of phosphorylated AKT subsequently activating canonical AKT signalling. These findings identify TRIM37/AKT axis as a potential target for overcoming sorafenib resistance in patients with HCC.
Constitutive activation of Akt signalling is a common event in human cancers and acts as a key factor in cancer development and progression [25, 26]. Therapeutic targeting of the Akt pathway has been aggressively pursued for the treatment of a wide range of malignant pathologies, including HCC [27,28,29,30]. For example, Fu et al. reported that Circ-IGF1R promotes proliferation and anti-apoptotic in HCC by activating the PI3K/AKT pathway [27]. Jondal and colleagues also reported that neoadjuvant PI3K/mTOR/AKT inhibition reduces tumour growth of hepatocellular carcinoma after laser thermal ablation in small-animal model [28]. Interestingly, it has been reported that dual inhibition of Akt and c-Met as a second-line therapy following acquired resistance to sorafenib in hepatocellular carcinoma cells [29]. Additionally, Tang demonstrated that overexpression INPP4B, an important negative regulation gene of AKT signalling pathway, suppressed cell proliferation, migration, invasion and EMT in human HCC cell lines [30]. Collectively, these findings establish a strong rationale for therapeutic targeting of the AKT pathway in HCC. Nevertheless, achieving improved treatment outcomes has been an insurmountable challenge to date. Although current therapeutic approaches, such as the use of Akt inhibitors, MK-2206 or GDC-0068, which are known to promote cell growth arrest and to sensitize cancer cells to radiotherapy, however, response rates in clinical trials with single-agent Akt inhibitors are typically low [31]. Therefore, there is an urgent need to identify more effective therapeutic targets that regulate Akt in an appropriate manner as an alternative to global AKT blockade. Herein, we found that TRIM37 was over-expressed in HCC and silencing TRIM37 significantly both inhibited the transcription activity of AKT and the levels of phosphorylated AKT, subsequently restored the sensitivity of HCC cells to sorafenib. Therefore, our results suggesting that TRIM37 could contribute to AKT activation and thereby represent a novel target for HCC treatment.
TRIM37 gene located on chromosome 17q22–23, which was originally found to be frequently mutated in patients with mulibrey nanism, a disease with dramatic growth impairment [16, 17]. Previous studies had been shown that TRIM37 overexpression in HCC was associated with advanced stage and tumour progression and TRIM37 may serve as an independent prognostic factor of HCC. For example, Jiang et al. reported that over-expression of TRIM37 promoted cell migration and metastasis and activates Wnt/beta-catenin signalling in HCC [32]. N Mir et al. demonstrated that TRIM37 mediated EMT in HCC cells and it was achieved by the activation of Wnt signalling [33]. Further studies demonstrated that TRIM37 plays vital roles in various biological processes depending on TRIM domain-dependent E3 ligase activity, such as promotion of peroxisomal matrix protein import via direct monoubiquitination of PEX5 at K464 and silencing of gene expression through monoubiquitination of histone H2A [34, 35]. However, overexpression of a TRIM37 mutant that lacks E3 ligase activity could not prevent the TRIM37 depletion-resulted supernumerary centrosomal-component foci [36]. Previous reports have been demonstrated that TRIM37 promotes lung cancer cell proliferation dependent on the regulation of BCL2 and BAX [23] and that knockdown of TRIM37 dramatically inhibited the proliferation, migration/invasion, and the epithelial-mesenchymal transition (EMT) phenotype in glioma cells [24]. However, the detailed mechanism of TRIM37 in cancer is still unclear. In this study, we showed that TRIM37 could activate the AKT signalling pathway in HCC cells and TRIM37 levels were significantly correlated with AKT activity in clinical HCC tissues. Interestingly, TRIM37 can be found in other TRIM family members, which might mediate activation of AKT signalling because of its E3 ubiquitin ligase activity. For example, several negative regulations of AKT signalling, PTEN, NPP5J, PPP2A, etc., were underlying ubiquitination and proteasome degradation, subsequently activating Akt signalling pathway. Therefore, elucidating the underlying mechanism whereby TRIM37 activates AKT signalling in HCC will require further investigation.
In conclusion, we unveiled a novel function of TRIM37 in HCC chemo-resistance by regulating AKT signalling. We found that TRIM37 confers Sorafenib resistance on HCC cells and inhibition of TRIM37 sensitized HCC cell lines to Sorafenib cytotoxicity. Mechanically, overexpression of TRIM37 upregulated AKT activity and the levels of phosphorylated AKT subsequently activating canonical AKT signalling. Our findings suggest that TRIM37 signalling may represent a promising strategy for Sorafenib-resistant HCC patients and enhance therapeutic effect for this malignant tumour.
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66:115–132
Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A (2016) Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 66:271–289
Keating GM (2017) Sorafenib: a review in hepatocellular carcinoma. Target Oncol 12:243–253
Kudo M (2018) Systemic therapy for hepatocellular carcinoma: latest advances. Cancers (Basel) 10:6
Giannini EG, Farinati F, Ciccarese F, Pecorelli A, Rapaccini GL, Marco M, Benvegnu L, Caturelli E, Zoli M, Borzio F, Chiaramonte M, Trevisani F (2015) Italian liver cancer, prognosis of untreated hepatocellular carcinoma. Hepatology 61:184–190
Ghouri YA, Mian I, Rowe JH (2017) Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog 16:1
Brandi G, de Rosa F, Agostini V, di Girolamo S, Andreone P, Bolondi L, Serra C, Sama C, Golfieri R, Gramenzi A, Cucchetti A, Pinna AD, Trevisani F, Biasco G (2013) Italian liver cancer, metronomic capecitabine in advanced hepatocellular carcinoma patients: a phase II study. Oncologist 18:1256–1257
Vivanco I, Sawyers CL (2002) The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2:489–501
Qiao M, Sheng S, Pardee AB (2008) Metastasis and AKT activation. Cell Cycle 7:2991–2996
Yuan TL, Cantley LC (2008) PI3K pathway alterations in cancer: variations on a theme. Oncogene 27:5497–5510
Bachman KE, Argani P, Samuels Y, Silliman N, Ptak J, Szabo S, Konishi H, Karakas B, Blair BG, Lin C, Peters BA, Velculescu VE, Park BH (2004) The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol Ther 3:772–775
Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD, Downward J (1994) Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature 370:527–532
Moscatello DK, Holgado-Madruga M, Emlet DR, Montgomery RB, Wong AJ (1998) Constitutive activation of phosphatidylinositol 3-kinase by a naturally occurring mutant epidermal growth factor receptor. J Biol Chem 273:200–206
Downward J (2003) Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer 3:11–22
Avela K, Lipsanen-Nyman M, Idanheimo N, Seemanova E, Rosengren S, Makela TP, Perheentupa J, Chapelle AD, Lehesjoki AE (2000) Gene encoding a new RING-B-box-Coiled-coil protein is mutated in mulibrey nanism. Nat Genet 25:298–301
Kallijarvi J, Avela K, Lipsanen-Nyman M, Ulmanen I, Lehesjoki AE (2002) The TRIM37 gene encodes a peroxisomal RING-B-box-coiled-coil protein: classification of mulibrey nanism as a new peroxisomal disorder. Am J Hum Genet 70:1215–1228
Hu X, Xiang D, Xie Y, Tao L, Zhang Y, Jin Y, Pinello L, Wan Y, Yuan GC, Li Z (2019) Suppresses invasion migration and metastasis of luminal breast cancer cells via activation of GATA3 and repression of TRIM37 expression. Oncogene 7:234
Han C, Xia X, Jiao S, Li G, Ran Q, Yao S (2019) Tripartite motif containing protein 37 involves in thrombin stimulated BV-2 microglial cell apoptosis and interleukin 1beta release. Biochem Biophys Res Commun 516:1252–1257
Brigant B, Metzinger-Le Meuth V, Rochette J, Metzinger L (2018) TRIMming down to TRIM37: relevance to inflammation, cardiovascular disorders, and cancer in MULIBREY nanism. Int J Mol Sci 20:9
Li Y, Deng L, Zhao X, Li B, Ren D, Yu L, Pan H, Gong Q, Song L, Zhou X, Dai T (2018) Tripartite motif-containing 37 (TRIM37) promotes the aggressiveness of non-small-cell lung cancer cells by activating the NF-kappaB pathway. J Pathol 246:366–378
Tao Y, Xin M, Cheng H, Huang Z, Hu T, Zhang T, Wang J (2017) TRIM37 promotes tumor cell proliferation and drug resistance in pediatric osteosarcoma. Oncol Lett 14:6365–6372
Dong S, Pang X, Sun H, Yuan C, Mu C, Zheng S (2018) TRIM37 targets AKT in the growth of lung cancer cells. Onco Targets Ther 11:7935–7945
Tang SL, Gao YL, Wen-Zhong H (2018) Knockdown of TRIM37 suppresses the proliferation, migration and invasion of glioma cells through the inactivation of PI3K/Akt signaling pathway. Biomed Pharmacother 99:59–64
Chan TO, Rittenhouse SE, Tsichlis PN (1999) AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem 68:965–1014
Potter CJ, Pedraza LG, Xu T (2002) Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol 4:658–665
Fu HW, Lin X, Zhu YX, Lan X, Kuang Y, Wang YZ, Ke ZG, Yuan T, Chen P (2019) Circ-IGF1R has pro-proliferative and anti-apoptotic effects in HCC by activating the PI3K/AKT pathway. Gene 716:144031
Jondal DE, Thompson SM, Butters KA, Knudsen BE, Anderson JL, Roberts LR, Callstrom MR, Woodrum DA (2019) Single-dose neoadjuvant AKT pathway inhibitor reduces growth of hepatocellular carcinoma after laser thermal ablation in small-animal model. Radiology 292:752–759
Han P, Li H, Jiang X, Zhai B, Tan G, Zhao D, Qiao H, Liu B, Jiang H, Sun X (2017) Dual inhibition of Akt and c-Met as a second-line therapy following acquired resistance to sorafenib in hepatocellular carcinoma cells. Mol Oncol 11:320–334
Tang W, Yang L, Yang T, Liu M, Zhou Y, Lin J, Wang K, Ding C (2019) INPP4B inhibits cell proliferation, invasion and chemoresistance in human hepatocellular carcinoma. Onco Targets Ther 12:3491–3507
Pretre V, Wicki A (2018) Inhibition of Akt and other AGC kinases: a target for clinical cancer therapy? Semin Cancer Biol 48:70–77
Jiang J, Yu C, Chen M, Tian S, Sun C (2015) Over-expression of TRIM37 promotes cell migration and metastasis in hepatocellular carcinoma by activating Wnt/β-catenin signaling. Biochem Biophys Res Commun 464:1120–1127
Pretre V, Wicki A (2017) Epithelial-to-mesenchymal transition: a mediator of sorafenib resistance in advanced hepatocellular carcinoma. Curr Cancer Drug Targets 17:698–706
Wang W, Xia ZJ, Farre JC, Subramani S (2017) TRIM37, a novel E3 ligase for PEX5-mediated peroxisomal matrix protein import. J Cell Biol 216:2843–2858
Bhatnagar S, Gazin C, Chamberlain L, Ou J, Zhu X, Tushir JS, Virbasius CM, Lin L, Zhu LJ, Wajapeyee N, Green MR (2014) TRIM37 is a new histone H2A ubiquitin ligase and breast cancer oncoprotein. Nature 516:116–120
Kallijarvi J, Lahtinen U, Hamalainen R, Lipsanen-Nyman M, Palvimo JJ, Lehesjoki AE (2005) TRIM37 defective in mulibrey nanism is a novel RING finger ubiquitin E3 ligase. Exp Cell Res 308:146–155
Acknowledgment
Natural Science Foundation of China (No. 81602701, No.81974443); Natural Science Foundation of Guangdong Province (No. 2017A030313547, No. 2018A030313176); Science and Technology Projects Foundation of Guangdong Province (No. 2015A070710006, No. 2016A020215053); Science and Technology Projects Foundation of Guangzhou City (No. 201507020037, No. 201607010260); Research project of Gannan Medical University, Gannan Medical University Key Cultivation project (No:ZD201802); Natural Science Foundation of Jiangxi Province (No:20202BAB206040)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Tan, G., Xie, B., Yu, N. et al. TRIM37 overexpression is associated with chemoresistance in hepatocellular carcinoma via activating the AKT signaling pathway. Int J Clin Oncol 26, 532–542 (2021). https://doi.org/10.1007/s10147-020-01832-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-020-01832-5